Abstract
Background. To evaluate the clinical characteristics, treatment regimens, and outcome of children with Hodgkin lymphoma in a developing country over a period of 34 years. Methods. This paper retrospectively evaluates the treatment and prognosis of 614 children with Hodgkin lymphoma disease between 1971 and 2005. All patients were treated with chemotherapy, and also received radiotherapy. Results. There were 452 males and 162 females with a median age of 8 years (2 to 21); 183 patients had B symptoms. There were 165, 185, 145, and 119 patients in stage I, II, III, and IV, respectively. Histopathologic subtypes were mixed cellularity (344 patients), nodular sclerosis (90), lymphocytic predominance (62), lymphocytic depletion (46), unclassified types (69), and nodular lymphocyte predominant Hodgkin lymphoma (3). Overall (OS) and event-free survival (EFS) rates were 83 and 60%, though OS rates varied according to chemotherapy protocol; age; presence of B symptoms, leukocytosis, anemia, and extranodal involvement; and stage at diagnosis. Over the years, the median age of patients increased, as did the frequency of the nodular sclerosing type of disease. Conclusions. This is one of the largest series in a single center. The increase in the median age and in the frequency of the nodular-sclerosing type are thought to be related to the development status of Turkey. The ABVD protocol yielded the best survival rates and should be used for treatment of patients with Hodgkin lymphoma.
Hodgkin lymphoma (HL) is a form of cancer involving the lymphatic system. Although the pathogenesis of HL still remains unknown, it is likely that combinations of environmental and genetic factors are at work; for example, the Epstein-Barr virus (EBV) may precipitate the disease in a genetically susceptible individual Citation[1].
Hodgkin lymphoma has different epidemiologic features in developed and developing countries. In developing countries, childhood HL is far more common than the adult manifestation, indicating the importance of environmental factors in causation. Other features of HL in developing countries are that the majority of the patients have mixed cellularity type, and in most cases, childhood HL is associated with EBV. Young adults who develop infectious mononucleosis have a significantly higher risk of later developing HL Citation[1–8]. This was shown in EBV-positive HL by Hjalgrim et al. Citation[9]. In contrast, in developed countries, HL tends to occur at a later age, the highest incidence is among 15- to 34-year-olds, and the disease is rare in children younger than age 10. The nodular sclerosis form of HL is seen most frequently in young women in developed countries Citation[1], Citation[10–17].
Advancements made in the treatment of HL mean that most patients with this diagnosis are cured or are able to control the disease for years. Therefore, reduction of long-term toxicity of chemotherapy treatment and maintenance of a good quality of life are the major issues in the present treatment of HL Citation[1].
The purpose of this study is to evaluate the outcome of HL in Turkish children and to identify HL's prognostic factors and changing epidemiologic features over a 34-year period.
Patients and methods
Enrolled in this study were 614 children with histologically proven untreated HD who were treated at our center from 1971 to 2005. We evaluated the patients’ epidemiologic characteristics, treatment regimen, and survival in four time periods: the first period (1971–1980), the second period (1981–1990), the third period (1991–2000), and the fourth period (2001–2005).
All the patients were staged according to the Ann Arbor classification system. Histopathological subtypes were defined according to WHO classification. At the time of diagnosis, pretreatment clinical staging included a chest x-ray and a computed tomography scan of the thorax, abdomen, and pelvis. Laboratory tests included a complete blood count, Westergren method of erythrocyte sedimentation rate, liver and renal chemistries, and serum lactate dehydrogenase level.
The pretreatment factors evaluated for prognostic significance were age; gender; stage; histology; presence of mediastinal and/or peripheral bulky disease, extranodal disease, and B symptoms; hemoglobin level (<10 g/dl and ≥10 g/dl); white blood count (WBC) (≤10 000/mm3 and >10 000/mm3), erythrocyte sedimentation rate (<20 mm/h and ≥20 mm/h), and serum lactate dehydrogenase level (LDH; ≤500 IU/L and >500 IU/L). Peripheral lymphadenopathy over 10 cm, or mediastinal lymphadenopathy greater than 33% of maximum intrathoracic cavity was accepted as bulky disease Citation[18].
Chemotherapy protocol
Each patient received chemotherapy and radiotherapy; however, treatment protocols were modified over the years. In the first period, all patients regardless of their stages or histopathology were treated with 6 courses of COPP (Cyclophosphamide 600 mg/m2 on days 1 and 7, intravenously, vincristine 1.4 mg/m2 on days 1 and 7, intravenously, prednisolone 40 mg/m2 peroral for 14 days, procarbasine 100 mg/m2 peroral for 14 days) protocol and radiotherapy. In the second period, stage I and II patients with mixed cellularity (MC) and lymphocyte predominant forms were treated with three courses of COPP with the same doses and involved field 2 250 cGy radiotherapy. Stage I and II patients with nodular sclerosing (NS) or lymphocyte-depleted histopathology were treated with three courses of ABVD (Adriamycin 25 mg/m2 on days 1 and 14, intravenously, bleomycin 10 mg/m2 on days 1 and 14, intravenously, vinblastine 6 mg/m2 on days 1 and 14, intravenously, dacarbazine 375 mg/m2 on days 1 and 14, intravenously) and involved field radiotherapy. All stage III and IV patients were treated with three courses of COPP+ involved field radiotherapy + three courses of COPP. During the third and fourth period, patients received ABVD chemotherapy and involved field radiotherapy. In those periods, early-stage patients received three courses of chemotherapy; those in advanced stages underwent a six-course sandwich chemotherapy protocol. The doses were as described in previously. In the last decade, to decrease infertility risk, boys with mediastinal disease were treated with the ABVD/COPP alternating regimen which also consists of three courses in early stages and 6 courses in advanced stages.
Radiotherapy protocol
Cobalt 60 teletherapy unit was used until 1993 and linear accelerator with 6 MV x-rays thereafter. Conventional fractionation was used using daily fractions of 150 to 180 cGy. No specific immobilisation device was used during treatment. In few patients vacuumed immobilization beds or thermoplastic immobilisation masks were used. Radiotherapy was applied as mantle, minimantle, and inverted Y according to disease extension in early years. Involved field radiotherapy conforming to the regions of the Ann Arbor classification was used later. Total radiotherapy dose was decided depending on response to the chemotherapy and initial volume of the disease. Total radiotherapy doses in the first period were between 3 000 and 4 000 Gy. Total radiotherapy doses afterwards ranged between 2 000 to 2 500 cGy (Median dose: 2 250 cGy). Sedation was used during radiotherapy when needed.
Median values were used to analyze demographic characteristics. Relationships between variables were assessed with Pearson correlation. Mortality and survival are expressed as percentages. Overall and event-free survival rates were estimated by using Kaplan-Meier analysis, and survival differences for prognostic factors were compared by using the log-rank test. Mulitvariate analysis was done by using Cox-regression method. All calculations were performed with SPSS version 10.0 statistical software.
Results
Patient characteristics
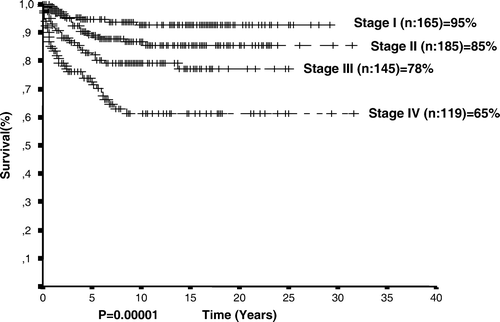
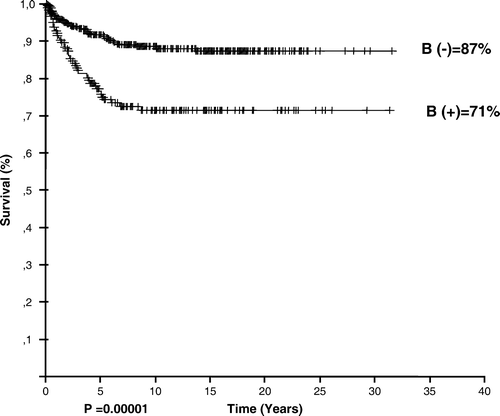
The clinical features of the 614 patients are given in . There were 452 boys and 162 girls with a median age of 8 years (range 2–21 years) at diagnosis; 183 patients had B symptoms (29.8%). The number of patients with localized disease (stage I and II) and advanced disease (stage III and IV) were 350 (58%) and 264 (42%), respectively. Mixed cellularity was the most common histological subtype (56%). Eigthy-seven patients had mediastinal and/or peripheral bulky disease (14.2%).
Table I. Clinical and demographic characteristics of all patients.
Patient characteristics according to years
Our study evaluates patients treated over 34 years. The median age of the patients increased in the fourth and most recent period (from 8 to 9.25 years), although the male/female ratio was nearly the same in all four periods. Mixed cellularity subtype was the most common histological type in all periods. However, in the fourth period, the percentage of the mixed cellularity subtype decreased and that of the nodular sclerosing subtype increased. In addition, the percentage of patients with advanced disease was lower in the second, third, and fourth periods than in the first period.
Changing characteristics of Hodgkin's disease in Turkish children
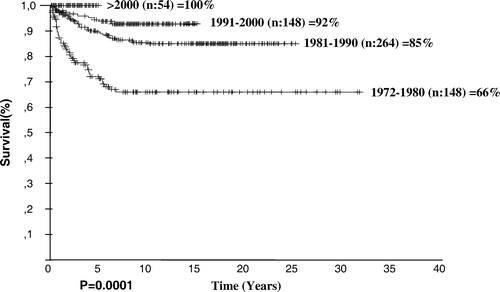
In the first period, the median age of the patients was 8 years, and 9.5% of the patients were 4 years of age or younger. The percentages of patients with bulky disease, extranodal involvement, and advanced stage (stage III and IV) were 17.6%, 45.9%, and 62.8%, respectively. In the fourth period, the median age of the patients was 9.25, and 5.6% of the patients’ age were 4 years or younger. The percentages of those with bulky disease, extranodal involvement, and advanced stage were 5.6%, 11.1% and 46.3%, respectively. The overall survival rates of the patients in the first and fourth periods were 66% and 100%, respectively ().
The median age increased from 8 to 9.25 years and the percentage of patients younger than age 5 decreased from the first to the fourth period; however, neither of these changes is significant. Changes in percentages of bulky disease (p = 0.02), extranodal disease (p = 0.0001), advanced stage (p = 0.03) and B symptoms (p = 0.001) are significant statistically. The frequency of the MC type in the first and the fourth periods was 54.1 and 42.6%, respectively, but this difference is not significant (p = 0.15). However, the frequency of NS type in the first and the fourth periods was 12.8 and 35.2%, respectively, which is significant (p = 0.001).
Survival analysis
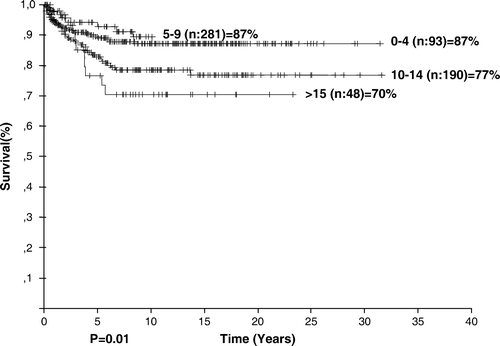
At a median follow-up of 9.96 years, 306 patients (49.8%) were disease free. The estimated overall and event-free survival rates of the entire group were 82.59 and 59.44%, respectively. OS rates were 79, 95.6, and 91.8% for patients receiving regimens of COPP (n = 402), ABVD (n = 94), and alternating ABVD/COPP (n = 65) (p = 0.02). The overall survival rate varied according to age (p = 0.01, ), stage (p = 0.00001, ), and the presence of B symptoms (p = 0.00001, ), leukocytosis (p = 0.04), anemia (p = 0.0009), extranodal involvement (p = 0.00001).
Cox regression analysis showed that high erythrocyte sedimentation rate, leukocytosis, older age, and advanced stage have been risk factors for prognosis ().
Table II. Multivariate analysis of the 614 patients with Hodgkin lymphoma.
Discussion
Hodgkin lymphoma is a malignant growth of cells in the lymphatic system. Its incidence continues to increase during the early teen and adolescent years. The age distribution in developed countries shows two modal peaks: young adults (15–34 years) and older adults (>50 years). In developing countries, the young adult peak is replaced by a childhood (0–14 years) form. This difference in age distribution between developed and developing countries has suggested to some investigators that exposure to an infectious disease plays a role in HL's etiology Citation[1]. These two peaks can also be seen in nasopharyngeal carcinoma. There are strong correlations between EBV infection and both HL and nasopharyngeal carcinoma Citation[19]. Although there is strong pivotal EBV effect on the etiopathogenesis of both diseases, it is not the sole factor. Hjalgrim et al.'s Citation[9] study has shown that infectious mononucleosis is associated only with EBV-positive Hodgkin lymphoma. They commented that EBV-negative Hodgkin lymphoma might have different etiopathology. The other interesting point is that EBV is associated with mixed cellularity histopathology Citation[20]. In our study, higher rate of mixed cellular histopathology could be associated with this infection.
In one large study of children and adolescents with HL in a developed country, 3% of patients were aged between 0 and 4 years, 12% were between 5 and 9 years, 44% of patients were between 10 and 14 years, and 41% were older than 15 years Citation[16]. Studies in developing countries have found that 17.6% to 25% of children with HL were aged 5 years or younger Citation[2], Citation[4]. Eleven percent of the patients enrolled in the United Kingdom Children's Cancer Study Group HD1 and HD2 were 5 years or younger Citation[21]. Nodular sclerosis (NS) type was the most common histological type, followed by MC type.
In developed countries, HL is therefore uncommon in children younger than 10 years. When seen, younger children have a higher incidence of lymphocyte-predominant and mixed cellularity histology and a lower incidence of nodular sclerosing histology than adolescents and adults Citation[1], Citation[10–23]. The study by Hsu et al. Citation[22] compared the epidemiology of HL in both developed and developing countries. The median age in developing countries (9 years) was lower than in developed countries (15.3 years). Interestingly, the NS type was the most common subtype in developing countries in this study.
The study previously reported by our center found that the most common subtype was MC (75.7%) Citation[4]. In another study from our country by Cavdar et al. Citation[2], MC was the most common subtype followed by NS. Mixed cellularity histology was the most common subtype (42.6%) in our study after 2000. Interestingly, frequency of the NS type has increased to 35.2%. The increase in the median age, decrease in the frequency of diagnosis in children younger than 5 years, and an increase in the frequency of the NS type are thought to be related to the development status of our country.
In this study, 15.1% of patients were aged 4 years or younger. However, the percentage of those younger than age 5 decreased to 5.6% after the year 2000. There was therefore an increase in the median age–from 8 years before 2000 to 9.25 in more recent years. This change is probably created by changing of the socio-economic status in our country. These changes are parallel to western countries. This comment is also true for histopathologic subgroups. Increased rate of nodular sclerosing type is parallel to the economical development in our country.
In our study, the median follow-up duration was 9.96 years (0.1–31.59 years). This follow-up time is one of the longest of any series. The overall survival was 83% in the entire group, but increased in recent years, reaching 92% in the 1991–2000 time period. This increased survival reflects our department's success in the last decade. The OS was 100% after 2000, but event-free survival rate in this period was 97%. In long-term follow-up, these rates may be decreased. Actual results of our department are reflected in the patient group treated between 1991 and 2000 period. EFS in this group was 77%. The overall survival rate varied according to age, stage, and the presence of B symptoms, leukocytosis, anemia, and extranodal involvement, which were poor prognostic factors. High erythrocyte sedimentation rate, leukocytosis, older age, and advanced stage have been risk factors for prognosis in multivariate analysis.
Treatment of HL in childhood usually consists of combined modality treatment, including chemotherapy and low-dose involved field radiotherapy. With this combined modality treatment, survival is achieved in up to 90% of patients with HL Citation[1]. In developed countries, OS rates were reported to be 89.3–100% Citation[10], Citation[17], Citation[22]. Low-dose involved field radiotherapy was successful in the COG study Citation[16], which stratified patients according to disease stage and patient characteristics. The treatment protocol was four or six courses of COPP/ABV. Patients with stage IV disease were treated with two additional courses of cytarabine and etoposide. Patients were divided two groups: those receiving radiotherapy or no radiotherapy. The survival rate in the radiotherapy group was 90%; it was slightly lower in the no-radiotherapy group, particularly in stage IV patients.
In some studies, chemotherapy alone was used as the initial treatment Citation[3], Citation[12], Citation[24]. In a study by Ertem et al. Citation[3] every patient received chemotherapy for the initial treatment, including the COPP, ABVD, and ABVD regimens alternating with COPP, to be given as a total of 12 courses. The overall survival rates for stage I–II and stage III–IV were 92.3 and 89.5%, respectively. In the Rotterdam-HD-84-protocol, children with stage I–IIA disease were treated with six courses of EBVD; those with stage IIB–IV disease received three to five alternating cycles of EBVD and MOPP. The 10-year overall survival rate was 95% Citation[12]. Arya et al. Citation[24] used four cycles of COPP alternating with four cycles of ABVD in all stages of childhood HD. The 5-year overall survival rate was 91.5%. Hudson et al. Citation[11] conducted a prospective study of the VAMP/COP chemotherapy protocol, which includes methotrexate. Their OS rate was 92.7% and EFS was 75.6, with a median follow-up time of 5.8 years. The VAMP/COP protocol consists of six alternating cycles. The UKCCSG protocol Citation[17] includes chlorambucil (ChlVPP) given in six to eight courses. Their treatment regimen was stratified according to whether radiotherapy was given as well; patients with stage I cervical region HL did not receive chemotherapy. This study took place from 1982–1992 and EFS and OS were relatively low. EFS for stage I–IV were 65%, 80%, 69%, and 45% in this study. The radiotherapy-alone group had lower survival.
Currently we are treating the patients with less toxic protocols. Current treatment plan consists of four courses of chemotherapy (ABVD) without radiotherapy in early stages (stage I–II). Radiotherapy is used in these patients in case of recurrent disease.
The epidemiologic features of HL are related to socioeconomic status. Increase in the median age, decrease in the frequency of the children aged less than 5 years, and a higher incidence of the NS type are thought to be related to the development status of our country. The overall survival rate varied according to age, stage, and the presence of B symptoms, leukocytosis, anemia, and extranodal involvement, which were poor prognostic factors. The ABVD regimen has the best survival rates and should be used for treatment of patients with HD.
References
- Hudson MM, Onciu M, Donaldson SS. Hodgkin lymphoma. Principles and Practice of Pediatric Oncology5th ed, PA Pizzo, DG Poplack. Lippincott Williams & Wilkins, Philadelphia 2006; p. 695–721
- Çavdar AO, Taçoy A, Babacan E, Gözdaşoğlu S, Arcasoy A, Topuz U, et al. Hodgkin's disease in Turkish children: A clinical and histopathological analysis. J Natl Cancer Inst 1977; 58: 479–81
- Ertem U, Duru F, Dağdemir A, Taçyildiz N, Pamir A, Akçayöz A, et al. Hodgkin's disease in 82 Turkish children diagnosed over a 10-year period: Epidemiological, clinical, and histopathologic features and prognosis with prolonged chemotherapy. Pediatr Hematol Oncol 1997; 14: 359–66
- Büyükpamukçu M, Atahan L, Çağlar M, Kutluk T, Akyüz C, Hazar V. Hodgkin's disease in Turkish children: Clinical characteristics and treatment results of 210 patients. Pediatr Hematol Oncol 1999; 16: 119–29
- Gözdaşoğlu S, Ünal E, Ertem M, Yavuz G, Pamir A, Taçyildiz N, et al. Modified OPPA/COPP chemotherapy and involved-field radiation therapy for the treatment of Hodgkin's disease in Turkish children. Turk J Haematol 2001; 18: 47–52
- Çavdar AO, Gözdaşoğlu S, Yavuz G, Unal E, Pamir A, Taçyildiz N, et al. Characteristics of early type-I pattern (0–6 years) Hodgkin's disease in Turkish children. Turk J Haematol 2002; 19: 55–62
- Karimi M, Yarmohammadi H, Ghavanini AA, Kumar PV. Epidemiological surveillance of pediatric Hodgkin's disease in southern Iran. Med Sci Monit 2002; 8: CR572–5
- Oguz A, Karadeniz C, Okur FV, Çitak EC, Pinarli FG, Bora H, et al. Prognostic factors and treatment outcome in childhood Hodgkin disease. Pediatr Blood Cancer 2005; 45: 670–5
- Hjalgrim H, Smedby KE, Rostgaard K, Molin D, Hamilton-Dutoit S, Chang ET, et al. Infectious mononucleosis, childhood social environment, and risk of Hodgkin Lymphoma. Cancer Res 2007; 67: 2382–8
- Smith RS, Chen Q, Hudson MM, Link MP, Kun L, Weinstein H, et al. Prognostic factors for children with Hodgkin's disease treated with combined-modality therapy. J Clin Oncol 2003; 21: 2026–33
- Hudson MM, Krasin M, Link MP, Donaldson SS, Billups C, Merchant TE, et al. Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin's disease. J Clin Oncol 2004; 22: 4541–50
- Hakvoort-Cammel FGAJ, Buitendijk S, van den Heuvel-Eibrink M, Hahlen K. Treatment of pediatric Hodgkin disease avoiding radiotherapy: Excellent outcome with the Rotterdam-HD-84-protocol. Pediatr Blood Cancer 2004; 43: 8–16
- Kung FH, Schwartz CL, Ferree CR, London WB, Ternberg JL, Behm FG, et al. POG 8625: Randomized trial comparing chemotherapy with chemoradiotherapy for children and adolescents with stages I, IIA, IIIA1 Hodgkin disease. J Pediatr Hematol Oncol 2006; 28: 362–8
- Tebbi CK, Mendenhall N, London WB, Williams JL, de Alarcon PA, Chauvenet AR; A report from the Children's Oncology Group. Treatment of stage I, IIA, IIIA1 pediatric Hodgkin's disease with doxorubicin, bleomycin, vincristine and etoposide (DBVE) and radiation: A Pediatric Oncology Group (POG) study. Pediatr Blood Cancer 2006;46:198–202.
- Clavel J, Steliarova-Foucher E, Berger C, Danon S, Valerianova Z. Hodgkin's disease incidence and survival in European children and adolescents (1978–1997): Report from the Automated Cancer Information System project. Eur J Cancer 2006; 42: 2037–49
- Nachman JB, Sposto R, Herzog P, Gilchrist GS, Wolden SL, Thomson J, et al. For the Children's Cancer Group. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin's disease who achieve a complete response to chemotherapy. J Clin Oncol 2002; 20: 3765–71
- Capra M, Hewitt M, Radford M, Hayward J, Weston CL, Machin D. On behalf of Children's Cancer and Leukaemia Group (formerly UKCCSG). Long-term outcome in children with Hodgkin's lymphoma: The United Kingdom Children's Cancer Study Group HD82 trial. Eur J Cancer 2007;43:1171–9.
- Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol 1989; 7: 1630–6
- Barista I, Varan A, Ozyar E. Bimodal age distribution in Hodgkin's disease and nasopharyngeal carcinoma. Med Hypotheses 2007; 68: 1421
- Gulley ML, Eagan PA, Quintanilla-Martinez L, Picado AL, Smir BN, Childs C, et al. Epstein-Barr virus DNA is abundant and monoclonal in the Reed-Sternberg cells of Hodgkin's disease: Association with mixed cellularity subtype and Hispanic American ethnicity. Blood 1994; 83: 1595–602
- Stoneham S, Ashley S, Pinkerton R, Hewitt M, Wallace WHB, Shankar AG. On behalf of Children's Cancer and Leukaemia Group (formerly UKCCSG). Hodgkin's lymphoma in children aged 5 years or less-the United Kingdom experience. Eur J Cancer 2007; 43: 1415–21
- Hsu SC, Metzger ML, Hudson MM, Pedrosa F, Lins M, Pedrosa M, et al. Comparison of treatment outcomes of childhood Hodgkin's lymphoma in two US centers and a center in Recife, Brazil. Pediatr Blood Cancer 2007; 49: 139–44
- Spitz MR, Sider JG, Cole Johnson C, Butler JJ, Pollack ES, Newell GR. Ethnic patterns of Hodgkin's disease incidence among children and adolsescents in the United States, 1973–82. J Natl Cancer Inst 1986; 76: 235–9
- Arya LS, Dinand V, Thavaraj V, Bakhshi S, Dawar R, Rath GK, et al. Hodgkin's disease in Indian children: Outcome with chemotherapy alone. Pediatr Blood Cancer 2006; 46: 26–34