Abstract
Background. Carcinoma ex pleomorphic adenoma (CXPA) is a rare parotid malignancy and until today no standardized concept exists for its therapy apart from recommendations for parotid carcinoma in general. Prognosis is thought to be poorer than for other parotid malignancies. We sought to describe a general diagnostic and therapy strategy and assess factors predicting the outcome. Methods. We retrospectively analysed the courses of 22 patients with a CXPA of the parotid gland treated at a tertiary medical care centre for otorhinolaryngology. We examined parameters of medical history, diagnostics, surgical and adjunctive therapy and analysed overall and disease-specific survival. Results. About half of the patients had evidence of a parotid mass of up to 1 year only while maximum of the others was 48 years. Nine patients were primarily operated without suspicion for malignancy. Both 5-year disease-specific and overall survival were 60%. Recurrence-free survival rate after 5 years was 85%. Any patients with a stage I or II disease had an uneventful follow-up. To date, no patient with a stage IV disease has survived longer than 5 years. Conclusion. Surgical therapy (total or radical parotidectomy) is the method of choice for CXPA of the parotid gland. Stage I tumors have a very good and stage IV tumors a bad prognosis.
Tumors of the salivary glands are rarely seen neoplasms with a yearly incidence of only 0.4–2.5 tumors per 100 000 residents Citation[1]. However, the salivary glands are beset by a greater variety of neoplasms than any other organ in the body. Approximately 80% of all salivary gland tumors occur in the parotid gland and again roughly 80% of these are said to be benign tumors. In respect to all neoplastic processes in the major salivary glands, the pleomorphic adenoma (PA) is the most frequent one with an incidence of 67.5% Citation[2]. As numerously mentioned in the literature, the benign PA can transform into a carcinoma ex pleomorphic adenoma (CXPA), however reported prevalences in the literature differ greatly (3–15%) Citation[3–5]. Many authors claim that the risk of malignant transformation increases with the duration of history as well as with tumor size Citation[1], Citation[4], Citation[6]. Due to the entity's low incidence, so far no standard concept exists for its treatment. Most literature sees CXPA in general having a very bad prognosis. As we were able to detect a rather young population with early diseases, we sought to investigate whether early diagnosis and treatment could lead to an improved clinical course and outcome.
Materials & methods
We retrospectively analysed 22 patients treated at the Department of Otorhinolaryngology, Head and Neck Surgery at the University Hospital of Cologne for a histopathologically verified carcinoma ex pleomorphic adenoma (CXPA) of the parotid gland from 1987 to 2006.
We recorded personal variables and preoperative clinical and diagnostic results. Follow-up included routine physical examination and if necessary imaging of the primary site. When required, the referring physician was contacted to obtain relevant data.
Statistical analyses were performed using SPSS software for medical statistics (release 12.0). When comparing two groups, unpaired t-tests or Mann-Whitney-u-tests were calculated. Tests of association of categorical variables were performed using a χ2 statistic or Fisher's exact test. The confidence interval (CI) was 95% and significance levels were p = 0.05. Percentage numbers are rounded in the text.
Results
Patients’ characteristics
The sex ratio among the affected persons was balanced (12 women, 10 men). The overall incidence peak was the fifth decade of life and continuously thereafter. Mean age was 57 years (range: 29–82 years). The mean lead time (clinical evidence of a parotid mass) was 9 years (range 0.1–48 years).
Preoperative findings are listed in .
Table I. Preoperative findings
Therapy and findings
At the end of all preoperative diagnostics (including at least the history, physical examination, ultrasound and FNA; where required added by CT and/or MRI) nine patients were thought to have a pleomorphic adenoma of the parotid gland before operation. Eight patients were under suspicion of having a parotid malignoma. In only three cases the suspected diagnosis was CXPA. Depending on the suspected diagnosis patients received either a superficial, total or radical parotidectomy. When histological analyses revealed the CXPA, six patients were re-operated. In sum, 15 patients received a total, four patients a radical parotidectomy. In three patients having received a superficial parotidectomy only, we abstained from further intervention due to the small size of the carcinomatous part. These patients were in disease stage I or II and to date remain with an uneventful follow-up.
In 20 patients the primary parotid tumor could be removed totally (R0). The two remainders with carcinoma unclear surgical margins (R1) were both found to have distant metastasis. Twelve patients received an additional ipsilateral neck dissection revealing a pathologic N-positive disease in half of them with one case of extracapsular extension.
All the T1 (n = 8) and T2 tumors (n = 7) were without any locoregional or distant metastasis and thus classified for their correlating TNM/AJCC Citation[7] disease stage I, respectively stage II. All others were classified stage IV (n = 7).
The carcinoma histology was adenocarcinoma in 14 cases. Second were adenoid (n = 3) and myoepithelial carcinomas (n = 2) with remaining malignancies being an anaplastic, an undifferentiated and a polymorph microinvasive carcinoma.
In total, twelve patients were postoperatively radiated and their mean age was 63.8 years compared to 50.2 years for the not radiated patients. The radiation dose ranged from 48 Gy to 72 Gy (mean dose 61 Gy). Four patients had a combined radiochemotherapy with carboplatinum.
shows operative and postoperative characteristics.
Table II. Operative and postoperative findings
Follow-up and survival
Mean observation time for all patients was 51 months (range 11–155 months). The overall survival after 5 years was 57% (mean 97 months; CI: 65–128 months).
The 5-year disease-specific survival was 60% with four patients having died of their disease. According to the Kaplan-Meier calculation, the mean postoperative disease-free time was 124 months (i.e. 10 years; CI: 97–152 months). In those subjects with a total resection of a CXPA (R0), the recorded recurrence-free survival rate after 5 years was 85%.
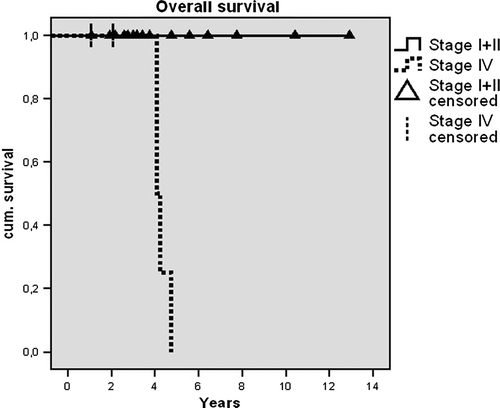
Of all patients with a stage I or II disease (n = 15), none had a tumor recurrence and no case of disease-specific death was recorded (mean follow-up 57 months; mean age 53 years). The group of stage IV tumors (n = 7) included all four cases of disease-specific death and both cases of tumor recurrence (mean follow-up 40 months; mean age 67 years). So far none of these patients has survived more than 5 years, though many lived for at least 4 years ().
Figure 1. Kaplan-Meier calculation shows none of the stage IV patients surviving more than 5 years after diagnosis, though many live for at least 4 years. Overall survival for stage I and stage II patients is excellent.
Overall recurrence-free survival rate of radiated patients was 50% after 5 years compared to 100% of unradiated patients (p = 0.029).
Patients receiving chemotherapy had a lower overall survival rate (50%) after 5 years than those receiving no chemotherapy (63%). This result however shows no significance due to the low number of patients receiving adjuvant chemotherapy at all.
The following variables were significantly associated with disease-specific death: disease stage (p = 0.007), age (p < 0.001), T stage (p = 0.11), M stage (p = 0.003), N stage (p = 0.002), tumor recurrence or residuum (p = 0.001).
No parameter was statistically associated with overall survival.
Discussion
The CXPA is the malignant derivative of the benign mixed tumor or pleomorphic adenoma. Literature says that CXPA usually emerges in the 6th or 7th decades of life span Citation[8–11]. In our sample about half of the patients were older than 50 years, but four of our 22 patients were less than 40 years old and with this our sample is younger than any other population previously described. Higher disease stage tended to be associated with older age (p = 0.058) with a mean age of 53 years for patients with stage I or stage II disease, compared to 67 years for patients with a stage IV disease (range: 45–82 years). This item has not been discussed before in the literature, and deserves further investigation.
Lead time in our sample was on average 9 years. The literature shows an inconsistently but meaningful picture with either rather short (9 months) or long durations of symptoms (23 years) Citation[10], Citation[11].
Compared to the literature we do not only seem to have acquired a rather young, but also a rather early CXPA group with 68% stage I or II patients. Statistical analysis suggests that there is a causal correlation between these two items. Taken together, the frequency of stage I and stage II of CXPA and the short history suggest that a synchronous development of carcinomas and pleomorphic adenomas is at least possible.
A quarter of our patients had undergone parotidectomy on the ipsilateral side before. Accordingly the repeated excisions of recurrent PA do not seem to engender malignancy Citation[12]. Apart from a very long history no other apparent risk factors are known for the development of a CXPA.
For the preoperative facial nerve palsy our number (23%) is comparable to others in the literature and numbers for the suspected microscopic or confirmed histologic infiltration of the facial nerve are likewise Citation[9–11], Citation[13].
The therapy of choice for a CXPA in the parotid gland is surgery. In our sample the follow-up has indicated that superficial parotidectomy can be a sufficient surgical approach for very small carcinomas inside PA, but otherwise a total if not radical parotidectomy is recommended. A neck dissection has a diagnostical and a therapeutical meaning, but still for very small carcinomas at a clinically inconspicuous neck the procedure might be dispensable.
In line with the literature we used the following indication criteria for postoperative irradiation in our sample: high grade disease, question of resection adequacy with possible histologic margin involvement, lymph nodes beset, perineural invasion or lymphangiosis carcinomatosa. Statistically, in our study the use of postoperative radiation therapy was associated with a significant reduction in recurrence-free survival rate, but this result largely depends on the fact that any recurrence occurred only in stage IV patients and all these had been radiated. We support the notion that, in general, surgery followed by postoperative radiation should be considered the standard of care for patients with CXPA Citation[9]. However, as unradiated patients with a stage I or II disease had an uneventful follow-up in our study, surgery alone can be a therapy option for small carcinomas.
The role of additional postoperative chemotherapy for CXPA remains unclear Citation[5]. Our decisions on whether treating patients with adjuvant chemotherapy or not were based on a combination of patients’ general condition, age, tumor stage and pathologic details. This field clearly needs more research exploration.
The prognosis of parotid tumors in general is said to decline with the extent of the tumor and the existence of locoregional metastases Citation[5]. Recurrence and R1 resection of a malignant parotid gland tumor indicates an unfavorable long-term prognosis Citation[10]. CXPA in general is said to have the worst prognosis of all parotid malignancies with a disease-specific 5-year survival rate of only 44% and a locoregional control of 66%. Our specific sample with low stage disease may explain the slight improvement in overall (57%) and disease-specific survival (60%) after 5 years compared to data published so far Citation[9], Citation[10].
Conclusions
The CXPA of the parotid gland is a rare tumor which has its peak incidence in the 6th and 7th decade of life. Depending on the lead time, patients either present with a low or high stage disease. Currently preoperative diagnostics cannot reliably predict the correct diagnosis. Adenocarcinoma is the malignant component in the majority of cases. Surgical therapy (total or radical parotidectomy) is the method of choice for CXPA of the parotid gland. For small carcinomas a pure surgical concept alone without any adjunctive therapy has shown no disadvantage in our sample, while for greater CXPA postoperative radiotherapy is recommended. The role of postoperative chemotherapy is still unclear. In general stage I tumors have a very good and stage IV tumors a comparatively bad prognosis.
References
- Böcker W, Denk H, Heitz PU. Pathologie. Urban & Fischer Verlag; 2003.
- Stojadinovic S, Reinert S, Philippou S. [Problems in diagnosis and therapy of carcinoma in stroma-abundant pleomorphic adenoma of the parotid gland]. Mund Kiefer Gesichtschir. 1998; 2(5)266–9
- Rice DH. Salivary gland disorders. Neoplastic and nonneoplastic. Med Clin North Am 1999;83(1):197–218, xi.
- Seifert G. [Carcinoma in pre-existing Warthin tumors (cystadenolymphoma) of the parotid gland. Classification, pathogenesis and differential diagnosis]. Pathologe 1997; 18(5)359–67
- Spiro RH. The management of salivary neoplasms: An overview. Auris Nasus Larynx 1985; 12(Suppl 2)S122–S127
- Riede UN, Werner M, Schäfer HE. Allgemeine und spezielle Pathologie. 41st ed. Deutsches Ärzteblatt; 2004.
- Major salivary glands (parotid, submandibular, and sublingual). American Joint Committee on Cancer: AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002. p 53–58.
- Altemani A, Martins MT, Freitas L, Soares F, Araujo NS, Araujo VC. Carcinoma ex pleomorphic adenoma (CXPA): immunoprofile of the cells involved in carcinomatous progression. Histopathology 2005; 46(6)635–41
- Chen AM, Garcia J, Bucci MK, Quivey JM, Eisele DW. The role of postoperative radiation therapy in carcinoma ex pleomorphic adenoma of the parotid gland. Int J Radiat Oncol Biol Phys 2007; 67(1)138–43
- Nouraei SA, Hope KL, Kelly CG, McLean NR, Soames JV. Carcinoma ex benign pleomorphic adenoma of the parotid gland. Plast Reconstr Surg 2005; 116(5)1206–13
- Olsen KD, Lewis JE. Carcinoma ex pleomorphic adenoma: a clinicopathologic review. Head Neck 2001; 23(9)705–12
- Batsakis JG. Recurrent mixed tumor. Ann Otol Rhinol Laryngol 1986; 95(5 Pt 1)543–4
- Lewis JE, Olsen KD, Sebo TJ. Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol 2001; 32(6)596–604
