Abstract
Purpose. Cytarabine (ara-C) has limited activity in solid tumours. CP-4055 (ELACYT™) is a novel ara-C-5’-elaidic acid ester that may circumvent this limitation. CP-4055 maximum tolerated dose (MTD), pharmacokinetics and antitumor activity have been investigated in patients with solid tumours. Material and methods. Thirty-four patients (19 malignant melanoma, 8 ovarian cancers and 7 NSCLC) received CP-4055 as a 30 min, or 2 hr intravenous (IV) infusion daily for 5 consecutive days every 3 or 4 weeks (D1-5 q3w or D1-5 q4w) in a dose escalation designed study with doses ranging from 30 to 240 mg/m2/day. Results. The most frequent CTC grade 1-2 adverse events (AEs) were nausea, fatigue, vomiting, anorexia and pyrexia. Most of the grade 3–4 AEs were neutropenia. The MTD was 200 mg/m2/day and 240 mg/m2/day for D1-5 q3w and D1-5 q4w, respectively. The MTD was independent of infusion time in the 4 week schedule. CP-4055 was maintained in plasma for up to 5-10 hr at dose levels >150 mg/m2/day. One objective partial response (PR) with time to progression (TTP) of 22 months was reported in an advanced malignant melanoma patient. Conclusion. CP-4055 was well tolerated; the majority of the AEs were of CTC grade 1. The 3 week schedule was not recommended due to neutropenic nadir between days 18–26. The recommended dose was 200 mg/m2/day in a D1-5 q4w schedule. Efficacy data suggest that CP-4055 might be active in treatment of solid tumours.
Stage IV malignant melanoma, ovarian cancer and non-small cell lung cancer (NSCLC) are diseases with poor prognoses Citation[1–8].
Ara-C, a well–known antimetabolite drug, is routinely used in combination with other chemotherapeutics to treat acute myeloid leukaemia (AML) Citation[9], acute lymphoblastic leukaemia (ALL) and non-Hodgkin's lymphoma Citation[10–13]. However, ara-C has limited cytotoxicity in solid tumours Citation[14–16]. This may be due to a poor penetration into the tumour cells Citation[16], a minimal accumulation in tumour cells Citation[17], a limited activation via intracellular phosphorylation of ara-C into ara-CTP (ara-C triphosphate, the active metabolite), or to a rapid deamination and inactivation of ara-C to ara-U (uracil arabinoside).
CP-4055, a fatty acid derivative (ara-C-5’-elaidic acid ester) of ara-C, was designed in order to facilitate cellular accumulation of ara-C in tissues, increase ara-C retention in tumour cells and potentially delay inactivation to ara-U. Unlike ara-C, cellular uptake of CP-4055 is independent of nucleoside transporters, and is believed to diffuse passively through the cellular membrane or to use an alternative internalization mechanism Citation[18]. CP-4055 is then hydrolyzed intracellularly by esterases to release free ara-C which is subsequently phosphorylated to the active ara-CTP. Interestingly, in contrast to ara-C described solely as a potent inhibitor of DNA synthesis Citation[19], CP-4055 also transiently inhibits RNA synthesis Citation[20]. Moreover CP-4055 is not a substrate for deoxycytidine deaminase Citation[21].
CP-4055 has demonstrated cytotoxicity in solid tumour and leukaemia cells in vitro and in vivo Citation[18], Citation[20], Citation[21]. Repeated treatment with CP-4055 in human leukaemia and in solid tumour models in vivo enhanced the antitumor effect Citation[18].
Based on the above preclinical data, a dose finding phase I study was designed to determine the MTD, safety profile, PK properties and possible antitumor effect of CP-4055 when given to patients with malignant melanoma, ovarian cancer or NSCLC.
Material and methods
Patient population
Patients with a histological confirmed diagnosis of advanced malignant melanoma, ovarian cancer or NSCLC who had failed standard therapy, were enrolled between November 2003 and May 2005. Further eligibility criteria were ECOG-WHO performance status 0–2, age ≥18 years, adequate bone marrow function (absolute neutrophil count ≥1.5×109/L, platelets ≥100×109/L, and haemoglobin ≥9 g/dL), hepatic function (transaminases ≤ 2.5×upper normal laboratory value (UNL) or < 5×UNL if documented liver metastases; bilirubin and alkaline phosphatase ≤1.5×UNL), renal function (creatinine ≤1.6 mg/dL), and no significant reduction in heart function, life expectancy > 3 months, no chemo-, radio-, or immunotherapy within 6 weeks prior to study and measurable lesions on computed tomography (CT) scans or magnetic resonance imaging (MRI). Major surgery, excluding biopsy, was not permitted within 4 weeks prior to study start. Pregnant or breast feeding women, and patients with previous or current malignancies at other sites (with the exception of adequately treated in situ carcinoma of the cervix uteri or basal or squamous cell carcinomas of the skin), known brain and/or leptomeningeal tumour involvement and history of allergic reactions to ara-C or eggs, uncontrolled intercurrent illnesses (including active infection, symptomatic congestive heart failure, unstable angina pectoris, cardiac arrhythmia, or a history of significant neurological or psychiatric disorders) were also excluded.
Ethics and consent
Prior to patient enrolment, the study has been approved by local institutional review boards. All patients gave written informed consent in accordance with national and institutional review board guidelines. All the procedures were following the Helsinki declaration of 1975, as revised in 1983.
Clinical assessments before and during treatment
Medical history and physical examination (height, weight, vital signs and ECOG performance status) were recorded at baseline. Cardiac function was monitored continuously during the infusion of CP-4055. Physical examinations, haematological chemistry and urinalysis were recorded at regular intervals. Safety was assessed based on data available from all cycles, and toxicity was graded using the NCI CTC version 2, April 1999. Tumour size was recorded at baseline and at the end of each second cycle. Tumour response was evaluated using the Response Evaluation Criteria in Solid Tumours (RECIST) Citation[22]. The evaluation of the lesions was defined as following: Complete Response (CR), disappearance of all target/non target lesions; Partial Response (PR), at least a 30% decrease in the sum of the longest diameter of target lesions, taking as reference the baseline sum longest diameter; Progressive Disease (PD), at least a 20% increase in the sum of the longest diameter of target lesions, taking as reference the smallest sum longest diameter recorded since the treatment started or the appearance of one or more new lesions; Absence of disease progression, neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum longest diameter since the treatment started.
Study design and methodology
The study was an open-label phase I, non-randomized multicentre (the Norwegian Radium Hospital, Oslo, Norway; the Royal Surrey County Hospital, Guildford, UK; the Princess Royal Hospital, Hull, UK), dose escalation study.
The objectives were to determine dose limiting toxicities (DLTs), the MTD, the recommended dose (RD), the CP-4055's PK profile and tumour response.
Drug administration
CP-4055 (5 or 10 mg/mL sterile solution) was diluted in 0.9% saline before a 30 min IV infusion daily for 5 consecutive days every 3 weeks (D1-5 q3w) or 4 weeks (D1-5 q4w). A 2 hr infusion was also investigated in the 4 week schedule in order to assess how it would affect the safety profile of CP-4055. Dose of CP-4055 started at 30 mg/m2/day and was escalated according to a modified Fibonacci regimen. Three patients were treated per dose level, and inclusion of two new patients at each dose level was pending until the tolerance of the first patient in the first cycle had been clinically evaluated. The number of treatment cycles per patient was unlimited in the absence of PD and/or unacceptable AEs.
Dose limiting toxicity
The DLT was defined either as a) any non-haematological AE CTC grade ≥3 other than alopecia, nausea, vomiting or fever, b) neutropenia grade 4 lasting for ≥7 days, c) febrile neutropenia defined as an absolute neutrophil count < 500/µL lasting for ≥3 days and fever at least 38.5 °C for 24 hr, or d) thrombocytopenia CTC grade 4.
After the occurrence of one DLT, up to 3 additional patients were enrolled at the same dose level. If no DLT was observed among the 3 new patients, the next dose level could be explored. If at least one additional DLT was observed among the 3 new patients, then the dose escalation was stopped and further enrolment was done at the dose level below.
Maximum tolerated dose and recommended dose
The MTD was defined per treatment schedule to be the dose of CP-4055 that caused a DLT in at least 2 of 6 patients in the first treatment cycle. The RD was defined as the highest dose below the MTD on which no more than 1 of 6 patients experienced DLT.
Pharmacokinetic analysis
Blood samples (2 mL) were collected on day 1 and 4 during the first cycle. Plasma concentrations of CP-4055, ara-C and ara-U were quantified using a validated LC-MS/MS procedure with a limit of quantification of 5 ng/ml.
Estimates of the PK parameters (peak concentration (Cmax), time of observed maximum (plasma) concentration (Tmax), t½, and area under the concentration-time curve (AUC), clearance (CL) and steady state volume of distribution (Vss) of CP-4055, ara-C and ara-U were derived from individual concentration-time data sets by non-compartmental analysis.
Clinical chemistry, haematology and urine assessments
Blood, plasma and serum samples as well as urine strip tests were analyzed to monitor bone marrow, liver, kidney and renal functionalities. These analyses were performed according to the hospitals’ routine procedures.
Results
Patient characteristics
The 34 enrolled patients were all Caucasian, with a mean age of 56 years (range 27–76), and with performance status ECOG-WHO 0–1 (). Nineteen patients had malignant melanoma, 8 ovarian cancer, and 7 patients NSCLC. Apart from 1 patient pre-treated with immunotherapy only, all others had received chemotherapy prior to the study.
Table I. Patient characteristics.
Twenty-one patients had undergone surgery; 11 had additional immunotherapy and 9 had additional radiotherapy for more than 6 weeks prior to the study ().
Table II. Patient pre treatment prior to enter in the study.
Dose escalation, DLT and MTD
3 week schedule (D1-5), 30 min infusion (n = 24)
Twenty-four patients with advanced malignant melanoma (n = 13), ovarian cancer (n = 5) and NSCLC (n = 6) were enrolled in the 30 min infusion, D1-5 q3w schedule. In total 61 cycles were administered (median 2, mean 2.5, range 1–8). Five patients received only one treatment cycle. Nineteen patients completed two cycles, of whom 7 patients continued beyond cycle 2. The dose was escalated up to 200 mg/m2/day ().
The first DLT, a fatigue CTC grade 3 caused by a reduction in haemoglobin (from 10.6 g/dL on day 1 to 7.3 g/dL on day 4), was reported at 175 mg/m2/day in a malignant melanoma patient. The treatment was discontinued, and the patient recovered rapidly after blood transfusion, completed two additional cycles without further problems. None of the 3 additional patients enrolled at the same dose level experienced severe toxicity. At the next dose level, a CTC grade 4 neutropenia (0.0x109 g/L observed on day 24 and 26) was classified as a DLT. The patient had no fever and recovered by day 30, but received no further CP-4055 treatment. No other severe toxicity was observed in the remaining five patients treated at the same dose level.
Among the 15 patients enrolled on and above 150 mg/m2/day only 2 patients were able to continue into cycle 2 without a delay. The remaining 13 patients experienced delays due to late neutropenic nadir grade 3 and 4 (days 22–24, n = 8), anaemia (n = 3), vomiting due to migraine (n = 1) and stomach pain (n = 1). The 2 patients who entered cycle 2 without delay were both withdrawn after 2 days of treatment, one due to neutropenia and one due to hemopneumothorax caused by a failed line insertion attempt. In view of these findings the MTD was established at 200 mg/m2/day in the 3 week schedule. Due to late neutropenic nadir, a decision was made to continue the study in a 4 week schedule.
4 week schedule (D1-5), 30 min infusion (n = 6)
Four patients with malignant melanoma and two with ovarian cancer were treated with CP-4055 240 mg/m2/day, 30 min infusion (). A total of 13 cycles were administered (median 1.5, mean 2.2, range 1–4). Only 3 patients continued into cycle 2, two of these continued beyond cycle 2. All 6 patients developed grade 4 neutropenia, whereas 1 patient experienced a fatigue grade 3 classified as a DLT. The dose escalation was discontinued and 240 mg/m2/day was considered to be the MTD for the D1-5 q4w schedule with a RD at 200 mg/m2/day.
4 week schedule (D1-5), 2 hr infusion (n = 4)
The infusion time was increased from 30 min to 2 hr for the last 4 patients in the study, 2 with malignant melanoma, 1 with ovarian cancer, and 1 with NSCLC (). A total of 11 cycles of CP-4055 240 mg/m2/day were administered (median 3, mean 2.75, range 1–4) in a D1-5 q4w setting.
Neutropenia CTC grade 4 (n = 2) and grade 3 (n = 1) with nadir on day 22–23 were reported. The fourth patient developed thrombocytopenia CTC grade 3.
Two patients continued into cycle 2 without a delay. One malignant melanoma patient and 1 NSCLC patient were withdrawn from the study after the first cycle. The patient with malignant melanoma was diagnosed with brain metastases, and the NSCLC patient did not meet the neutrophil inclusion criteria. This patient, however, was subsequently retreated with 3 additional cycles of CP-4055 as compassionate use.
Safety
All patients experienced at least one treatment related AE. Of 446 AEs registered in total, the most frequent related AEs were nausea, fatigue, vomiting, anorexia, pyrexia and neutropenia. The majority of AEs, independent of causality, were of CTC grade 1 or 2 whereas the most frequent grade 3 and 4 AE was neutropenia with 24 events occurring in 19 patients ().
Table III. Number of adverse events with > 10 occurrences and their CTC grade, whether the AEs were related to study treatment or not.
Among the 32 patients treated with a 30 min infusion, 1 of 3 patients developed neutropenia CTC grade 3 or 4 at 150 mg/m2/day, 4 of 6 patients at 175 mg/m2/day, 5 of 6 at 200 mg/m2/day, all 6 at 240 mg/m2/day. As for the patients treated with 2 hr infusion, 3 of the 4 patients developed neutropenia CTC grade 3 and 4 at 240 mg/m2/day. Three anaemic events, CTC grade 3 (7.4 ≤ Hb ≤ 7.7 g/dL) were observed at the doses 150, 175 and 200 mg/m2/day. In addition, 3 thrombocytopenia CTC grade 3 (platelets 28, 42 and 47×109/L) at daily doses of 175, 200 mg/m2, and 240 mg/m2, and 3 events of fatigue (one CTC grade 4 and two grade 3) were seen at doses 30, 150 and 240 mg/m2/day, respectively.
Three CTC grade 3 infections (2 lung infections and 1 line infection) were possibly related to CP-4055 in three different patients. One of the patient with lung infection and the patient with line infection had neutropenia at the time of the infection, whereas the second patient with lung infection did not. Other infections were all of grade 1–2 and either unlikely related to the study drug or not associated to neutropenia.
Pharmacokinetic analysis
PK analyses were performed for all patients, except for 3 patients (2 treated during 30 min and 1 patient treated during 2hr at the highest dose level, 240 mg/m2/day) ().
Table IV. Mean (SD) PK parameters for CP-4055, ara-C and ara-U for all dose levels on Day 1.
In the 30 min infusion, the Cmax and AUC0-∞ for CP-4055 increased linearly with increasing dose and CP-4055 reached its highest concentration at the end of the infusion () with occasional very low concentrations being observed in some subjects up to 24 hr after the start of the infusion (). The concentration of CP-4055 decreased rapidly in plasma after the end of the infusion (t½=0.26–0.40 hr). Ara-C rapidly appeared in plasma (30–45 min after the start of infusion) and was quickly eliminated (t½=0.36–0.63 hr). Maximum concentrations of ara-C in plasma were over an order of magnitude lower than those of CP-4055. Ara-U was detected in plasma 30 min after the start of the infusion, reaching its maximum concentration (up to 10 fold higher than that of ara-C) after 1–2 hr. The elimination of ara-U was slower than that observed with ara-C and CP-4055 (t½=5.69–8.38 hr; ). Ara-U could still be detected in plasma 24 hr after end of infusion ().
Figure 1. Concentrations of CP-4055 (♦), ara-C (▪) and ara-U (•) after a 30 min (—) and 2 hr (---) infusion of CP-4055 at dose level 240 mg/m2.
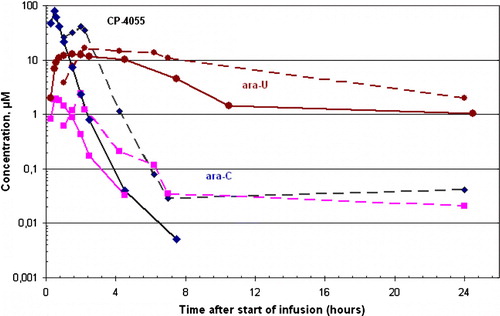
Overall, the PK of CP-4055, ara-C and ara-U were broadly linear with increasing dose level of CP-4055, but the plasma clearance of CP-4055 was up to 2-fold slower at the higher dose levels.
There were no obvious and consistent differences in the plasma PK of CP-4055, ara-C and ara-U on day 1 and day 4 (data not shown).
The Cmax for CP-4055 observed after 2 hr infusion with 240 mg/m2/day was approximately 2.5 times lower than the Cmax obtained after 30 min infusion (, ). The AUC of ara-C was slightly higher after 2 hr infusion time as compared to 30 min infusion (). No other PK parameters differences could be seen between 30 min and the 2 hr infusion of CP-4055.
Antitumor activity
Among the 34 patients enrolled in the study, 1 PR was observed and 12 patients had no progression of their disease at evaluation after 2 cycles ().
Table V. Occurrence and duration of partial response and stable disease by tumour type and CP-4055 dose.
The PR, a 39% reduction in the sum of the longest tumour diameters, was observed in a malignant melanoma 56 years old male patient with lymph node metastases. This patient had hypertension at study start, and he received chemotherapy (DTIC) three months prior to the CP-4055 treatment at 240 mg/m2/day. The treatment was stopped after 3 days in cycle 2 due to an allergic reaction (CTC grade 3). The PR was reported 6 weeks after the first dose and was confirmed at several time points by CT, the latest 17 months after treatment start (). Two PET-CT scans, at 12 and 14 months post-treatment showed no metabolic activity. Furthermore, the patient experienced complete cessation of clinical symptoms during this period. Twenty-two months after treatment start both CT and PET-CT scans showed a new lesion (TTP). The patient received laparascopic surgery of an abdominal lymph node metastasis and was still alive more than 3 years after inclusion in this study.
Disease was not progressing at evaluation after 2 cycles of treatment in 7 patients with advanced malignant melanoma at doses ranging from 30 to 240 mg/m2/day. The same was reported for 2 ovarian cancer patients, and 3 NSCLC patients at doses ranging from 60 to 240 mg/m2/day (). There was no progression for up to 11, 16 and 33 weeks in patients with ovarian cancer, malignant melanoma and NSCLC, respectively.
Discussion
In the current first-in-man clinical study with CP-4055, a late neutropenic nadir (day 18–26) was observed when the drug was administered daily from day 1–5. At the dose of 150 mg/m2/day or higher only 2 of 15 patients were able to continue the 3 week schedule as planned, suggesting that this schedule was not feasible. However, a 4 weeks schedule was long enough to regain an acceptable neutrophil count prior to entering the next treatment cycle. Four different CP-4055 schedules have been tested in another study (D1,8 q3w; D1,8,15 q4w; D1,15 q4w and D1-2 q4w; all 2 hr infusion) in order to investigate the possibility to find more convenient schedules for further combination studies. MTD was not reached in any of the schedule and neutropenia was not a major concern at doses of up to more than 440 mg/m2 /week Citation[23].
In the current D1-5 q4w schedule study bone marrow toxicity counts for 76% of all grade 3–4 toxicities (neutropenia). Neutropenia was of no concern in the other schedule finding studies and seems therefore to be more dependent on schedule (weekly/biweekly vs. q3w/q4w) than dose intensity (mg/m2/week). On the other hand the safety profile of CP-4055 seems to be both schedule and dose dependent. The day 1–5 schedule might therefore be difficult to combine with other drugs causing neutropenia.
The infusion time (30 min vs. 2 hr) seems to have little or no impact on safety profile of CP-4055. Beside neutropenia, the most frequent AEs related to CP-4055 treatment (nausea, fatigue, vomiting, anorexia and pyrexia) were mostly of CTC grade 1 and 2 and easily manageable. The frequency of thrombocytopenia and mucositis was much lower with CP-4055 at a dose of up to 240 mg/m2 in the D1-5 q4w schedule compared to ara-C alone at doses of 60 to 100 mg/m2/day Citation[24]. Moreover, the ara-C syndrome seen with standard to high doses of ara-C was not observed at the doses administered in the current study Citation[25], Citation[26]. However, if CP-4055 would have a better tolerability profile than ara-C alone, may only be determined when biological equivalent effective doses have been established.
The inter-subjects variability in the extent of systemic exposure to CP-4055, ara-C and ara-U was generally low. The PK parameters for CP-4055 and its metabolites did not allow distinction to be drawn between intracellular and extracellular (including plasma) specific processes. Nevertheless, the conversion of CP-4055 into ara-C seems to be catalyzed by esterase activity in blood and/or tissues, probably including intracellular conversion in tumour cells.
Conversion of ara-C into ara-U occurs intracellularly in tumour cell lines Citation[27]. However it might be possible that a portion of the ara-U which appears in plasma could be formed to some extent directly from ester hydrolysis induced-deamination of CP-4055.
Except from one patient who only received immunotherapy prior to be enrolled in this study, all other patients were previously treated with one to four lines of chemotherapy. CP-4055 showed some clinical activity with one PR and several patients without disease progression from first evaluation after 2 cycles (8 weeks) and up to 33 weeks of treatment. However, the low number of patients receiving potentially biologically active doses does not allow conclusion regarding any possible biological activity of CP-4055 in solid tumours. In addition, the unknown number of patients with progressive disease at the time of entering the study makes it difficult to interpret the efficacy data. Overall, the results from this phase I study are encouraging and support the initiation of phase II studies with CP-4055 as a single agent or in combination with other drugs. CP-4055 combined with sorafenib is now being explored in a phase II study in patients with malignant melanoma. The combination is feasible, without any particular increase in neutropenia observed so far (unpublished data).
Conclusion
The data from the first-in-man clinical study showed that CP-4055 was well tolerated with a favourable and predictable safety profile. The MTD for the D1-5 q4w schedule was 240 mg/m2/day and the RD 200 mg/m2/day. The infusion time did not seem to influence the safety profile. Signs of antitumor activity were seen across the three solid tumour indications investigated; one patient with PR for up to 17 months as the best response. The data support further clinical development of CP-4055 in a variety of solid tumours and in haematological malignancies.
Acknowledgements
The PK analyses were carried out at York Bioanalytical Services, UK; their valuable contribution is greatly acknowledged. And we are very thankful to Chris Franks who revised the manuscript. The co-authors Marit Liland Sandvold, Jean-Michel Gaullier and Wenche Rasch are employees at the company Clavis Pharma ASA. The other co-authors have no conflict of interest.
References
- Francis SO, Mahlberg MJ, Johnson KR, Ming ME, Dellavalle RP. Melanoma chemoprevention. J Am Acad Dermatol 2006; 55: 849–61
- Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: Time for a change?. Cancer 2007; 109: 455–64
- Kasper B, D'Hondt V, Vereecken P, Awada A. Novel treatment strategies for malignant melanoma: A new beginning?. Crit Rev Oncol Hematol 2007; 62: 16–22
- Chan JK, Cheung MK, Husain A, Teng NN, West D, Whittemore AS, et al. Patterns and progress in ovarian cancer over 14 years. Obstet Gynecol 2006; 108: 521–8
- Herzog TJ, Pothuri B. Ovarian cancer: A focus on management of recurrent disease. Nat Clin Pract Oncol 2006; 3: 604–11
- Colombo N, Van Gorp T, Parma G, Amant F, Gatta G, Sessa C, et al. Ovarian cancer. Crit Rev Oncol Hematol 2006; 60: 159–79
- Subramanian J, Govindan R. Lung cancer in never smokers: A review. J Clin Oncol 2007; 25: 561–70
- Juergens RA, Brahmer JR. Adjuvant treatment in non-small cell lung cancer. Where are we now?. J Natl Compr Canc Netw 2006; 4: 595–600
- Lister TA, Rohatiner AZ, Bassan R, Gregory W, Willis L, Barnett MJ, et al. Conventional dose cytosine arabinoside in combination chemotherapy for acute myelogenous leukaemia. Semin Oncol 1987; 14: 53–4
- Bolwell BJ, Cassileth PA, Gale RP. High dose cytarabine: A review. Leukaemia 1988; 2: 253–60
- Plunkett W, Gandhi V. Cellular pharmacodynamics of anticancer drugs. Semin Oncol 1993; 20: 50–63
- Rustum YM, Raymakers RA. 1-Beta-arabinofuranosylcytosine in therapy of leukaemia: Preclinical and clinical overview. Pharmacol Ther 1992; 56: 307–21
- Grant S. Ara-C: Cellular and molecular pharmacology. Adv Cancer Res 1998; 72: 197–233
- Gailani S, Nussbaum A. Correlation of response to 1-beta-D-arabinofuranosyl cytosine and metabolism of drug by tumour. J Med 1976; 7: 93–102
- Bajetta E, Verusio C, Bonfante V, Bonadonna G. Cytarabine and cisplatin in advanced malignant melanoma. Cancer Treat Rep 1986; 70: 1441–2
- Chabner BA. Anticancer drugs. Cancer: Principles and Practice of Oncology4th ed, VT DeVita, S Hellman, SA Rosenberg. JB Lippincott Co, Philadelphia 1993; 365–369
- Someya H, Waud WR, Parker WB. Long intracellular retention of 4’-thio-arabinofuranosylcytosine 5’-triphosphate as a critical factor for the antisolid tumour activity of 4’-thio-arabinofuranosylcytosine. Cancer Chemother Pharmacol 2006; 57: 772–80
- Breistøl K, Balzarini J, Sandvold ML, Myhren F, Martinsen M, De Clercq F, et al. Antitumour activity of P-4055 (Elaidic Acid-Cytarabine) compared to cytarabine in metastatic and s.c. human tumour xenograft models. Cancer Res 1999; 59: 2944–9
- Ross DD, Chen SR, Cuddy DP. Effects of 1-beta-D-arabinofuranosylcytosine on DNA replication intermediates monitored by PH-step alkaline elution. Cancer Res 1990; 50: 2658–66
- Bergman AM, Kuiper CM, Voorn DA, Comijn EM, Myhren F, Sandvold ML, et al. Antiproliferative activity and mechanism of action of fatty acid derivatives of arabinofuranosylcytosine in leukaemia and solid tumour cell lines. Biochem Pharmacol 2004; 67: 503–11
- Bergman AM, Kuiper CM, Myhren F, Sandvold ML, Hendriks HR, Peters GJ. Antiproliferative activity and mechanism of action of fatty acid derivatives of arabinosylcytosine (ara-C) in leukaemia and solid tumour cell lines. Nucleosides Nucleotides Nucleic Acids 2004; 23: 1523–6
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumours. J Natl Cancer Inst 2000; 92: 205–16
- Delaunoit T, Raymond E, Awada A, Savinelli F, Culine S, Rasch W, et al A three schedule phase I trial of CP-4055, weekly and q2 weeks in patients with advanced or metastatic solid tumors. J Clin Oncol 2006;(Suppl): 2067 (abstract).
- Stentoft J. The toxicity of cytarabine. Drug Safety 1990; 5: 7–27
- Peters WG, Colly LP, Willemze R. High-dose cytosine arabinoside: Pharmacological and clinical aspects. Blut 1988; 56: 1–11
- Herzig RH, Wolff SN, Lazarus HM, Phillips GL, Karanes C, Herzig GP. High-dose cytosine arabinoside therapy for refractory leukaemia. Blood 1983; 62: 361–9
- Capizzi RL, White JC, Powel BL, Perrino F. Effect of dose on the pharmacokinetic and pharmacodynamic effects of cytarabine. Semin Hematol 1991; 28: 54–69
