Abstract
Introduction. Image guided adaptive brachytherapy (IGABT) for cervical cancer, using mainly MRI, is an evolving method, increasingly replacing the 2D approach based on conventional radiography. During the complex 4D chain of this procedure image-assistance is provided for disease assessment, provisional treatment planning (“pre-planning”), applicator placement and reconstruction, as well as for contouring, definitive treatment planning and quality control of dose delivery. With IGABT changes of topography adjacent to the applicator, caused by tumour regression, oedema, organ changes and dilation are identified. Thus, the CTV for IGABT is primarily based on the tumour volume at the time of BT and takes into account both time and spatial domains. IGABT requires systematic concepts for target, OAR, biological modelling, DVH analysis, and dose-volume-adaptation. Methods and Results. This report focuses on the advantages and uncertainties, dose-effect relations and clinical results of the IGABT procedure addressing the current status and future perspectives. Uncertainties during the 4D chain of IGABT are mainly related to target contouring, applicator reconstruction, as well as to inter-fraction, intra-fraction and inter-application variability, as caused by tumour response and organ changes. Different from EBRT where set-up uncertainties are compensated by adding a margin to the CTV, no margins to the lateral and anterior-posterior directions can be used for IGABT. Discussion. By 3D treatment planning for IGABT significant improvement of the DVH parameters is achieved compared to 2D library plans. In small tumours the benefit is primarily obtained by a decrease of dose to nearby OAR while in large tumours the use of supplementary interstitial techniques and optimization may double the target volume that can be treated at a therapeutic dose level. The clinical impact of IGABT could recently be demonstrated by the establishment of some correlations between target- and organ-related DVH parameters versus disease control and side effects, which need further clarification. Overall, a very high local control rate can be achieved with minor treatment related morbidity. This favourable therapeutic ratio seems to be now reproducible under different conditions at various treatment centres. These results have to be validated within the upcoming multi-centre prospective IntErnational study on MRI-guided brachytherapy in locally advanced cervical cancer (EMBRACE).
Image guided adaptive brachytherapy (IGABT) in locally advanced cervix cancer has recently been introduced Citation[1–3] and is now gaining momentum as this emerging technique has been shown to provide major improvements in dose volume parameters and in clinical outcome primarily due to a high precision of the dose delivered by gynaecological IGABT Citation[3–6]. The advancement of this technique is primarily based on the use of repetitive MRI performed before and during treatment. Other imaging modalities such as ultrasound Citation[7] and functional imaging are also under evaluation but so far only very limited data are available Citation[8], Citation[9].
This status report will first address the principles applying for IGABT in general and will then provide a specific overview of the use of MRI based IGABT in the treatment of locally advanced cervical cancer with regard to all steps involved in the treatment chain.
The sources of information for this overview were obtained from a Pub Med search for articles published before June 2008 by use of the following key-words: cervical cancer or cervix cancer; image-guided brachytherapy, 3D brachytherapy, CT-based brachytherapy or MRI-based brachytherapy. Updating and new analysis of clinical material from our departments as well as clinical data obtained from major collaborating departments in the field (Institut Gustave Roussy, Paris, University Hospital Leuven) were also included to further illustrate the current status and potential perspectives of MRI based IGABT in the treatment of locally advanced cervical as of 2008.
Basic principles applying for IGABT
There are some fundamental features of brachytherapy (BT) which have to be taken into account for image guidance in general and for gynaecological BT in particular. These conditions are only described here in broader terms Citation[10] but they definitively will need further attention in future image orientated research and development.
Clinical use of BT always involves the placement of an applicator near to or into a selected tumour (site) by a radiation or organ specific oncologist experienced in introducing applicators for a specific site applying manual, tactile, optical and mental skills acquired through a specific period of learning (“micro-invasive surgery”; “radiotherapeutic intervention”). This implies variation both at the institutional level according to tradition/school preferences (Manchester, Paris, Stockholm, Fletcher etc.) but also at the personal level with intra-person and inter-person variation in applicator placement.
As BT is performed through applicators adjacent to or inside the Clinical Target Volume (CTV), images for BT have to be taken with the applicator in place. The inherent aim of BT imaging is therefore to offer on one and the same high quality image all the important elements: applicators, potential source positions, Gross Tumour Volume (GTV), CTV and adjacent organs at risk (OAR). This complex aim implies significant interactions between image demands for applicators, treatment planning (source position), and contouring of regions of interest. It may also imply the need to use various imaging methods, which have to be integrated (“fusion”) during the process of treatment planning (conventional radiography, CT, MRI, US). This is specific for organ site (e.g. prostate, gynaecology, breast: for imaging GTV and OAR) and for applicator type and mandates adapted solutions. A significant effort in research and development (e.g. feasibility, visibility, validity) is therefore needed to obtain appropriate images.
The applicator has impact on the adjacent topography, which may be relevant for both the relation to the GTV, the tumour bearing organ and/or the adjacent OAR. This is most pronounced in endoluminal and intracavitary BT (dilatation) but is also known for interstitial BT (oedema). With oedema a time component has to be taken into account, as it develops and resolves within hours to days. This impact may be different from application to application and/or from fraction to fraction during the same application which implies inter-application and inter-fraction uncertainty.
The process of treatment planning in BT always starts from identification of source position(s) and applicators on the image in the individual patient (“3D reconstruction”), which has to be performed as precise as possible and especially the systematic error inherent in this procedure has to be minimized to be able to deliver high-precision BT safely.
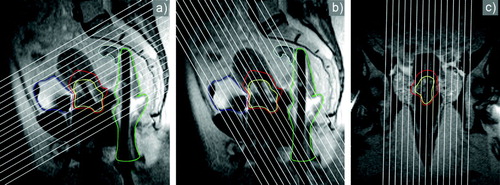
Imaging for BT needs a systematic relation between source (axis or axes of the applicator) and adjacent topography (), which implies a “Brachytherapy Eye View” (BEV). This “BEV” defines a joint geometry system between the image orientation in the patient and the BT applicator which is fundamental to obtain appropriate image guidance during applicator implantation, target contouring, applicator reconstruction and treatment planning.
Figure 1. The “Brachytherapy Eye View” illustrating a systematic relation between applicator geometry and image orientation. White lines indicate the selection of the appropriate image orientation parallel and orthogonal to the axis of the intrauterine tandem, as well as to the sagittal (a, b) and coronal (c) midplane of the ring: (a) para-transverse, (b) para-coronal and (c) para-sagittal sequences. Contours of GTV, HR CTV and OAR are given.
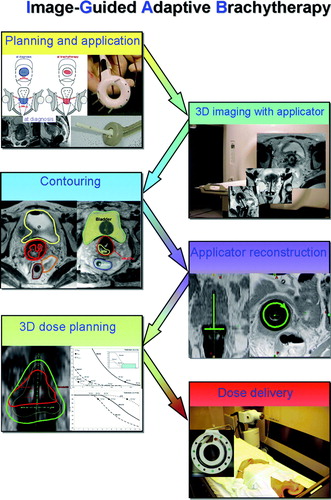
According to the specific characteristics of BT, imaging alongside the clinical assessment plays an important role at different steps in different ways within the treatment chain such as image assisted provisional treatment planning (“pre-planning”), image guided application, image assisted definitive treatment planning and image assisted quality control of dose delivery (imaging during/after brachytherapy). In this context it is important to stress that imaging and all related procedures (e.g. contouring) needs a higher precision than with conventional EBRT due to the very sharp dose fall-off in all directions, in particular in the region adjacent to the BT applicators. Thus, variation in millimetres in target contouring has a major impact on dose application (in 10 percentiles).
“In Room Imaging” as introduced for external beam radiotherapy (EBRT) with a CT-scanner available in the treatment room has to be looked at specifically for BT, as the applicator in place (“treatment machine”) is imaged together with GTV, CTV and OAR. Therefore imaging with the applicator in place always means to some degree “In Room Imaging”. However, there is some uncertainty, how far this situation corresponds to the situation during BT irradiation, which seems to be-under many circumstances-minimal for the fixed relation between the applicator and the tumour bearing organ, but may be significant for certain adjacent organs with significant movement relevant for BT.
Due to specific patterns of growth (e.g. expansive and infiltrative) and due to different patterns of treatment response there may be or may be not significant changes of tumour and adjacent topography during a fractionated course of EBRT. Major changes occur e.g. in case of pre-brachytherapy EBRT (+/− chemotherapy) in cervix cancer, as the tumour may shrink significantly during this time period. Thus, the GTV in cervix cancer during 40–50 Gy EBRT is reduced to mean less than 20–30% of its volume at the time of diagnosis Citation[11], Citation[12]. Therefore IGABT takes into account the situation at diagnosis and the tumour size and configuration as it presents at the time of BT with the applicator in place, based on images at diagnosis and at BT. This definition of “adaptive” is different from the contemporary definition used mainly for image guided EBRT and intra-fractional EBRT changes as seen by Cone beam CT. With IGABT adaptation is taken in both spatial (3D) and time (4D) domains. By use of the response adapted CTV defined at the time of BT it is possible to obtain much higher doses than currently possible with EBRT Citation[13].
The treatment chain of image guided adaptive brachytherapy in cervix cancer
The 4D treatment chain () starts with disease assessment at diagnosis including clinical drawing and MRI before any treatment begins. Through repetitive clinical examinations, complemented by imaging as necessary, the course of disease is evaluated and documented in order to follow the patient and to prepare comprehensively the IGABT procedure.
The process of IGABT is described in detail elsewhere Citation[3], Citation[9]. In this context it has to be underlined that this 4D chain includes many variations in regard to application, applicators, imaging, reconstruction, contouring, treatment planning, dose prescription, dose constraints for OAR. These variations will need to be understood as comprehensively as possible during the further evolution of this image based procedure in order to enable a thorough discussion of the different uncertainties based on evidence. To appropriately evaluate the clinical outcome of IGABT in cervical cancer a thorough follow-up of patients with 3D assessment of (complete) response, of local disease recurrence and of major events with regard to morbidity in relation to the complete 3D dose distribution of EBRT and BT is also needed.
Uncertainties in target contouring
The concept of HR CTV and IR CTV Citation[1] has been developed and validated through inter-observer studies from its beginning. The inter-observer variation, as expressed by the Conformitiy Index (CI) was from 0.5 to 0.8 for most of the presented material Citation[14–17]. There was only one report from a teaching workshop with participants with very limited experience, where the CI was only 0.1–0.3. This underlines the importance of experience in radiological assessment of disease which can only be gained within some learning period necessitating time and an interdisciplinary approach, integrating MRI radiologists with interest in gynaecology. It has been shown, that CT results in far more uncertainties for target contouring as compared to MRI Citation[18].
The issue of target contouring needs major attention, as the amount of uncertainty will have a major impact on the whole therapeutic chain. More comprehensive data is therefore needed to further illuminate the various aspects of this complex issue (e.g. topographical distribution of variation). The two published papers include only a limited number of patients (Lang et al. (n = 3); Viswanathan et al. (n = 10) Citation[15], Citation[18]), whereas more material is available according to data in publication process Citation[16], Citation[17].
Furthermore, it has to be kept in mind, that image quality itself has a major impact on the precision of target contouring (“validity”), which implies that further radiological research is needed to improve image quality and interpretation, also at the time of brachytherapy after response to radio–chemotherapy Citation[19]. This is true for any imaging method used, however in particular for MRI in the context of cervix cancer, as MRI represents the imaging method of choice for target contouring. The role of functional imaging has not been sufficiently addressed yet Citation[9].
Uncertainties in applicator reconstruction and use of margins for brachytherapy
Precise definition of the BT applicator is important for IGABT as the applicator functions as dose carrier and the dose calculation is relying solely on the geometry of source positions. Reconstruction on CT images is straightforward since source channels and dummy markers can be visualised on CT Citation[20–23]. However, for MRI special reconstruction techniques are needed in order to define the applicator geometry as accurate as for x-rays and CT. Currently there are several ongoing projects dedicated to this field of research. In the meanwhile, the use of marker tubes in the source channel, templates for the outer applicator surface and registration between radiographs or CT and MRI are showing promising results and are described in recent studies on IGABT Citation[3–5], Citation[24], Citation[25].
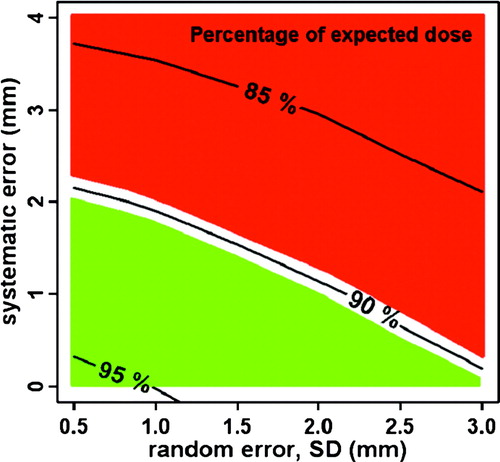
Reconstruction uncertainties in BT are of the same character as set-up uncertainties in EBRT since both define uncertainties in the relation between the radiation source and the target and OARs. The impact of reconstruction uncertainties on delivered dose depend on the dose gradients, the size of random and systematic reconstruction uncertainties, and on the number of BT fractions. Systematic errors should be avoided as much as possible since they have much more impact on delivered dose than random errors. This is illustrated in where the curves show the percentage dose (relative to TPS values) that 90% of patients will receive. When systematic errors are reduced to 1–2 mm, it is possible to limit the impact of reconstruction uncertainties (“set up”) on delivered dose to less than 10% Citation[26].
Figure 3. The curves show the percentage dose (relative to TPS values) as a function of random and systematic longitudinal reconstruction uncertainties. Reconstruction errors in the red area should be avoided (>10% dose deviation for at least 10% of the patients). The green area indicates a “safe” region of longitudinal reconstruction errors (<10% dose deviation for at least 90% of patients).
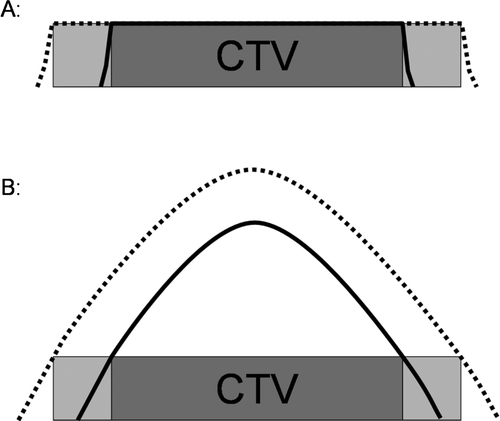
In conventional EBRT, set-up uncertainties are compensated by adding a margin to the CTV. The size of the prescription dose plateau is thereby increased, and the dose delivered to the CTV will be unaffected by geometric errors which are smaller than the size of the extra margin. In BT it is not possible to create a dose plateau in lateral and anterior-posterior directions, since the dose gradients cannot be manipulated to become less steep Citation[26]. As the addition of margins in lateral and anterior-posterior directions is directly equivalent to a BT dose escalation, the uncertainty of DVH parameters would not improve (). The use of margins is not recommended in these directions. However, in longitudinal direction the situation is basically different and it seems to be possible to create a plateau by loading the tandem above the target. This will make the dose distribution more robust to uncertainties in the longitudinal direction i.e. towards the uterine corpus and the vagina.
Figure 4. Effect of margins on dose distribution in external beam radiotherapy and intracavitary cervix cancer brachytherapy: A: In external beam radiotherapy a margin for PTV (light grey) will result in an increase of the field size. The plateau of the dose distribution covering the whole PTV (dashed line) remains unchanged. B: For intracavitary brachytherapy any margin into the lateral and anterior-posterior direction will result in a change of the dose distribution within the CTV with a volume and dose increase in the high dose regions of the CTV (from solid line to dashed line dose profile).
Inter-fraction, inter-application and intra-fraction variation: applicator, CTV and OAR movement
A prerequisite for analysis and understanding of this kind of variations is a clear terminology with regard to the specific characteristics of BT. Variation (geometric/dosimetric) occurs between fractions within one BT application (e.g. PDR) or between different applications. In order to classify these basically different situations with regard to terminology we propose to call the first type of variation “inter-fraction variation” and the second type “inter-application variation”. Inter-fraction variation is then defined as the topographic and/or dosimetric change between fractions without removal and re-insertion of the applicator. There is no direct comparison to EBRT for this type of variation. Inter-application variation is then defined as variation between different applications. This may be compared to the situation in EBRT, as the patient/organ position in relation to the “treatment device” (linac/applicator) may vary for each individual treatment.
For inter-fraction variation data for gynaecologic brachytherapy are very limited. One study compared two sets of MRI, scanned prior to each fraction, for an HDR schedule with 2 fractions given within 12–24 hours using the same BT application Citation[27]. The findings revealed a stable situation for target and rectum, while the position of the sigmoid changed significantly. In case of the bladder, a filling protocol, before each imaging procedure and dose delivery could reduce the variations substantially. Inter-fraction variability may also be prominent for PDR, especially for protracted schedules lasting 50–60 hours, but detailed analysis is scarce. So far the data is limited to indirect evidence for a stable geometry between BT applicator and rectum during a 10 hour PDR treatment Citation[28]
There is some evidence for inter-application variation. Studies used one treatment plan for different intracavitary applications. The superimposition showed major deviations for the dose to the target and to the OARs Citation[29–31]. In general the dose to the target structures becomes higher, as the treated volume is kept constant and the tumour is regressing between the applications. The geometrical relationship (“set-up”) between the applicator and the OAR can vary substantially for different reasons. Due to this significant inter-application variation the current suggestion is to follow adaptive treatment planning, with MR or CT imaging for each application.
Intrafraction variation is defined as the topographical variation during irradiation as in EBRT. This has not been investigated directly for BT. Based on the available data from inter-fraction variation analysis it seems likely that the situation for target, bladder and rectum can be taken as stable for HDR with little variation between dose plan and dose delivery, in particular for the parameters of interest as D90, 2 ccm and 0.1 ccm Citation[27].
Dose-volume benefit from 3D optimisation in IGABT
Dose optimisation involves manipulation of dwell positions and dwell times. In this way the size and the shape of the classical pear shaped isodose can be changed. In small tumours the irradiated volume can be reduced in order to reduce dose to OARs. In larger tumours the depth of the prescription isodose can be expanded by typically 5 mm in intracavitary applications Citation[3]. By introduction of additional interstitial needles, parametrial involvement can be further targeted and it becomes possible to provide prescription dose up to 15 mm from point A without increasing dose to OARs significantly Citation[32].
Non-optimised dose distributions in brachytherapy result in highly variable tumour doses. Lindegaard et al. showed that non-optimised standard plans with dose prescription to point A would result in target doses (D90 of HR CTV) ranging from 52 to 160% of prescribed dose with mean and standard deviation of 113±30%. With dose optimisation the delivered doses became more homogeneous: 117±22% (62–141%). At the same time the dose to OARs could be improved such that DVH constraints could be overall respected in 16/21 patients in optimised plans as compared to 3/21 patients in standard plans Citation[5]. Similar results were obtained by De Brabandere et al. where bladder and sigmoid DVH constraints could be respected after dose optimisation in 16 patients, whereas standard dose plans resulted in exceeding of constraints for 10/16 and 7/16 patients, for bladder and rectum respectively. At the same time they could obtain a mean target dose increase of 3 Gy Citation[4].
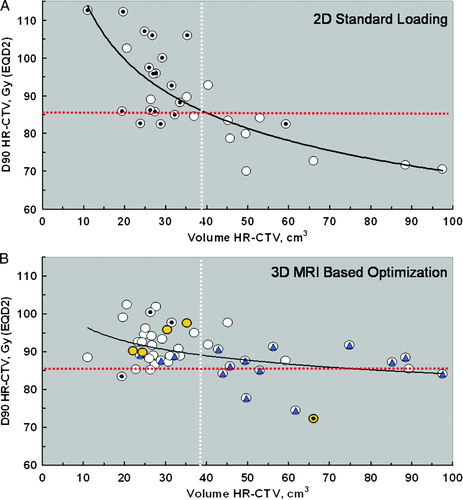
A new analysis of the relationship between D90 and volume of HR CTV of 50 patients treated at Aarhus University Hospital () show that the combined use of IGABT and optimization more or less doubles the volume that can be effectively treated to 85 Gy (α/β = 10). For standard library plans (2D) the effect of the inverse square law is clearly seen with a negative potency function describing the data points and the D90 decreases below 85 Gy (α/β = 10) when the volume of HR CTV increases above 40 cm3 (A). It is also noticeable that the high D90 obtained with 2D standard plans in small tumours with a HR CTV < 40 cm3 also seem to impose an over dosage of the OAR in most cases. By optimization and use of interstitial needles in selected patients (B) the curve is much more flat such that overdosing of OAR in smaller tumours is avoided and the intended dose of at least 85 Gy (α/β = 10) is reached even in large tumours with a HR-CTV up to 90 cm3.
Figure 5. D90 as a function of HR CTV volume comparing standard 2D and optimized 3D treatment plans in a consecutive series of 50 locally advanced cervix cancer patients treated at Aarhus University with tandem and ring intracavitary brachytherapy plus additional interstitial needles in selected cases. The horizontal red dotted line indicates the desired D90 of 85 Gy for HR CTV. The vertical white dotted line indicatse the mean volume of HR CTV. A: Standard library plans (2D). The 18 dotted data points (z) indicate cases in which the dose volume constraints for organs at risk were superseded. B: Optimized (3D) plans. Additional interstitial needles were applied in 15 cases (blue triangle), and a vaginal cylinder in 4 cases (yellow circle). The 4 dotted data points (z) indicate cases in which the dose volume constraints for organs at risk where superseded.
Correlation of DVH parameters with clinical outcome: Possibilities and pitfalls
In order to validate IGABT and the suggested DVH parameters for tumour control and constraints for OAR currently used (see below), this complex treatment technique has to be investigated within the clinical setting. This implies many assumptions and limitations which have to be considered when switching from 2D point based BT to image guidance and optimization. This implies also clinical follow-up of patients treated with this approach, 3D image based assessment of any failure (local recurrence or morbidity) and topographic and dosimetric correlation of these findings.
To our knowledge, presents the largest compilation of DVH data reported so far from about 300 patients treated according to the GEC ESTRO guidelines at 4 institutions using different dose rates (HDR, PDR) and 3 different applicators (Tandem/ring, Ovoids, Mould). Despite these differences the new GEC ESTRO guidelines provide an opportunity for a meaningful communication and comparison of these central DVH parameters between different “schools of BT” and underline the central role of the GEC ESTRO guidelines as a research tool. may therefore serve as a provisional reference material until data from prospective observational studies with link to clinical outcome become available. However, as discussed below there are several underlying assumptions which have to be taken into account when calculating and comparing these DVH values. Furthermore these results represent mean values for specific experiences, while maximum dose limits have to be based on clear dose effect relationships.
Table I. Dose volume parameters (± SD) from four institutions for the High Risk Clinical Target Volume (HR CTV) and organs at risk in cervix cancer brachytherapy treated and reported according to the GEC ESTRO guidelines. *Data from C. Haie-Meder, personal communication; §Data from E. van Limbergen, personal communication
DVH parameters for a combined dose assessment of EBRT and IGABT
A major obstacle for progress in IGABT for cervical cancer has been the basic difficulty to “add” doses from EBRT and from one or multiple fractions of BT. This was due to spatial uncertainties and to a large variation in different doses per fraction, which applied for both the CTV and the OAR. The Gyn GEC ESTRO group then proposed for current clinical practice to use some assumptions that may give a reasonable working platform for the vast majority of clinical situations Citation[2].
Physics
The first “assumption” is that the HR CTV receives at least the dose from EBRT as reported for the ICRU point. The same applies for the small volume of adjacent OAR receiving the highest dose from brachytherapy. Furthermore it is assumed that in fractionated brachytherapy the identical small volume always receives the highest dose (“worst case”). Based on these assumptions, the physical doses are recommended to be “summed up”, taking into account the different doses per fraction Citation[2].
Radiobiology
Secondly, it was proposed to apply the linear-quadratic model and to express the dose as physical dose and as biologically weighted dose, both for the CTV with a uniform alpha beta value of 10 Gy and for the OAR with a uniform alpha beta value of 3 Gy Citation[2], Citation[33]. For the underlying biological model, the major draw backs are the limited knowledge about alpha-beta values for cervix cancer and for OAR. It is quite possible that the alpha beta value for cervix cancer is a heterogenous parameter and may be lower than 10 in many cases. It therefore seems somewhat questionable, that the assumed mean alpha beta value of 10 is representative Citation[34]. Something analogue may apply for the alpha-beta values in OAR, which may be slightly higher, in particular for the bladder Citation[35]. Furthermore, the half-time of repair for normal tissue is taken as 1.5 hours, which seems reasonable but may also represent some underestimation. If this is true, this would have a clear impact on calculations for the iso-effects from LDR and PDR brachytherapy, making these dose rates “relatively more toxic” Citation[36]. Finally, it has been recently discussed, with upcoming use of high doses per fraction in stereotactic radiotherapy that the linear-quadratic model only allows to model tissue effects appropriately, up to doses of about 5 Gy per fraction Citation[37]. For higher doses, in particular for OAR, the calculated dose is likely overestimating the effect. This may be in particular true then for the values as found for the bladder in HDR cervix brachytherapy (5 − 7+ Gy per fraction in 2 ccm).
General considerations
Apparently, there is much rationale in using the current assumptions and the simplistic model approach, in particular as this has been a strong basis for the rapid development of this field during the last decade. These assumptions and these models seem to work reasonably well in clinical practice, as long as a conservative attitude towards 3D optimization and dose volume constraints is respected. However, it is evident that this method has shortcomings, especially with regard to cross institutional comparisons. Therefore, research is needed, to further advance appropriate spatial dose allocation (e.g. by better understanding uncertainties and by developing registration techniques) and to further advance biological modelling based on clinical data to reduce the uncertainties in the estimation of the radiobiological tumour and normal tissue effects.
Dose response relationship for local control
For IGABT of cervical cancer it is essential to investigate whether relationships between DVH parameters for the targets and local control exist. The D90 and the D100 have been introduced by the Gyn GEC ESTRO group both for the HR and the IR CTV Citation[2]. So far, few data are available on these DVH parameters, mostly from small patient series (). In regard to the published material, there is some variation from mean 79 Gy to mean 90 Gy as the minimum dose for the HR CTV Citation[3–5], Citation[38]. It will be important to find out the clinical impact of these different doses on local control.
In a recent study including 141 patients, Dimopoulos et al. investigated the impact of D90 and D100 for the targets on local control. It was demonstrated that if the D90 for the HR CTV was ≥87 Gy EQD2 true pelvic control rates of >95% can be achieved Citation[39]. However, a clear dose effect was found only in large tumours not responding well to primary EBRT (+/ − chemotherapy).
According to the limited evidence, available so far, the major effect to be expected for small tumours at diagnosis and for bulky tumours (limited to the cervix/proximal parametria and responding well) is to assure local control for almost all of these patients and at the same time to not induce major treatment related side effects (< < 5%). However, at present, there is no clear indication, which dose level is needed in terms of D90 for the HR CTV to reach this aim for any of these subgroups. To find out, it is mandatory to perform prospective multi-centre clinical research on IGABT, allowing for different dose levels for prescription (e.g. EMBRACE study, see below) according to long standing clinical experience Citation[40].
Dose volume constraints for organs at risk
The main OAR in cervix cancer brachytherapy are rectum, bladder, sigmoid colon and relevant parts of the bowel loops adjacent to the target volumes. In clinical routine the minimum doses to the most exposed 2 cc tissue volume (D2cc) is taken as a parameter for treatment plan optimization. Dose effect relationship for the rectum was available for the ICRU 38 reference point. As mean values for the ICRU 38 dose and the D2cc are comparable, the tolerance level to start IGABT was taken from these experiences Citation[40–42]. As there were no evidence based limits for the sigmoid, the rectum constraints were applied. For the bladder the available literature data on dose effects were not straightforward Citation[40], Citation[42]. Dose constraints were determined based on the current and traditional clinical practice. For the vagina no dose volume parameters and constraints have been recommended so far.
Rectum
For the rectum recently first dose-volume constraints have been proposed based on clinical evidence. Koom et al. showed that patients with more severe rectal side effects (endoscopy score ≥2) had significantly higher D2cc (75±10 Gy versus 69±9 Gy, α/β = 3), D1cc (80±12 Gy versus 73 ±10 Gy, α/β = 3), and D0.1cc (93±20 Gy versus 85±14 Gy, α/β = 3) Citation[43]. A similar trend has been demonstrated by the Vienna group for 141 patients: the incidence of G1-G4 late toxicity for rectum was significantly higher when the D2cc for the rectum was >75 Gy (α/β = 3) (20% versus 4%) Citation[44]. These findings were even more significant for the Vienna rectoscopy study in 35 patients Citation[45]. Nowadays a dose limit of 70–75 Gy EQD2 is applied in most centres.
Sigmoid
For the sigmoid, dose volume constraints applied until now have not been clearly linked to clinical outcome. In the Vienna series with 141 patients some dose effect relation could be shown for an overall number of 3 sigmoid events with 9% (2/22) compared to 1% (1/119) applying a cut off level of 75 Gy Citation[44]. However, in the Vienna rectosigmoidoscopy study the frequency of teleangiectasia was for a similar mean dose of 65±7 and 66±8 Gy to the 2 cc volume of sigmoid and rectum, respectively only 3 of 29 (10%) in the sigmoid compared to 26 of 35 (74%) in the rectum Citation[45]. In a recent small study by Sturzda et al. major inter-fraction variation was found for the sigmoid in 15 of 22 patients Citation[46]. Therefore, due to more underlying uncertainties for assessing the dose to the sigmoid colon (e.g. movement), the total calculated dose using the “worst case assumption” has to be handled with caution. Another interesting finding is, that in different series published so far, the dose to the sigmoid seems to be significantly different for a comparable dose to the HR CTV: e.g. 63 Gy in Vienna Citation[3] versus 69 Gy in Aarhus Citation[5], both using the tandem-ring applicator and the same method for reporting (). The reasons for these differences need to be elucidated.
Bladder
For the bladder, the overall situation for dose volume assessment applying 3D based parameter (2 ccm, 0.1 ccm) presents still with little clinical evidence. This is in line with the difficulty of showing dose effects for cervix cancer BT for the bladder in the past Citation[42]. For centres changing from radiography to 3D based dose volume assessment it has been striking that in the past high doses have been applied in small volumes on a regular basis, often far beyond what has been shown by the ICRU bladder point. However, even in the Vienna series with 141 patients, no dose effect could be shown for this large series applying different cut-off levels for small volumes Citation[44]. Therefore, there is much research and development needed in this particular field. In a small retrospective study in Vienna of symptomatic and asymptomatic patients (n = 34) receiving a dose >90 Gy (EQD2, a/b of 3) the location of the high dose volume was investigated as additional parameter. The posterior bladder wall was divided into a low and medium segment (including and not including the bladder neck). In 18 patients with a high dose in the medium segment only 3 experienced bladder side effects, whereas for 16 patients with a high dose in the lower segment 10 suffered from late adverse bladder side effects (p < 0.05) [Munandar A et al., unpublished material]. These findings indicate that some additional parameter may be useful in future clinical research to increase the value of “pure” DVH parameters for assessing bladder morbidity.
Vagina
For the vagina no dose volume parameters had been recommended by the Gyn GEC ESTRO group. The reasons for this was that DVH parameters that are commonly used to report dose for other OAR can not be directly applied for the vagina due to high uncertainties in dose assessment Citation[47]. Furthermore, no clinical evidence had been reported. In a recent small study performed for the assessment of vaginal morbidity in Vienna, different endpoints were studied like teleangiectasia, bleeding, fibrosis and shortening and correlated to different dose levels in absolute small volumes (2 cc, 1 cc, 0.1 cc). No significant correlation was found between a given endpoint (e.g. teleangiectasia yes/no) and a certain dose level Citation[48]. Innovative research approaches seem to be necessary to better understand the underlying mechanisms and to define parameters and a model which may be feasible for predicting outcome.
Clinical results and future perspectives for IGABT
At present IGABT for cervical tumours is introduced as a routine treatment only in a limited number of institutions worldwide, which explains why there are relatively few published articles on clinical outcome. Nevertheless, reports on clinical experience of different centres indicate the feasibility and potential of IGABT in cervical cancer patients Citation[3–6], Citation[18], Citation[32], Citation[39], Citation[43–45], Citation[47], Citation[49], Citation[50].
The first available clinical results Citation[6] demonstrated that both local control and morbidity were significantly influenced by systematic application of the MRI-based approach. The improvement of local control was achieved in limited and advanced disease. Thus, for limited disease (IB1/IIB proximal less than 4–5 cm) high local control rates (in the range of 95–100%) and associated low rates of severe late morbidity (<5%) can be expected. It also seems to be possible achieving high local control rates (∼90%) with low rates of severe late morbidity (<5%) for advanced disease (IB2, IIB more than 5 cm, IIIB). There is furthermore growing evidence from other single institution series that these very high local control rates seem to be reproducible under different conditions at various places: Paris IGR, Leuven, Aarhus, London Mount Vernon. Altogether, according to a recent survey within the EMBRACE study group (see below), an overall number of about 800 patients have been treated so far worldwide applying IGABT for cervical cancer.
Future clinical research will have to focus on the further improvement of high precision technology and methodology of this 3D adaptive approach and on the validation of these results in a prospective multi-centre setting with systematic application of IGABT applying the GEC ESTRO recommendations for target definition and for dose volume assessment. Thus, a collaborative IntErnational study on MRI-guided brachytherapy in locally advanced cervical cancer (EMBRACE) was designed which is starting in 2008. The most important aim of this EMBRACE study is to implement cervix cancer IGABT worldwide under high quality standards and then to correlate local control and dose volume parameters for GTV, HR CTV and IR CTV as well as late morbidity and dose volume parameters for OARs. Declaration of interest: Medical University of Vienna receives financial and/or equipment support for research and educational purposes from Nucletron B.V., Varian Medical Systems, Inc., and Isodose Control B.V.
References
- Haie-Meder C, Pötter R, Van Limbergen E, Briot E, De Brabandere M, Dimopoulos J, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 2005; 74: 235–45
- Pötter R, Haie-Meder C, Van Limbergen E, Barillot I, De Brabandere M, Dimopoulos J, et al. Recommendations from Gynaecological (GYN) GEC ESTRO Working Group (II): Concepts and terms in 3D image based treatment planning in cervix cancer brachytherapy: Aspects of 3D imaging, radiation physics, radiobiology, and 3D dose volume parameters. Radiother Oncol 2006; 78: 67–77
- Kirisits C, Pötter R, Lang S, Dimopoulos J, Wachter-Gerstner N, Georg D. Dose and volume parameters for MRI based treatment planning in intracavitary brachytherapy of cervix cancer. Int J Radiat Oncol Biol Phys 2005; 62: 901–11
- De Brabandere M, Mousa AG, Nulens A, Swinnen A, Van Limbergen E. Potential of dose optimisation in MRI-based PDR brachytherapy of cervix carcinoma. Radiother Oncol 2007; doi:10.1016/j.radonc.2007.10.026
- Lindegaard JC, Tanderup K, Nielsen SK, Haack S, Gelineck J. MRI-guided 3D optimization significantly improves DVH parameters of pulsed-dose-rate brachytherapy in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys 2008; 71: 756–64
- Pötter R, Dimopoulos J, Georg P, Lang S, Waldhäusl C, Wachter-Gerstner N, et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol 2007; 83: 148–55
- Van Dyk S, Bernshaw D. Ultrasound-based conformal planning for gynaecological brachytherapy. J Med Imaging Radiat Oncol 2008; 52: 77–84
- Lin LL, Mutic S, Low DA, LaForest R, Vicic M, Zoberi I, et al. Adaptive brachytherapy treatment planning for cervical cancer using FDG-PET. Int J Radiat Oncol Biol Phys 2007; 67: 91–6
- Pötter R, Fidarova E, Kirisits C, Dimopoulos J. Image-guided adaptive brachytherapy for cervix carcinoma. Clin Oncol 2008; doi:10.1016/j.clon.2008.04.011.
- Pötter R. Modern imaging in brachytherapy. The GEC ESTRO Handbook of Brachytherapy, A Gerbaulet, R Pötter, JJ Mazeron, H Meertens, E Van Limbergen. ESTRO, Brussels 2002; 123–151
- Lim K, Chan P, Dinniwell R, Fyles A, Haider M, Cho YB, et al. Cervical cancer regression measured using weekly magnetic resonance imaging during fractionated radiotherapy: Radiobiologic modeling and correlation with tumor hypoxia. Int J Radiat Oncol Biol Phys 2008; 70: 126–33
- Dimopoulos J, Schard G, Kirisits C, Lang S, Baldinger A, Helbich ThH, et al MRI assessment of cervical cancer for adaptive radiotherapy Strahlenther Onkol 2008, (in press).
- Georg D, Kirisits C, Hillbrand M, Dimopoulos J, Pötter R. Image-guided radiotherapy for cervix cancer: High-tech external beam therapy versus high-tech brachytherapy Int J Radiat Oncol Biol Phys 2008; doi:10.1016/j.ijrobp.2008.03.032
- Nulens A, Lang S, Briot E, De Brabandere M, Kirisits C, Dimopoulos J, et al. Evaluation of contouring concepts and dose volume parameters of MR based brachytherapy treatment plans for cervix cancer: Results and conclusions of the GEC-ESTRO GYN working group delineation workshops. Radiother Oncol 2005; 75(S1)S9
- Lang S, Nulens A, Briot E, Kirisits C, De Brabandere M, Dumas I, et al. Intercomparison of treatment concepts for MR image assisted brachytherapy of cervical carcinoma based on GYN GEC-ESTRO recommendations. Radiother Oncol 2006; 78: 185–93
- Dimopoulos J, De Vos V, Berger D, Petric P, Dumas I, Kirisits C, et al. Interobserver comparison of target delineation for MRI-assisted cervical cancer brachytherapy: Application of the GYN GEC-ESTRO recommendations Radiother Oncol 2008 (in press).
- Petric P, Dimopoulos J, Kirisits C, Berger D, Hudej R, Pötter R. Inter- and intraobserver variation in HR-CTV contouring: Intercomparison of transverse and paratransverse image orientation in 3D-MRI assisted cervix cancer brachytherapy Radiother Oncol 2008, (in press).
- Viswanathan AN, Dimopoulos J, Kirisits C, Berger D, Pötter R. CT- versus MRI-based contouring in cervical cancer brachytherapy: Results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys 2007; 68: 491–8
- Dimopoulos J, Schard G, Berger D, Lang S, Goldner G, Helbich T, et al. Systematic evaluation of MRI findings in different stages of treatment of cervical cancer: Potential of MRI on delineation of target, patho-anatomical structures and organs at risk. Int J Radiat Oncol Biol Phys 2006; 64: 1380–8
- Fellner C, Pötter R, Knocke TH, Wambersie A. Comparison of radiography- and computed tomography-based treatment planning in cervix cancer in brachytherapy with specific attention to some quality assurance aspects. Radiother Oncol 2001; 58: 53–62
- Kim RY, Pareek P. Radiography-based treatment planning compared with computed tomography (CT)-based treatment planning for intracavitary brachytherapy in cancer of the cervix: Analysis of dose-volume histograms. Brachytherapy 2003; 2: 200–6
- Pelloski CE, Palmer M, Chronowski GM, Jhingran A, Horton J, Eifel PJ. Comparison between CT-based volumetric calculations and ICRU reference-point estimates of radiation doses delivered to bladder and rectum during intracavitary brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2005; 62: 131–7
- Hellebust TP, Tanderup K, Bergstrand ES, Knutsen BH, Røislien J, Olsen DR. Reconstruction of a ring applicator using CT imaging: Impact of the reconstruction method and applicator orientation. Phys Med Biol 2007; 52: 4893–904
- Berger D, Dimopoulos J, Pötter R, Kirisits C. The reconstruction of brachytherapy ring applicators directly on MR images. Radiother Oncol 2008 (in press).
- Haack S, Lindegaard JC, Nielsen SK, Gelineck J, Tanderup K. Applicator reconstruction in MRI 3D image based dose planning of brachytherapy for cervical cancer, Radiother Oncol 2008, (in press).
- Tanderup K, Hellebust TP, Lang S, Granfeldt J, Pötter R, Lindegaard JC, et al Consequences of random and systematic reconstruction uncertainties in 3D image based brachytherapy in cervical cancer. Radiother Oncol 2008 (in press).
- Lang S, Georg P, Kirisits C, Dimopoulos J, Kuzucan D, Georg D, et al. Uncertainty analysis for 3D image-based cervix cancer brachytherapy by repeated MRI examinations: DVH variations between two HDR fractions within one applicator insertion. Radiother Oncol 2006; 81(S1)S79
- Tanderup K, Christensen JJ, Granfeldt J, Lindegaard JC. Geometric stability of intracavitary pulsed dose rate brachytherapy monitored by in vivo rectal dosimetry. Radiother Oncol 2006; 79: 87–93
- Hellebust TP, Dale E, Skjønsberg A, Olsen DR. Inter fraction variations in rectum and bladder volumes and dose distributions during high dose rate brachytherapy treatment of the uterine cervix investigated by repetitive CT-examinations. Radiother Oncol 2001; 60: 273–80
- Kirisits C, Lang S, Dimopoulos J, Oechs K, Georg D, Pötter R. Uncertainties when using only one MRI-based treatment plan for subsequent high-dose-rate tandem and ring applications in brachytherapy of cervix cancer. Radiother Oncol 2006; 81: 269–75
- Davidson MT, Yuen J, D'Souza DP, Batchelar DL. Image-guided cervix high-dose-rate brachytherapy treatment planning: Does custom computed tomography planning for each insertion provide better conformal avoidance of organs at risk?. Brachytherapy 2008; 7: 37–42
- Kirisits C, Lang S, Dimopoulos J, Oechs K, Georg D, Poetter R. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: Design, application, treatment planning and dosimetric results. Int J Radiat Oncol Biol Phys 2006; 65: 624–30
- Lang S, Kirisits C, Dimopoulos J, Georg D, Pötter R. Treatment planning for MRI assisted brachytherapy of gynecologic malignancies based on total dose constraints. Int J Radiat Oncol Biol Phys 2007; 69: 619–27
- Bentzen SM, Baumann M The linear-quadratic model in clinical practice In: Steel GG.“Basic clinical radiobiology”, 3rd edn. London: 2002. p 134–146.
- Dörr W, Bentzen SM. Late functional response of mouse urinary bladder to fractionated X-irradiation. Int J Radiat Biol 1999; 75: 1307–15
- Orton CG. High-dose-rate brachytherapy may be radiobiologically superior to low-dose rate due to slow repair of late-responding normal tissue cells. Int J Radiat Oncol Biol Phys 2001; 49: 183–9
- Thames H. Estimation of changes in tolerance dose with the large fraction sizes typical of stereotactic radiotherapy. Oral communication. ICRO ÖGRO 8, Salzburg, May 2007.
- Chajon E, Dumas I, Touleimat M, Magné N, Coulot J, Verstraet R, et al. Inverse planning approach for 3-D MRI-based pulse-dose rate intracavitary brachytherapy in cervix cancer. Int J Radiat Oncol Biol Phys 2007; 69: 955–61
- Dimopoulos J, Lang S, Kirisits C, Fidarova E, Berger D, Georg P, et al. Dose-volume histogram parameters and local tumour control in MR image guided cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys 2008 (in press).
- Gerbaulet A, Pötter R, Haie-Meder C. Cervix cancer. The GEC ESTRO Handbook of Brachytherapy, A Gerbaulet, R Pötter, JJ Mazeron, H Meertens, E Van Limbergen. ESTRO, Brussels 2002; 301–363
- ICRU Report 38. Dose and volume specification for reporting intracavitary therapy in gynaecology. International Commission on Radiation Units and Measurements, Bethesda, Maryland,1985.
- Pötter R, Van Limbergen E, Gerstner N, Wambersie A. Survey of the use of the ICRU 38 in recording and reporting cervical cancer brachytherapy. Radiother Oncol 2001; 58: 11–8
- Koom WS, Sohn DK, Kim JY, Kim JW, Shin KH, Yoon SM, et al. Computed tomography-based high-dose-rate intracavitary brachytherapy for uterine cervical cancer: Preliminary demonstration of correlation between dose-volume parameters and rectal mucosal changes observed by flexible sigmoidoscopy. Int J Radiat Oncol Biol Phys 2007; 68: 1446–54
- Georg P, Dimopoulos J, Kirisits C, Lang S, Berger D, Pötter R. Dose volume parameters in cervical cancer patients treated with MRI based brachytherapy and their predictive value for late adverse side effects in rectum, sigmoid and bladder. Radiother Oncol 2006; 81(S1)S38–S39
- Georg P, Kirisits C, Goldner G, Dörr W, Hammer J, Pötzi R, et al Correlation of dose volume parameters, endoscopic and clinical rectal side effects in cervix cancer patients treated with definitive radiotherapy including MRI based brachytherapy. Radiother Oncol 2008, (in press).
- Sturdza AE, Berger D, Lang S, Dimopoulos J, Thomas G, Georg P, et al. Uncertainties in assessing sigmoid dose volume parameters in MRI-guided fractionated HDR brachytherapy. Brachytherapy 2008; 7: 109
- Berger D, Dimopoulos J, Georg P, Georg D, Pötter R, Kirisits C. Uncertainties in assessment of the vaginal dose for intracavitary brachytherapy of cervical cancer using a tandem-ring applicator. Int J Radiat Oncol Biol Phys 2007; 67: 1451–9
- Fidarova E, Schüssler S, Dimopoulos J, Bachtiary B, Georg P, Berger D, et al An investigation into the relationship between MRI-based DVH parameters and vaginal morbidity in cervical cancer brachytherapy Radiother Oncol 2008, (in press).
- Wachter-Gerstner N, Wachter S, Reinstadler E, Fellner C, Knocke TH, Pötter R. The impact of sectional imaging on dose escalation in endocavitary HDR-brachytherapy of cervical cancer: Results of a prospective comparative trial. Radiother Oncol 2003; 68: 51–9
- Dimopoulos J, Kirisits C, Petric P, Georg P, Lang S, Berger D, et al. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: Clinical feasibility and preliminary results. Int J Radiat Oncol Biol Phys 2006; 66: 83–90