Abstract
Introduction. Rectal cancer is a common disease in Western populations. Improved treatment modalities have resulted in increased survival and tumour control. With increasing survival there is a growing need for knowledge about the long-term side effects and functional results after the treatment. Aim. To describe the long-term functional outcome in patients treated for rectal cancer through a systematic review of the current literature and to provide an outline of the promising developments within this area. Results. Standard resectional surgery with loss of the rectal reservoir function results in poor functional results in up to 50–60% of the patients. New methods of surgery including the construction of a neoreservoir and improvement of the technique for local excision have been developed to minimize the functional disturbances without compromising the oncological result. The addition of chemo and/or radiotherapy approximately doubles the risk of poor functional results. During the last decades the techniques for chemo/radiotherapy has been markedly improved with a positive impact on functional outcome. New methods for treatment of functional disturbances e.g. bowel irrigation and sacral nerve stimulation are currently under development. Perspectives. To improve the functional outcome in this growing patient population several approaches can be taken. The primary cancer treatment must be improved by minimizing the surgical trauma and optimizing the imaging and radiation techniques. Population screening should be considered in order to find the cancers at an earlier stage, hereby increasing the proportion of patients eligible for local excision without the need for chemo/irradiation. All patients recovering from rectal resection should be examined and registered systematically regarding their functional results and treatment should be offered to the severely affected patients. More studies are still needed to evaluate the efficacy of irrigation and nerve stimulation in this patient group.
Recent years’ developments have resulted in improved survival and growing use of multimodality treatment for cancer. This has caused an increasing attention to the importance of knowledge on long-term side effects caused by the treatment. Knowledge of long-term side effects is essential to tailor treatment to each patient in order to achieve the right balance between optimizing tumour control and minimizing the side effects, and it is essential for providing sufficient information to the patients and the medical staff involved in the treatment and control. This knowledge is also crucial for the understanding of the underlying mechanisms and for the treatment of long-term side effects Citation[1].
In light of this, Acta Oncologica held a multi disciplinary symposium in Oslo in the autumn 2006 discussing functional disturbances and side effects after treatment of cancer. This review on bowel dysfunction after treatment for rectal cancer was inspired by the symposium.
Functional disturbances constitute a major problem for many surviving rectal cancer patients. The majority of research in rectal cancer has been directed at improving survival, reducing local recurrence rate and increasing the number of patients receiving sphincter preserving resection. New surgical techniques, and the use of individualized preoperative radio-chemotherapy have resulted in a markedly improved overall 5 year survival rate which is now about 70% Citation[2]. With increasing survival, the long-term functional outcome is becoming more and more important. Studies have indicated that, following a traditional restorative resection, a large proportion of the surviving patients experience major bowel, urinary and sexual dysfunctions on a daily basis resulting in low quality of life Citation[3–5]. The extent of these problems varies greatly with some patients obtaining normal function after a postoperative recovery of a few months and others being severely disabled physically, mentally and socially for the rest of their lives.
In the present work the focus will be the long-term and often chronic disturbances in bowel function after treatment for rectal cancer.
Surgical treatment
Sphincter Preserving Resection (SPR)
Anterior resection with Total Mesorectal Excision (TME) is the golden standard for rectal cancer surgery. The resection is done Ad Modum Heald where the tumour and the mesorectum are excised by sharp dissection with preservation of the autonomic nerves of the pelvis Citation[6]. Recently, laparoscopic techniques have been introduced but the advantages and limitations are still under investigation Citation[7], Citation[8]. For tumours located 10 to 15 cm from the anal verge, a partial mesorectal resection (PME) dividing the mesorectum at least 5 cm distally from the tumour is preferable. Tumours located 5 to 10 cm from the anal verge are excised by a TME with a low anterior resection (LAR) and an ultralow anterior resection (UAR) is used on tumours located 3–5 cm from the anal verge, with a coloanal anastomosis at the dentate line.
Low Anterior Resection Syndrome
Traditional anterior resection with sphincter preservation often results in impaired functional results. Low anterior resection syndrome (LARS) is associated with the loss of the rectal reservoir function leading to urgency, frequent bowel movements and occasional faecal incontinence in a large group of the patients (see ) Citation[3–5], Citation[9–14]. The functional disturbances are often most pronounced immediately after surgery, decrease during the post-operative months and reach a plateau approximately one year after surgery. Risk factors for developing LARS are low colorectal or ultra low coloanal anastomosis, end-to-end anastomosis, anastomotic leakage, acute or chronic inflammation and adjuvant radiotherapy, with the level of anastomosis the being most important factor Citation[7].
Table I. Functional outcome after 12 months after LAR.
Damage to the sphincteric apparatus also contributes to the development of LARS. In LAR and UAR the sphincter muscles and/or the intrinsic rectal innervation may be compromised leading to disruption of the recto-sphincteric reflexes which causes decreased anal pressures Citation[7]. In addition mechanical lesions to the internal sphincter may occur during the transanal introduction of the circular stapler Citation[7], Citation[15].
Abdomino-Perineal Resection
All rectal cancers not suitable for sphincter-preserving surgery should be excised by abdominoperineal resection (APR) with removal of the entire distal rectum and anal canal including the sphincters and with the creation of a colostomy. The stoma is permanent and can markedly interfere with the patient's body image as well as physical and social functioning. It has therefore been suggested that sphincter-preserving surgery should be used whenever possible. Many studies have investigated the quality of life (QoL) after APR and sphincter-preserving surgery, but the results are inconclusive Citation[16]. The majority of these studies conclude that the decrease in QoL caused by negative body image and impaired sexuality after APR is counterbalanced by impairment caused by diarrhoea and other bowel symptoms after SPR Citation[17], Citation[18].
In general, APR patients have worse QoL than patients with a high anterior resection, but they have better QOL than patients with a low coloanal anastomosis.
Adjuvant and neo-adjuvant therapy
During the last decades, radiotherapy and chemo-irradiation have increased their role as adjuvant therapy in the treatment of rectal cancer. For years, radiotherapy has been a keystone in the treatment of locally advanced cancers reducing the risk of local recurrence in primarily resectable cancers and inducing tumour regression in primarily irresectable cancers, thus facilitating later radical surgery. Radiotherapy has been administered in many different regimens; pre- or postoperative, long- or short-course treatment. Randomised trials have shown that preoperative radiotherapy decreases local recurrence rates more than postoperative irradiation while also decreasing the side effects Citation[2], Citation[19].
Irradiation to the pelvis can cause a large variety of symptoms, often divided into acute and chronic complications. The acute toxicity comprises diarrhoea, perineal dermatitis, anal incontinence and cystitis. These symptoms occur shortly after the beginning of radiation and often last 3–10 weeks. In few patients, the symptoms are so severe that treatment must be stopped and very rarely deaths have occurred Citation[20]. Late toxicity develops several months after treatment. Depending on the irradiation field and dose given to surrounding tissues, the symptoms vary. Because of the anatomical proximity it can be difficult to completely avoid the irradiation of the small bowel that leads to small bowel toxicity. With modern irradiation techniques, the risk of severe small bowel toxicity is expected to be below 5% Citation[21]. The most common symptoms of small bowel toxicity include diarrhoea, abdominal pain, nausea and vomiting. Less common but more severe symptoms include small bowel obstruction or stricture, bleeding, fistulation, necrosis, bowel perforation and risk of adhesions. It can also affect the absorptional function of the bowel, leading to malnourishment and weight loss Citation[21].
Improving functional results
During the last decades, much has been done to improve the treatment for rectal cancer both with regards to improved survival and tumour control, and to improved functional outcome. The surgical techniques have been adjusted with more emphasis on sphincter and nerve sparing. Autonomic nerves should be preserved by visual identification and sparing of the superior and inferior hypogastric plexus Citation[22]. Hereby the innervation of the sphincteric apparatus is preserved and a better functional result is to be expected.
Neoreservoir
Patients receiving SPR benefit considerably by the creation of a neoreservoir, by markedly reducing the risk and severity of LARS Citation[7]. Several surgical methods have been developed to address this problem: colonic J-pouch, end-to-side anastomosis a.m. Baker or coloplasty.
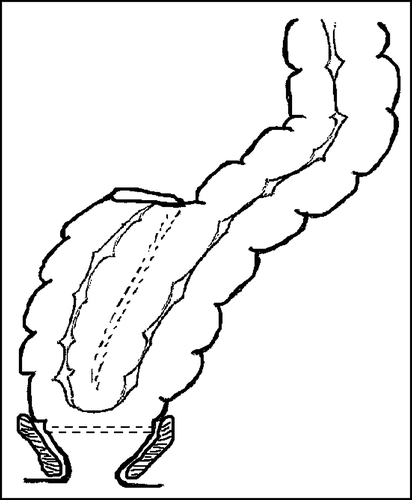
The colonic J-pouch is created by folding a short segment of the colon and making a side-to-side anastomosis between the two loops (). This restores volume and improves rectal wall compliance and sensory function of the neorectum. A few randomised trials have been performed comparing functional results after colonic J-pouch and a straight end-to-end anastomosis, all showing significantly better functional outcome in the pouch group (see ) Citation[23–27].
Table II. Bowel function after colonic J-pouch and straight anastomosis.
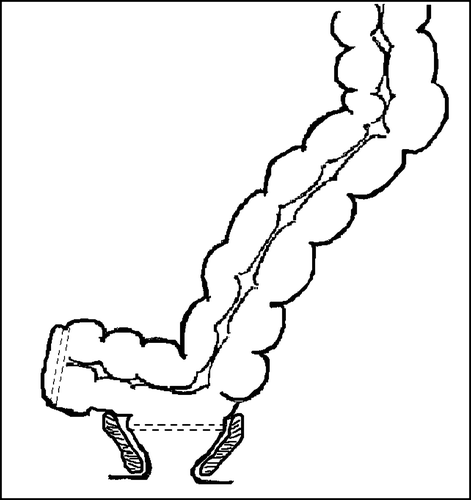
Different sizes of pouches have been explored, and though large pouches gave a higher reservoir capacity and rectal compliance, they also caused severe problems with evacuation. Small pouches resulted in less evacuational problems but increased urgency and stool frequency Citation[7]. Time has shown that a colonic J-pouch of 6 cm is optimal with regard to functional results, but the size of the pouch may be customised for each patient taking into account their anal sphincteric function and the tendency to constipation/diarrhoea Citation[3]. In patients where the pelvis is too narrow for a large colonic J-pouch, an end-to-end anastomosis combined with a coloplasty can be used. The coloplasty is formed by a 7–10 cm longitudinal incision which is closed transversally hereby creating a reservoir which is consequently connected to the rectal remnant by circular stapling (). The result is a more voluminous neorectum with interrupted antegrade colonic peristalsis and therefore better faecal holding capacities Citation[7], Citation[28], Citation[29]. A randomised study comparing colonic J-pouch and coloplasty showed no significant differences in functional results but a significantly higher risk of anastomotic leakage in the coloplasty group Citation[30].
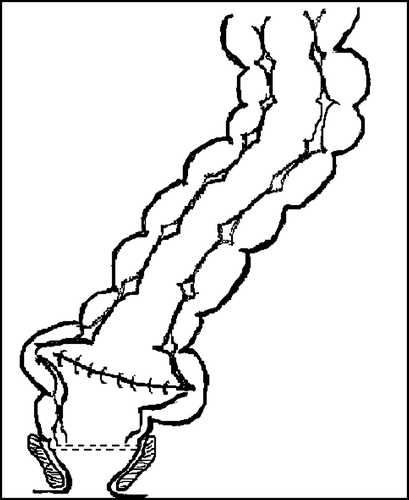
The anastomosis can also be achieved by an end-to-side anastomosis a.m. Baker in which a dilated distal blind end of the colon may increase the neorectal volume and change the motility patterns (). The advantage of this technique is a faster surgical procedure, fewer stapler lines and therefore less risk of anastomotic leakage. Finally it is less bulky and thereby fits easier into a narrow pelvis. A Swedish randomised trial have investigated the functional outcome of the colonic J-pouch in comparison to end-to-side anastomosis, and found similar functional outcome except for a faster recovery in evacuation time, in favour of the pouch Citation[12]. Another prospective randomized study also showed similar long-term functional outcome between colonic J-pouch and end-to-side anastomosis, with only minor differences in the recovery period Citation[31].
In conclusion, all three types of neoreservoir results in comparable functional results. Both the coloplasty and the end-to-side anastomosis have the advantage of being less bulky. However, the coloplasty has a higher risk of anastomotic leakage, and has therefore in our opinion no place in standard surgery. In Scandinavia today, the preferred neorectal construction is the end-to-side anastomosis since it decreases time of surgery and lessens the number of stapler lines whereby it reduces the risk of leakage.
Both the descending and the sigmoid colon can be used for the anastomosis with or without a pouch Citation[3]. When using the sigmoid colon, mobilisation of the left colonic flexure is not mandatory. Using the descending colon always requires mobilisation of the left colonic flexure to obtain adequate length and gives higher risk of splenic lesions and damaging of autonomic nerves in front of the aorta. The wall of the descending colon is regularly thinner and more indolent while the sigmoid is often diseased with diverticulae and thickened by muscular hypertrophy and is therefore less compliant. One study compared the use of sigmoid and descending colon in ultra-low colonic J-pouch and found no significant differences in functional outcome in the two groups Citation[32]. Still there is a general consensus that in case of multiple diverticulae in the sigmoid, the descending colon is used for the anastomosis Citation[32].
Perineal Colostomy
Especially among the young and active patients, a permanent abdominal colostomy is considered a mutilating procedure. A new surgical approach with construction of a perineal colostomy (PC) has been developed and is still being adjusted. The technique has been combined with smooth muscleplasty to create a pseudo-continent function (pseudo-continent perineal colostomy PCPC), and recently a total anorectal reconstruction with single or double dynamic graciloplasty has been developed. This creation of a neosphincter is a complicated surgical procedure and the morbidity is high Citation[33]. All patients with a perineal colostomy require lifelong colonic irrigation to regulate stool evacuation 341. This method has proven to obtain acceptable continence and a satisfying QoL by improving the patients’ body image and may in the future become an acceptable alternative to a permanent abdominal colostomy Citation[35–37].
Patients with tumours involving the sphincter have so far all been candidates for APR. With recent developments, some of these patients might be eligible for chemoradiation followed by a local excision, whereby the anal canal and the bowel continuity are preserved.
Curative management by rectum-conserving methods
Smaller rectal tumours can be curatively treated by non-resectable methods, hereby avoiding the detrimental effects of resectional surgery. This can be done as a local excision from within the lumen by a transanal excision (TE) or transanal endoscopic microsurgery (TEM) depending on the height of the tumour with tumours located within 5 cm from the dentate line amenable for TE, while tumours located higher than this require a TEM Citation[38]. The tumour is visualised either by anal retraction or by insertion of a specialised resectoscope. In both cases, the tumour is removed with a circumferential margin of 1–2 cm with a full-wall incision, which can be closed transversely with sutures. Tumours located as high as 20 cm posteriorly can be excised by this technique Citation[38]. The major problem with local excision is the inability to assess the occurrence of microscopic spreading of the disease since regional lymph nodes cannot be excised Citation[39]. So far imaging techniques cannot reliably detect metastases in regional lymph nodes Citation[39]. In many centres TE/TEM is recommended for T1 tumours without high risk features (poorly differentiated carcinoma, deep submucosal invasion, lymphovascular invasion and lesions in the lower third of the rectum) Citation[40]. With careful selection of patients, TEM can achieve similar oncological outcome to TME while limiting mortality and morbidity Citation[41]. The incidence of defaecational disorders after TEM is significantly lower than after TME Citation[42].
Local excision can be combined with neoadjuvant therapy to eradicate any nodal disease, hereby decreasing the risk of local recurrence Citation[41], Citation[43]. An Italian study showed comparable results between TEM and TME after neoadjuvant chemoradiation in treatment of T2 cancers with regards to probability of failure and survival with a median follow-up period of 56 months Citation[44].
Chemo and radiotherapy
The combination of chemo/radiotherapy and surgery is a major cause of poor functional result. A large number of patients are given chemo/radiotherapy to prevent a few cases of locally recurrent cancer. Patient selection with regards to risk of recurrence is essential to find the patients most likely to benefit from this treatment, hereby minimizing the number of patients receiving chemo/irradiation Citation[45–47].
The technique for radiotherapy has improved markedly over the last decades. Imaging techniques (CT and MRI) are used for 3D dose planning. The use of new techniques for dynamic radiation with tighter margins restrict the irradiation volume to the target volume, hereby maintaining the efficacy on the tumour and minimising the side effects by minimising the radiation dose given to the surrounding tissues. Due to these new techniques, the anal sphincters have recently been excluded from the radiation field when possible in the hope of decreasing the risk of anal incontinence. Through the use of postoperative radiotherapy, the neorectum is irradiated and this causes frequent and severe side effects with increased number of stools, urgency of defecation, faecal incontinence and frequent use of pads Citation[48]. With preoperative radiotherapy, the irradiated rectum and sigmoid colon are removed during surgery, hereby decreasing the risk of developing severe side effects in these segments of the gut.
A Danish prospective, randomized multicenter study of postoperative radiotherapy showed a significant median delay in time to local recurrence in radiated patients compared with non-radiated patients Citation[49]. A study performed 14 years later in the surviving patients showed a long-term detrimental effect on anorectal function with high stool frequency, faecal urgency and faecal incontinence in patients receiving postoperative radiation Citation[50]. By use of rectal impedance planimetry it was shown that the addition of radiotherapy had induced rigidity of the rectum with reduced reservoir capacity and a weakened, less sensitive anal sphincter causing major anal dysfunction Citation[48].
In Sweden, two large randomized prospective trials including 1 406 patients were performed between 1980 and 1993 evaluating short course preoperative radiotherapy in rectal cancers (see ). In 2002, the 252 patients that were still alive were asked to participate in a study investigating QoL and medical history, and of these 139 patients were interviewed and examined by anorectal examination by sigmoidoscopy and anorectal physiological testing. The investigators found significantly more late complications including faecal and urinary incontinence in patients treated with adjuvant radiotherapy in comparison to non-radiated patients Citation[51]. Another Swedish study followed-up on a randomized study including 1 147 patients randomly assigned to preoperative irradiation or surgery alone. The patients without recurrence were matched against the Swedish Hospital Discharge Register to identify patients admitted to hospital after the primary treatment of the rectal cancer. They found an increase in the occurrence of bowel obstruction, nausea and abdominal pain in radiated patients Citation[52]. A large Dutch study including 597 patients randomized to surgery with or without short course preoperative radiotherapy showed a significant increase in long-term bowel dysfunction following radiotherapy with a faecal incontinence rate of 38% in the non-radiated group and 62% in the radiated group Citation[53]. In all studies mentioned, the sphincteric structures were included in the irradiation fields.
Table III. Long term side effects of preoperative short course radiotherapy.
An alternative strategy is to use long-course preoperative radiotherapy administering smaller fractions to a higher dose over 5–6 weeks followed by surgery 4–6 weeks later. This approach has been used in an effort to increase local control and to downstage the primarily irresectable cancers. The effect of long-course irradiation is enhanced when combined with chemotherapyCitation[54], Citation[55]. Several chemotherapeutical drugs are known to have a radio sensitizing effect besides the conventional cytotoxic effect Citation[45], Citation[56]. This combination has been proved to result in a 73.3–84% conversion rate for primarily irresectable cancers and to decrease the risk of local recurrence Citation[57], Citation[58].
Preoperative compared to postoperative chemoradiation shows the same overall survival rates but a significant reduction in local recurrence rate and treatment morbidity Citation[19], Citation[59]. The addition of chemotherapy to irradiation increases the risk of severe acute toxicity especially diarrhoea Citation[20]. The Polish Colorectal Study Group compared preoperative long-course chemoradiation with short-course radiation. They found no differences in survival, local control or late toxicity between the two groups with a median follow up of 48 months Citation[60]. Approx. 12–13 months after surgery the patients’ QoL and anorectal and sexual functioning were investigated through questionnaires and showed no significant differences Citation[61]. No systematic studies have investigated the long-term functional outcome of chemoradiation, and no studies have evaluated the effect of short-course vs. long-course radiotherapy on functional outcome.
Neoadjuvant chemoradiation without surgery can lead to complete pathological response in some patients. Few studies have indicated a complete response rate of 10–25% with marked effect on survival and local recurrence Citation[59]. A controversial Brazilian study suggests that complete responders could be treated by chemoradiation alone Citation[62]. What currently limits this approach is the problem of identifying the complete responders. This has so far been done postoperatively by examining the excised tumour and comparing it to the preoperative clinical staging. The standard imaging techniques for staging of the tumour are unreliable after chemoradiation. Transanal ultra sound, usually considered the most accurate method for staging T1 and T2 rectal tumours, has difficulties distinguishing between residual tumour and post-irradiation fibrosis Citation[63], Citation[64]. MRI has also proven inaccurate in re-staging rectal cancers after chemoradiation Citation[65]. Recent studies indicate that F-18 fluorodeoxyglucose positron emission tomography (FDG-PET) more accurately assesses the treatment response and stages the irradiated cancers Citation[59], Citation[66]. More research is needed to verify these findings before complete responders accurately can be identified and be spared the detrimental effects of resectional surgery.
Contact radiotherapy and brachytherapy are both methods for endocavitary irradiation with delivery of high dose irradiation to the tumour but low dose to the normal surrounding tissues. Local control has been achieved in 85–90% of patients with T1N0 tumours by endocavitary irradiation alone. By combining it with external beam irradiation, local control can be achieved in 80% of T2 tumours and 60% of T3 tumours Citation[67].
Treatment modalities for bowel dysfunction
Conservative management
To date, most functional disturbances are treated with conservative therapy including dietary advice, fibres, laxatives, Loperamide and suppositories, although the evidence for these regimens has never been provided. A large group of patients obtain unsatisfactory results, and this group may require more invasive therapy in order to obtain good functional outcome and high quality of life.
Bowel irrigation
Patients with an abdominal colostomy often complain of odour, flatus and peri-stomal skin conditions caused by stomal incontinence. Many of these patients can improve their function significantly by colostomy irrigation.
Colostomy irrigation can be done through retrograde irrigation with instillation of lukewarm water into the lumen of the gut by a catheter inserted through the stoma, or through antegrade irrigation with instillation through an appendicostomy. Studies have shown that up to 92–97% of patients with abdominal colostomies may achieve continence with retrograde colostomy irrigation hereby improving their physical wellbeing and QoL Citation[68], Citation[69]. The technique is time consuming and requires thorough instruction and training. Even after correct irrigation, episodes with faecal discharge between washouts can occur Citation[68].
Patients with a perineal colostomy require lifelong colonic irrigation to obtain acceptable functional results Citation[35], Citation[37]. This is usually done as a retrograde colonic irrigation, but newer approaches include cecal access for antegrade irrigation sometimes in shape of an appendicostomy, a cecal flap conduit or an ileal tubularisation. Retrograde irrigation has proved efficient resulting in faecal continence in 71% of these patients Citation[34]. The newer approach with antegrade irrigation through an appendicostomy or cecal access (Malone procedure) in perineal stoma patients has been shown to improve the continence rate to 84.6% Citation[35].
Retrograde transanal irrigation has proven valuable in relieving continence disturbances in a variety of patients including rectal cancer patients Citation[70], Citation[71]. The technique is similar to the colostomy irrigation except the catheter is inserted through the anal canal. In patients with defecation disturbances after low anterior resection, retrograde transanal irrigation proved efficient in up to 79% of patients in small series Citation[72], Citation[73].
Antegrade irrigation through an appendicostomy has been investigated and has proven to be safe and efficient in patients suffering from defaecational disturbances where it induces highly effective emptying of the large bowel even in patients with severe constipation Citation[74–76].
Nerve stimulation
Sacral Nerve Stimulation (SNS) is a new method for relieving defaecational disturbances. It has been tested on a variety of patients suffering from faecal incontinence due to sphincteric injury, neuronal damage and idiopathic incontinence and studies have shown that up to 80% of patients experience markedly improved function after implantation of the stimulator Citation[77], Citation[78].
Before the implantation of a permanent stimulator (the SNS), an external stimulator is implanted for testing the correct position and the functional results. This is obtained by Percutaneous Nerve Evaluation (PNE) with implantation of a small electrode into a sacral. The electrode is tested and if a significant reduction in incontinence episodes occurs, a permanent stimulator is implanted. The mode of action of SNS is unknown since it has no or only minor measurable effect on anal manometry and rectal volume tolerability. It has been hypothesized that the effect is due to modulation of the anorectal and/or colorectal motility, but this remains to be investigated Citation[77]. Only few studies have investigated the use of SNS in rectal cancer patients, but they have shown positive effects Citation[79], Citation[80]. The procedure of implantation and testing the device is uncomplicated and safe, which makes the method interesting for future studies in bowel disturbances after the treatment for rectal cancer.
Future Perspectives
Treatment for rectal cancer is in rapid development. Much research has been done improving survival and tumour control, and during the last decade functional outcome has been gaining more attention. In order to improve and treat the functional outcome several approaches can be chosen:
Improving primary cancer treatment:
Finding the tumours earlier by population screening, hereby increasing the number of patients eligible for non-resectional surgery without the need for neoadjuvant treatment.
Lessen the surgical trauma by optimizing the surgical procedures with minimal nerve and sphincter damage.
Optimizing the radiation techniques to ensure maximal irradiation of the tumour with minimal irradiation of the surrounding tissues.
Improving imaging techniques including MRI and CT for better detection of regional lymph node metastases and hereby correctly N-staging the tumours for better selection of patients eligible for local excision.
Improving imaging techniques for identifying complete responders to chemoradiation, hereby sparing them the surgery.
The use of molecular markers to identify the tumours sensitive to adjuvant therapy hereby limiting the risk of over treatment.
Improving secondary treatment of functional problems:
Prospective evaluations of functional deficits in all patients using validated questionnaires to improve knowledge of incidence and severity of the problems.
Better understanding of the impact of the functional problems on the patients’ QoL.
Prospective randomised trials evaluating all aspects of treatment, including conservative bowel management and newer treatment modalities such as TAI and SNS/PNE, to achieve high level of evidence for the efficacy of treatment.
Acknowledgements
The authors thank Rune Sjödahl and Jacob Lindegaard for their excellent advice and help in the writing and outlining of this article.
References
- Aziz NM. Cancer survivorship research: State of knowledge, challenges and opportunities. Acta Oncol 2007; 46: 417–32
- Glimelius B, Gronberg H, Jarhult J, Wallgren A, Cavallin-Stahl E. A systematic overview of radiation therapy effects in rectal cancer. Acta Oncol 2003; 42: 476–92
- Hallbook O, Sjodahl R. Surgical approaches to obtaining optimal bowel function. Semin Surg Oncol 2000; 18: 249–58
- Lewis WG, Martin IG, Williamson ME, Stephenson BM, Holdsworth PJ, Finan PJ, et al. Why do some patients experience poor functional results after anterior resection of the rectum for carcinoma?. Dis Colon Rectum 1995; 38: 259–63
- Williamson ME, Lewis WG, Finan PJ, Miller AS, Holdsworth PJ, Johnston D. Recovery of physiologic and clinical function after low anterior resection of the rectum for carcinoma: Myth or reality?. Dis Colon Rectum 1995; 38: 411–8
- MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993; 341: 457–60
- Ho YH. Techniques for restoring bowel continuity and function after rectal cancer surgery. World J Gastroenterol 2006; 12: 6252–60
- Nicum S, Midgley R, Kerr DJ. Colorectal cancer. Acta Oncol 2003; 42: 263–75
- Williams NS, Price R, Johnston D. The long-term effect of sphincter preserving operations for rectal carcinoma on function of the anal sphincter in man. Br J Surg 1980; 67: 203–8
- Lewis WG, Holdsworth PJ, Stephenson BM, Finan PJ, Johnston D. Role of the rectum in the physiological and clinical results of coloanal and colorectal anastomosis after anterior resection for rectal carcinoma. Br J Surg 1992; 79: 1082–6
- Vironen JH, Kairaluoma M, Aalto AM, Kellokumpu IH. Impact of functional results on quality of life after rectal cancer surgery. Dis Colon Rectum 2006; 49: 568–78
- Machado M, Nygren J, Goldman S, Ljungqvist O. Similar outcome after colonic pouch and side-to-end anastomosis in low anterior resection for rectal cancer: A prospective randomized trial. Ann Surg 2003; 238: 214–20
- Rasmussen OO, Petersen IK, Christiansen J. Anorectal function following low anterior resection. Colorectal Dis 2003; 5: 258–61
- Ho P, Law WL, Chan SC, Lam CK, Chu KW. Functional outcome following low anterior resection with total mesorectal excision in the elderly. Int J Colorectal Dis 2003; 18: 230–3
- Horgan PG, O'Connell PR, Shinkwin CA, Kirwan WO. Effect of anterior resection on anal sphincter function. Br J Surg 1989; 76: 783–6
- Pachler J, Wille-Jorgensen P. Quality of life after rectal resection for cancer, with or without permanent colostomy. Cochrane Database Syst Rev 2005;CD004323.
- Schmidt CE, Bestmann B, Kuchler T, Longo WE, Kremer B. Prospective evaluation of quality of life of patients receiving either abdominoperineal resection or sphincter-preserving procedure for rectal cancer. Ann Surg Oncol 2005; 12: 117–23
- Schmidt CE, Bestmann B, Kuchler T, Longo WE, Kremer B. Ten-year historic cohort of quality of life and sexuality in patients with rectal cancer. Dis Colon Rectum 2005; 48: 483–92
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–40
- Bosset JF, Calais G, Daban A, Berger C, Radosevic-Jelic L, Maingon P, et al. Preoperative chemoradiotherapy versus preoperative radiotherapy in rectal cancer patients: assessment of acute toxicity and treatment compliance. Report of the 22921 randomised trial conducted by the EORTC Radiotherapy Group. Eur J Cancer 2004; 40: 219–24
- Guckenberger M, Flentje M. Late small bowel toxicity after adjuvant treatment for rectal cancer. Int J Colorectal Dis 2006; 21: 209–20
- McNamara DA, Parc R. Methods and results of sphincter-preserving surgery for rectal cancer. Cancer Control 2003; 10: 212–8
- Park JG, Lee MR, Lim SB, Hong CW, Yoon SN, Kang SB, et al. Colonic J-pouch anal anastomosis after ultralow anterior resection with upper sphincter excision for low-lying rectal cancer. World J Gastroenterol 2005; 11: 2570–3
- Hallbook O, Pahlman L, Krog M, Wexner SD, Sjodahl R. Randomized comparison of straight and colonic J pouch anastomosis after low anterior resection. Ann Surg 1996; 224: 58–65
- Ho YH, Seow-Choen F, Tan M. Colonic J-pouch function at six months versus straight coloanal anastomosis at two years: Randomized controlled trial. World J Surg 2001; 25: 876–81
- Araki Y, Isomoto H, Tsuzi Y, Matsumoto A, Yasunaga M, Yamauchi K, et al. Functional results of colonic J-pouch anastomosis for rectal cancer. Surg Today 1999; 29: 597–600
- Dehni N, Tiret E, Singland JD, Cunningham C, Schlegel RD, Guiguet M, et al. Long-term functional outcome after low anterior resection: Comparison of low colorectal anastomosis and colonic J-pouch-anal anastomosis. Dis Colon Rectum 1998; 41: 817–22
- Ulrich A, Z'graggen K, Schmitz-Winnenthal H, Weitz J, Buchler MW. The transverse coloplasty pouch. Langenbecks Arch Surg 2005; 390: 355–60
- Z'graggen K, Maurer CA, Birrer S, Giachino D, Kern B, Buchler MW. A new surgical concept for rectal replacement after low anterior resection: The transverse coloplasty pouch. Ann Surg 2001; 234: 780–5
- Ho YH, Brown S, Heah SM, Tsang C, Seow-Choen F, Eu KW, et al. Comparison of J-pouch and coloplasty pouch for low rectal cancers: A randomized, controlled trial investigating functional results and comparative anastomotic leak rates. Ann Surg 2002; 236: 49–55
- Jiang JK, Yang SH, Lin JK. Transabdominal anastomosis after low anterior resection: A prospective, randomized, controlled trial comparing long-term results between side-to-end anastomosis and colonic J-pouch. Dis Colon Rectum 2005; 48: 2100–8
- Heah SM, Seow-Choen F, Eu KW, Ho YH, Tang CL. Prospective, randomized trial comparing sigmoid vs. descending colonic J-pouch after total rectal excision. Dis Colon Rectum 2002; 45: 322–8
- Rullier E, Zerbib F, Laurent C, Caudry M, Saric J. Morbidity and functional outcome after double dynamic graciloplasty for anorectal reconstruction. Br J Surg 2000; 87: 909–13
- Lasser P, Dube P, Guillot JM, Elias D. Pseudocontinent perineal colostomy following abdominoperineal resection: Technique and findings in 49 patients. Eur J Surg Oncol 2001; 27: 49–53
- Farroni N, Van den BA, Haustermans K, Van Cutsem E, Moons P, D'hoore A, et al. Perineal colostomy with appendicostomy as an alternative for an abdominal colostomy: Symptoms, functional status, quality of life, and perceived health. Dis Colon Rectum 2007.
- Marchal F, Doucet C, Lechaux D, Lasser P, Lehur PA. Secondary implantation of an artificial sphincter after abdominoperineal resection and pseudocontinent perineal colostomy for rectal cancer. Gastroenterol Clin Biol 2005; 29: 425–8
- Portier G, Bonhomme N, Platonoff I, Lazorthes F. Use of Malone antegrade continence enema in patients with perineal colostomy after rectal resection. Dis Colon Rectum 2005; 48: 499–503
- Wolff BG, Fleshman JW, Beck DE, Pemberton JH, Wexner SD. Surgical treatment of rectal cancer. The ASCRS Textbook of Colon and Rectal Surgery. 2007. p. 413–36.
- Paty PB, Nash GM, Baron P, Zakowski M, Minsky BD, Blumberg D, et al. Long-term results of local excision for rectal cancer. Ann Surg 2002; 236: 522–9
- Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum 2002; 45: 200–6
- Neary P, Makin GB, White TJ, White E, Hartley J, MacDonald A, et al. Transanal endoscopic microsurgery: A viable operative alternative in selected patients with rectal lesions. Ann Surg Oncol 2003; 10: 1106–11
- Doornebosch PG, Tollenaar RA, Gosselink MP, Stassen LP, Dijkhuis CM, Schouten WR, et al. Quality of life after transanal endoscopic microsurgery and total mesorectal excision in early rectal cancer. Colorectal Dis 2007; 9: 553–8
- Chakravarti A, Compton CC, Shellito PC, Wood WC, Landry J, Machuta SR, et al. Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg 1999; 230: 49–54
- Lezoche E, Guerrieri M, Paganini AM, D'Ambrosio G, Baldarelli M, Lezoche G, et al. Transanal endoscopic versus total mesorectal laparoscopic resections of T2–N0 low rectal cancers after neoadjuvant treatment: A prospective randomized trial with a 3-years minimum follow-up period. Surg Endosc 2005; 19: 751–6
- Sauer R. Adjuvant and neoadjuvant radiotherapy and concurrent radiochemotherapy for rectal cancer. Pathol Oncol Res 2002; 8: 7–17
- Blomqvist L, Glimelius B. The ‘good’, the ‘bad’, and the ‘ugly’ rectal cancers. Acta Oncol 2008; 47: 5–8
- Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol 2008; 47: 20–31
- Lundby L, Krogh K, Jensen VJ, Gandrup P, Qvist N, Overgaard J, et al. Long-term anorectal dysfunction after postoperative radiotherapy for rectal cancer. Dis Colon Rectum 2005; 48: 1343–9
- Balslev I, Pedersen M, Teglbjaerg PS, Hanberg-Soerensen F, Bone J, Jacobsen NO, et al. Postoperative radiotherapy in Dukes’ B and C carcinoma of the rectum and rectosigmoid. A randomized multicenter study. Cancer 1986; 58: 22–8
- Lundby L, Jensen VJ, Overgaard J, Laurberg S. Long-term colorectal function after postoperative radiotherapy for colorectal cancer. Lancet 1997; 350: 564
- Pollack J, Holm T, Cedermark B, Altman D, Holmstrom B, Glimelius B, et al. Late adverse effects of short-course preoperative radiotherapy in rectal cancer. Br J Surg 2006; 93: 1519–25
- Birgisson H, Pahlman L, Gunnarsson U, Glimelius B. Adverse effects of preoperative radiation therapy for rectal cancer: Long-term follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol 2005; 23: 8697–705
- Peeters KC, van de Velde CJ, Leer JW, Martijn H, Junggeburt JM, Kranenbarg EK, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients–a Dutch colorectal cancer group study. J Clin Oncol 2005; 23: 6199–206
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006; 355: 1114–23
- Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol 2006; 24: 4620–5
- Marijnen CA, Glimelius B. The role of radiotherapy in rectal cancer. Eur J Cancer 2002; 38: 943–52
- Rodel C, Grabenbauer GG, Schick C, Papadopoulos T, Hohenberger W, Sauer R. Preoperative radiation with concurrent 5-fluorouracil for locally advanced T4-primary rectal cancer. Strahlenther Onkol 2000; 176: 161–7
- Sun XN, Yang QC, Hu JB. Pre-operative radiochemotherapy of locally advanced rectal cancer. World J Gastroenterol 2003; 9: 717–20
- Balch GC, De Meo A, Guillem JG. Modern management of rectal cancer: A 2006 update. World J Gastroenterol 2006; 12: 3186–95
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006; 93: 1215–23
- Pietrzak L, Bujko K, Nowacki MP, Kepka L, Oledzki J, Rutkowski A, et al. Quality of life, anorectal and sexual functions after preoperative radiotherapy for rectal cancer: Report of a randomised trial. Radiother Oncol 2007; 84: 217–25
- Habr-Gama A, Perez RO, Proscurshim I, Campos FG, Nadalin W, Kiss D, et al. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg 2006; 10: 1319–28
- Kahn H, Alexander A, Rakinic J, Nagle D, Fry R. Preoperative staging of irradiated rectal cancers using digital rectal examination, computed tomography, endorectal ultrasound, and magnetic resonance imaging does not accurately predict T0,N0 pathology. Dis Colon Rectum 1997; 40: 140–4
- Williamson PR, Hellinger MD, Larach SW, Ferrara A. Endorectal ultrasound of T3 and T4 rectal cancers after preoperative chemoradiation. Dis Colon Rectum 1996; 39: 45–9
- Chen CC, Lee RC, Lin JK, Wang LW, Yang SH. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy?. Dis Colon Rectum 2005; 48: 722–8
- Melton GB, Lavely WC, Jacene HA, Schulick RD, Choti MA, Wahl RL, et al. Efficacy of preoperative combined 18-fluorodeoxyglucose positron emission tomography and computed tomography for assessing primary rectal cancer response to neoadjuvant therapy. J Gastrointest Surg 2007; 11: 961–9
- Gerard JP, Romestaing P, Chapet O. Radiotherapy alone in the curative treatment of rectal carcinoma. Lancet Oncol 2003; 4: 158–66
- Christensen P, Olsen N, Krogh K, Laurberg S. Scintigraphic assessment of colostomy irrigation. Colorectal Dis 2002; 4: 326–31
- Dini D, Venturini M, Forno G, Bertelli G, Grandi G. Irrigation for colostomized cancer patients: A rational approach. Int J Colorectal Dis 1991; 6: 9–11
- Christensen P, Bazzocchi G, Coggrave M, Abel R, Hultling C, Krogh K, et al. A randomized, controlled trial of transanal irrigation versus conservative bowel management in spinal cord-injured patients. Gastroenterology 2006; 131: 738–47
- Christensen P, Olsen N, Krogh K, Bacher T, Laurberg S. Scintigraphic assessment of retrograde colonic washout in fecal incontinence and constipation. Dis Colon Rectum 2003; 46: 68–76
- Gosselink MP, Darby M, Zimmerman DD, Smits AA, van KI, Hop WC, et al. Long-term follow-up of retrograde colonic irrigation for defaecation disturbances. Colorectal Dis 2005; 7: 65–9
- Iwama T, Imajo M, Yaegashi K, Mishima Y. Self washout method for defecational complaints following low anterior rectal resection. Jpn J Surg 1989; 19: 251–3
- Christensen P, Olsen N, Krogh K, Laurberg S. Scintigraphic assessment of antegrade colonic irrigation through an appendicostomy or a neoappendicostomy. Br J Surg 2002; 89: 1275–80
- Christensen P, Buntzen S, Krogh K, Laurberg S. Ileal neoappendicostomy for antegrade colonic irrigation. Br J Surg 2001; 88: 1637–8
- Krogh K, Laurberg S. Malone antegrade continence enema for faecal incontinence and constipation in adults. Br J Surg 1998; 85: 974–7
- Rasmussen OO, Buntzen S, Sorensen M, Laurberg S, Christiansen J. Sacral nerve stimulation in fecal incontinence. Dis Colon Rectum 2004; 47: 1158–62
- Pillinger SH, Gardiner A, Duthie GS. Sacral nerve stimulation for faecal incontinence. Dig Surg 2005; 22: 1–5
- Matzel KE, Stadelmaier U, Bittorf B, Hohenfellner M, Hohenberger W. Bilateral sacral spinal nerve stimulation for fecal incontinence after low anterior rectum resection. Int J Colorectal Dis 2002; 17: 430–4
- Ratto C, Grillo E, Parello A, Petrolino M, Costamagna G, Doglietto GB. Sacral neuromodulation in treatment of fecal incontinence following anterior resection and chemoradiation for rectal cancer. Dis Colon Rectum 2005; 48: 1027–36