Abstract
Background. Both adjuvant therapy and mammography screening can decrease breast cancer mortality and there is a need of knowing to what extent those two modalities are used in the population. Screening coverage is well documented but there is a scarcity of population-based data on use of systemic adjuvant treatment. Aim. To describe the introduction, and trends in the use of adjuvant systemic therapy for breast cancer in two of six public health regions in Sweden. Material & methods. Population-based data on use of adjuvant therapy were available from databases with documented high quality and high coverage data for Stockholm (1976–2005) and North Sweden (1980–2003, and 2005). Results. The use of systemic treatment was infrequent before the late 1980s in both regions, but increased during the 1990s. In 2005, the proportion of operable breast cancer patients treated with adjuvant endocrine therapy in the ages 40–59 was around 60 to 80%. The proportion adjuvant chemotherapy was less than 15% for the ages 70–74. For the north region the use of endocrine therapy increased successively over time, with an exception for age group 40–49 were a more rapidly increase occurred in the late 1990s. In Stockholm the increment was higher and more rapidly. There was no clear difference in chemotherapy use between the regions, and the use increased from the mid 1980s in age group 40–49, and in the early 1990s for women aged 50–59. In age group's 60–69 and 70–74 the use was relatively infrequent. Conclusions. Trends in, and levels of the use of adjuvant systemic therapy for breast cancer varied over time in the two study regions, particularly for endocrine therapy. We consider that the differences between the regions mainly reflect different interpretations of new scientific evidence. We stress the importance of a good documentation of all new treatment protocols.
The secular trend for age-adjusted breast cancer mortality has shifted in several western countries during the 1990s. The trend is now distinctly downward in, for instance, the UK and the US, despite continuing increases in the breast cancer incidence, except for the last 2–3 years Citation[1]. There has been considerable controversy regarding the extent to which preventive measures such as early diagnosis and widespread use of routine adjuvant systemic therapy has contributed to the decreasing trend in breast cancer mortality. Over the years several randomised controlled trials (RCTs) and overviews and meta-analyses of such trials have convincingly demonstrated that both mammography screening and adjuvant systemic therapy can decrease breast cancer mortality and case fatality Citation[2–7].
In Sweden, breast cancer mortality has been stable or slightly downward during past decades while at the same time breast cancer incidence has increased substantially. This has been explained by the introduction of population-based mammography screening as well as the increasing use of systemic adjuvant therapy. The population-based screening program with mammography in Sweden is well documented in several studies Citation[8–11]. However, as in many other countries, there are limited data on time trends in the use of adjuvant systemic therapy. The time trends in adjuvant therapy has so far only been reported from the Netherlands (1973–1997) Citation[6], southeast England (1996–2003) Citation[12], and from the US (1975–1999) Citation[13], where all authors expressed problems in obtaining high quality and high coverage data on adjuvant therapy.
Both mammography screening and adjuvant systemic therapy were introduced as part of routine health care in many countries during roughly the same time period, i.e., adjuvant systemic therapy in the late 1970s and mammography screening in the late 1980s through the early 1990s. The aim of the present study was to analyse secular trends in the use of adjuvant systemic therapy among patients with breast cancer, in two of six public health care regions in Sweden (Stockholm and North Sweden) 1976–2005.
Material and methods
Cancer care in Sweden is coordinated by central units organised at a regional level in the six different public health care regions. Despite a nationwide common view of what would be the golden standard and national recommendations and guidelines in breast cancer treatment, the different regions may have their guidelines adjusted for regional differences. Data in the present study derives from the two regions, Stockholm and North Sweden.
Stockholm
Study area
Stockholm is a mainly urban area with a population of about 1.8 million. The annual number of incident cases of invasive breast cancer increased from about 800 in the late 1970s to about 1 500 in the year 2005 Citation[14].
Breast cancer care program
Breast cancer care in the region is coordinated through a comprehensive breast cancer care program initiated in the mid 1970s. The program is organised by the multidisciplinary Stockholm Breast Cancer Study Group (SBCSG) and includes clinical practice guidelines implemented at the five breast cancer detection and treatment centres. Over the years, the SBCSG has initiated several RCTs on adjuvant therapy.
Breast cancer care database
A clinical database was initiated in 1976 aiming at prospectively include all women with primary breast cancer diagnosed in the region. It is based on reports from all clinicians collaborating with the SBCSG. The data registered include information on stage of disease at primary diagnosis, type of surgery, postoperative treatment (including adjuvant systemic therapy), histopathological data, hormone receptor status and follow-up data. The completeness of the registers is checked through record linkage with data from the Regional Cancer Registry in the Stockholm-Gotland region which reports to the nationwide Swedish Cancer Registry. The completeness of the register at the National Cancer Registry has been estimated at 96% Citation[15].
The breast cancer database includes prospectively collected information on the use of adjuvant systemic treatment among individual patients. Reporting was based on forms filled out by the responsible clinician in conjunction with the multidisciplinary, postoperative clinical conference. The recommended treatment was then discussed with the patient at the out-patient clinic a few days later. This implied that the treatment actually received by the patient may have deviated from the intended treatment reported on the form sent to the SBCSG secretariat. However, such inconsistencies have been found to be rare. For instance, among patients who were recommended treatment with tamoxifen, 96% were found to have initiated such treatment, and among those not recommended such treatment, only 1% actually received the drug Citation[16]. Non-compliance to a recommendation of adjuvant chemotherapy was in 1976–1984 found to be about 6 and 10% among pre- and post menopausal patients, respectively Citation[17].
Breast cancer care guidelines
The clinical practice guidelines issued by the SBCSG are intended to be evidence-based. Consequently, they have been continuously updated to comply with the increase of evidence from the RCTs on adjuvant systemic therapy and overviews of such RCTs. Routine adjuvant systemic therapy was not recommended until 1990. At that time, the survival benefits observed in the Oxford overviews with both cytotoxic chemotherapy and adjuvant endocrine therapy were considered mature and clinically worthwhile Citation[2], Citation[3].
North Sweden
Study area
North Sweden is a mainly rural area. It covers about half of Sweden but the population is only 0.9 million. The annual number of incident cases of invasive breast cancer increased from about 400 in the late 1970s to about 600 in the year 2005 Citation[14].
Breast cancer care program
As in Stockholm breast cancer care in the region is coordinated through a comprehensive breast cancer program. The program was initiated in 1980 and is organised by the multidisciplinary North Sweden Breast Cancer Group (NSBG). Similar to the SBCSG the NSBG has initiated several RCTs on adjuvant therapy as part of the program.
Breast cancer care database
As part of the regional breast cancer program a clinical database was initiated in 1980 aiming at prospectively include women with primary breast cancer diagnosed in the region. The format of the database is similar to that in Stockholm. The completeness of registration is checked through record linkage with data from the Regional Cancer Registry, also delivering the regional data to the National Cancer Registry.
In North Sweden, registered data were also based on forms filled out by the responsible clinician. However, all information on adjuvant systemic treatment has prospectively been checked for accuracy by data managers who have compared the registered information with clinical records. This routine has been implemented since September 1987 when the database was changed. The registered information from 1988 and onwards thus concerns the type of adjuvant systemic therapy that was actually initiated in a patient. However, before that year, information about treatment mostly concerned the treatment allocated to patients included in the RCTs. As adjuvant systemic therapy during this time was rarely used outside the trials, it was assumed that patients for whom data were unavailable had not received adjuvant systemic therapy. During 1980–1987 the county of Jämtland, which then constituted 15% of the female population in the region did not belong to the region and were not included in the clinical breast cancer database. Therefore, Jämtland was excluded from the analysis during that period. The registration in the breast cancer care database for the year 2004 was not yet complete and could therefore not be included in the analyses.
Breast cancer care guidelines
In North Sweden the regional clinical practice guidelines issued by the NSBG have been continuously updated. Routine adjuvant systemic therapy in selected patient groups was recommended first in 1987.
Adjuvant therapy
Study population/period
For this study we selected women aged 40–74 years who were reported to the breast cancer database 1976–2005 in Stockholm and 1980–2005 in North Sweden with a uni- or bilateral, primary, invasive breast cancer, and who were considered to have operable disease. Adjuvant treatment with tamoxifen, megestrol acetate, medroxyprogesterone acetate, and goserelin or oophorectomy among pre menopausal patients, was recorded as ‘adjuvant endocrine therapy’ irrespective of whether they also were treated with adjuvant chemotherapy. Adjuvant therapy with cytotoxic drugs (typically CMF-type regimens during the 1970s and early 1980s, and anthracycline-containing regimens during the late 1980s and 1990s) was recorded as ‘adjuvant chemotherapy’ irrespective of whether the patient also received adjuvant endocrine therapy. Patients receiving both treatment modalities, either in combination or sequentially were recorded as having had ‘adjuvant chemo-hormonal therapy’.
The study was approved by the Karolinska Institute's Research Ethics Committee (KI Dnr: 03-630). The approval was valid for both regions.
Results
Coverage of the breast cancer care databases
During the study period 1976–2005, 23 156 invasive breast cancer cases aged 40–74 years were reported to the Regional Cancer Registry in Stockholm and out of these, 21 639 were notified in the breast cancer care database, thus giving registration coverage of 93%. The coverage was lower during the first three years (in the late 70s), but remained at a high and stable level thereafter. A total of 92% had undergone primary surgery, and among them information on adjuvant systemic therapy was available in 98% of the patients between the years 1991 and 2005.
During the period 1976 to 1990 the registration of the data variable “no adjuvant therapy given” could not be separated from the variable “adjuvant therapy unknown”. This explains why the curve in is at a lower level before 1991. There is no reason to believe that the registration was less complete before 1990, therefore the level shown would likely be false too low. The proportion of operable cases with information on adjuvant therapy remained high and stable during the entire period ().
Figure 1. The annual number of invasive breast cancer cases in Stockholm county 1976–2005 (Stockholm), and in North Sweden 1980–2005 (North Sweden), in the ages 40–74: 1) reported to the Swedish Cancer Registry, 2) included in the regional breast cancer database, 3) registered as operable, and 4) with information on adjuvant therapy.
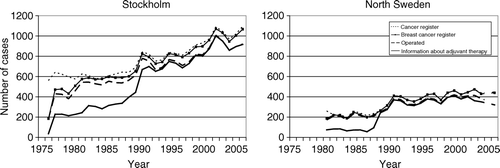
In North Sweden 8 714 invasive breast cancer cases aged 40–74 years were reported to the Regional Cancer Registry 1980–2005 (2004 excluded) compared to 8 543 in the breast cancer care database (98% coverage). The proportion of cases that underwent primary surgery was 91%. As in Stockholm this proportion did not change substantially during the study period (). Data on adjuvant systemic therapy was available in 96% of the patients for the period 1988 to 2005, but during 1980 to 1987 data were available only for patients included in RCTs. However, the vast majority of the patients treated outside the trials did not receive adjuvant therapy as it was not recommended in routine practice at the time.
Ages 40–49 years
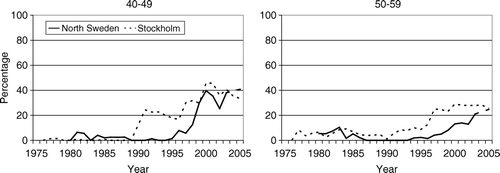
Before 1990 few patients in Stockholm below the age of 50 years were treated with adjuvant endocrine therapy () whereas 10–20% received adjuvant chemotherapy (). In Northern Sweden 15–25% received endocrine treatment during the 1980s whereas the use of chemotherapy was less prevalent, 5–10%.
Figure 2. The proportion of operable breast cancer cases aged 40–49, 50–59, 60–69 and 70–74 years treated with adjuvant endocrine therapy according to year of diagnosis and region.
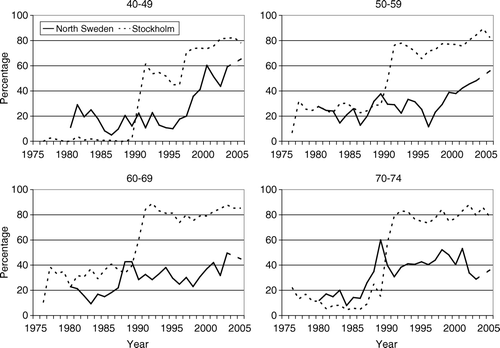
Figure 3. The proportion of operable breast cancer cases aged 40–49, 50–59, 60–69 and 70–74 years treated with adjuvant chemotherapy according to year of diagnosis and region.
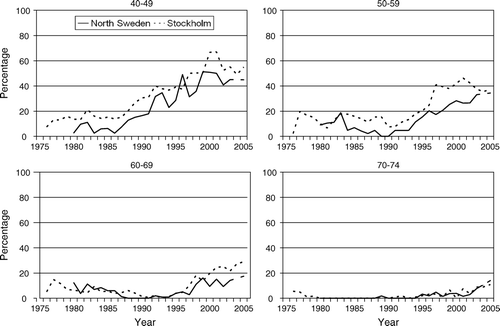
During the 1990s the use of both adjuvant endocrine and cytotoxic therapy increased substantially in both regions. The proportion who received chemotherapy was about the same in Stockholm and North Sweden, and about 50% received such therapy in the year 2005. The use of adjuvant endocrine therapy increased markedly in Stockholm during the early 1990s to about 50–60%. In approximately 40% of these cases the treatment was given in conjunction with chemotherapy (). A similar sharp increase in the use of adjuvant endocrine therapy was observed in North Sweden but not until the late 1990s. About half, to two-thirds of the patients received chemo-hormonal therapy.
Ages 50–59 years
During the late 1970s and 1980s the use of adjuvant endocrine therapy in both regions was fairly stable and covered 20–30% of the patients (). However, during the early 1990s the use increased sharply in Stockholm up to 70–80%, and remained stable during the remaining study period. No similar sharp upward trend was observed in North Sweden although there was a small increase in the use of endocrine therapy in the late 1990s and a continuing increment up to 55% in 2005.
The use of adjuvant chemotherapy was 10–15% until the mid 1990s in both regions and increased gradually to approximately 35% the year 2005 (). About half of the therapy was combined chemo-hormonal therapy ().
Ages 60–69 years
In Stockholm the trend in the use of endocrine therapy in age group 60–69 was similar to that observed in the women aged 50–59 (). In contrast, only a small increase was observed in North Sweden.
Adjuvant chemotherapy was rarely used in this age group during most of the study period (). The trends were not substantially different between the two regions. During the 1980s only 5–10% of the cases received such treatment and the time trends were actually slightly downward. However, since the late 1990s there appeared to be some increase in usage. In the year 2005, 15–30% of the women received adjuvant chemotherapy in both regions.
Because of the infrequent use of chemotherapy in this age group, rates for combined chemo-hormonal therapy were also low (data not shown).
Ages 70–74 years
Trends in the use of endocrine therapy in this age group were roughly similar to those observed at ages 50–59 years and 60–69 years: i.e. relatively stable around 10–20% until the late 1980s thereafter it increased sharply in Stockholm to about 80% (). In North Sweden use increased to some extent also during late 1980s to early 1990s, but levelled off at 40–50% during the 1990s. In the year 2005 the proportion was under 40%.
Only few patients in this age group received adjuvant chemotherapy during the study period ().
Discussion
Before conducting this study we had an assumption that new scientific evidence on systemic breast cancer therapy would have an immediate impact on treatment regimens, however, that assumption could not be confirmed.
Already in the mid 1970s preliminary results from controlled trials suggested significant and clinically worthwhile treatment benefits with adjuvant chemotherapy in early stage breast cancer Citation[18], Citation[19]. A few years later encouraging results were also reported from trials of adjuvant endocrine therapy with tamoxifen Citation[20], Citation[21]. Against this background one would perhaps have expected upward trends in use of adjuvant systemic therapy starting already in the late 1970s to early 1980s. It would also seem reasonable to assume that such upward trends were accentuated after 1985 following the recommendations of the National Institutes of Health/National Cancer Institute (NIH/NCI) Consensus Development Conference about routine use of adjuvant chemotherapy and adjuvant tamoxifen Citation[22]. Despite the availability of such guidelines there is evidence that adherence to and implementation of treatment recommendations is less than optimal. This has also been described by other authors Citation[23]. The present study shows that all types of adjuvant systemic therapy were used relatively infrequently before the late 1980s. Upward trends in the use of adjuvant therapy did not occur before the early 1990s. However a pattern in the use of adjuvant therapy reflecting a high degree of compliance to the regional practice guidelines who did not recommend adjuvant systemic therapy as a routine treatment before 1987 in North Sweden, and before 1990 in Stockholm were also seen in this study. In Stockholm, the correlation between regional guidelines and endocrine therapy is striking. This is most obvious in the youngest age group where endocrine therapy was not recommended to pre menopausal women before 1990. However, after 1990 a large increase was seen in all the age groups. After 1996 a slight change in the curves is seen due to a revision version of the guidelines. In the north region the changes in the curves were not as large and instantaneous as in Stockholm. Another difference between the two regions was the larger variability in North Sweden, which could be due to the lower number of cases there, in comparison to Stockholm.
We also studied the pTNM stage, and oestrogen receptor (ER) status distributions for the two regions in order to search explanation for the differences in adjuvant systemic therapy between the two regions. However, both the stage distribution and the distribution of ER were rather similar between the regions (data not shown), and none of them could explain the differences between the regions.
In the present study the figures of systemic adjuvant therapy is presented as a proportion of operable breast cancer cases. The reason for using this proportion instead of all breast cancer cases as the denominator used in other studies Citation[6], Citation[12], Citation[13] was the fact that inoperable cases would not benefit from receiving adjuvant therapy. The disadvantage with this proportion is that it is not easily compared with other studies, the advantage of this proportion is that it is more refined and should better be related to the outcome. In this study 84 to 93% of the breast cancer cases where operated throughout the study period.
The trend in North Sweden and Stockholm concerning adjuvant chemotherapy was similar to that in the south east of the Netherlands Citation[6]. Despite similar health care organisation and access to region wide cancer statistics in Sweden and in the Netherlands, the proportion of women below 50, treated with chemotherapy in the Netherlands was higher than in Sweden before the 1990. In that age group the percentage of women treated with tamoxifen was around 5% at maximum for the whole period, which was completely different from what was seen in the two Swedish regions.
Data reported from the southeast England Citation[12], was not divided into age groups which obstruct the comparability with the present study. Tataru et al. reported a relatively steep decrease in hormone therapy during the late 1990s and early 2000s, which was not seen in the two Swedish regions. For chemotherapy the differences were not as striking as for hormonal therapy. It's reasonable to believe that the differences seen on hormonal therapy are due to stricter treatment criteria in England during this period. In Sweden, also pre menopausal women with ER positive breast cancers have been treated with tamoxifen, where in England, only post menopausal women receive tamoxifen.
Trends in adjuvant therapy in the US were reported from eight population-based registries Citation[13], for a time period similar to the present study. The health care organisation in the United States is more privately based allowing physicians to act more freely in the decision on type of treatment to recommend the individual patient. This makes data from that study difficult to compare with ours. The US data was presented by stage: still a very sharp increase in hormonal therapy was seen a couple of years before the increase occur in Stockholm. This could probably be due to the already mentioned difference in health care organisation where in the US, new methods and recommendations was adopted earlier than in more centralised health care system.
The study had several strengths. It was based on almost three decades of population-based clinical data of diagnosed breast cancer cases, covering a third of the Swedish population. Prospectively collected data were available from the late 1970s until the mid 2000s. Initially adjuvant systemic therapy was only used in selected patients included in controlled clinical trials. Later, it was routinely used in a larger proportion of all the cases.
It might be seen as a limitation that we did not analyse the trends in more detail. Such an approach would have been necessary if the aim of this study had included assessing the extent to which the treatment offered to the patients adhered to the clinical practice guidelines issued by the regional breast cancer groups. North Sweden reported adjuvant therapy as “actually initiated” to the patient whereas in Stockholm treatment was reported as “recommended” to the patient. However, in reality the possible differences between the regions in whether the patients had received treatment or not are probably small because previous experience from Stockholm indicated that the patients’ compliance to recommended treatment was high, particularly in the case of adjuvant endocrine therapy. There is therefore no reason to assume any differences between the two regions in the percentages of patients having received complete treatment Citation[16], Citation[17]. Nevertheless, we can not exclude that this circumstance may cause a small overestimate of the reported proportions treated patients in Stockholm in comparison to North Sweden.
The upward trends in use of adjuvant systemic therapy in the early 1990s occurred roughly simultaneously with the introduction of population-based mammography screening. The screening program in Stockholm started 1989 (age 50–69) and the first round was completed in 1991. In North Sweden screening started 1989–1990 (age 40–74) in two of the counties (59% of the total population). Their first round was completed in early 1992. The remaining two counties in the northern region started mammography screening in the mid 1990s (age 50–69), and in 1998 all women in the target group in the region had been screened at least once. This implies that service screening was introduced in both regions concurrently with a more widespread use of adjuvant systemic therapy. In fact the introduction of screening which is believed to result in a down staging of the diagnosed breast cancers Citation[24], may also have had an influence on both type and amount of therapy that has been given thereafter.
The trends in adjuvant systemic therapy in the North Sweden and Stockholm, reported in the present study have to our knowledge not been shown before. Actually given adjuvant systemic therapy varied between the regions, particularly for endocrine therapy. We consider that these differences between the regions were mainly due to different interpretations of new scientific evidence. However, we were unable to verify this with information back in time when new treatment protocols had been issued. In order to maximise the use of individual data registers for evaluation of treatment effects we would emphasize the need of a systemic documentation of when, and for which patient groups new treatment regimens are agreed and issued.
Acknowledgements
This study was supported by a grant from the Swedish Cancer Society.
None of the authors have declared any conflict of interest
References
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365:1687–717.
- Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet 1992;339:1–15.
- Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet 1992;339:71–85.
- Nystrom L, Rutqvist LE, Wall S, Lindgren A, Lindqvist M, Ryden S, et al. Breast cancer screening with mammography: Overview of Swedish randomised trials. Lancet 1993; 341: 973–8
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005; 353: 1784–92
- Vervoort MM, Draisma G, Fracheboud J, van de Poll-Franse LV, de Koning HJ. Trends in the usage of adjuvant systemic therapy for breast cancer in the Netherlands and its effect on mortality. Br J Cancer 2004; 91: 242–7
- Sarkeala T, Heinavaara S, Anttila A. Organised mammography screening reduces breast cancer mortality: A cohort study from Finland. Int J Cancer 2008; 122: 614–9
- Jonsson H, Tornberg S, Nystrom L, Lenner P. Service screening with mammography in Sweden–evaluation of effects of screening on breast cancer mortality in age group 40–49 years. Acta Oncol 2000; 39: 617–23
- Duffy SW, Tabar L, Chen HH, Holmqvist M, Yen MF, Abdsalah S, et al. The impact of organized mammography service screening on breast carcinoma mortality in seven Swedish counties. Cancer 2002; 95: 458–69
- Swedish Organised Service Screening Evaluation Group. Reduction in breast cancer mortality from organized service screening with mammography: 1. Further confirmation with extended data. Cancer Epidemiol Biomarkers Prev 2006;15:45–51.
- Swedish Organised Service Screening Evaluation Group. Reduction in breast cancer mortality from the organised service screening with mammography: 2. Validation with alternative analytic methods. Cancer Epidemiol Biomarkers Prev 2006;15:52–6.
- Tataru D, Robinson D, Moller H, Davies E. Trends in the treatment of breast cancer in Southeast England following the introduction of national guidelines. J Public Health (Oxf) 2006; 28: 215–7
- Mariotto A, Feuer EJ, Harlan LC, Wun LM, Johnson KA, Abrams J. Trends in use of adjuvant multi-agent chemotherapy and tamoxifen for breast cancer in the United States: 1975–1999. J Natl Cancer Inst 2002; 94: 1626–34
- Centre of Epidemiology. Cancer Incidence in Sweden 2006. National Board of Health and Welfare, Stockholm 2007;
- Mattsson B, Wallgren A. Completeness of the Swedish Cancer Register. Non-notified cancer cases recorded on death certificates in 1978. Acta Radiol Oncol 1984; 23: 305–13
- Rutqvist LE, Cedermark B, Glas U, Johansson H, Nordenskjold B, Skoog L, et al. The Stockholm trial on adjuvant tamoxifen in early breast cancer. Correlation between estrogen receptor level and treatment effect. Breast Cancer Res Treat 1987; 10: 255–66
- Rutqvist LE, Cedermark B, Glas U, Johansson H, Rotstein S, Skoog L, et al. Randomized trial of adjuvant tamoxifen combined with postoperative radiation therapy or adjuvant chemotherapy in postmenopausal breast cancer. Cancer 1990; 66: 89–96
- Fisher B, Carbone P, Economou SG, Frelick R, Glass A, Lerner H, et al. 1-Phenylalanine mustard (L-PAM) in the management of primary breast cancer. A report of early findings. N Engl J Med 1975; 292: 117–22
- Bonadonna G, Brusamolino E, Valagussa P, Rossi A, Brugnatelli L, Brambilla C, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 1976; 294: 405–10
- Baum M, Brinkley DM, Dossett JA, McPherson K, Patterson JS, Rubens RD, et al. Improved survival among patients treated with adjuvant tamoxifen after mastectomy for early breast cancer. Lancet 1983; 2: 450
- Ribeiro G, Palmer MK. Adjuvant tamoxifen for operable carcinoma of the breast: report of clinical trial by the Christie Hospital and Holt Radium Institute. Br Med J (Clin Res Ed) 1983; 286: 827–30
- NIH/NCI. Adjuvant chemotherapy for breast cancer. NIH Concensus Statement 1985;5:1–19.
- Thuerlimann B, Koeberle D, Senn HJ. Guidelines for the adjuvant treatment of postmenopausal women with endocrine-responsive breast cancer: Past, present and future recommendations. Eur J Cancer 2007; 43: 46–52
- Swedish Organised Service Screening Evaluation Group. Effect of mammographic service screening on stage at presentation of breast cancers in Sweden. Cancer 2007;109:2205–12.