Abstract
Background. Somatostatin receptors (SSTR1-5) are inhibitory G-protein coupled receptors that are ubiquitously expressed in both normal and cancer cells. Activation of SSTRs results in inhibition of hormone secretion and cell proliferation. Loss-of-expression of SSTR2 in tumor tissues has been suggested to correlate with tumor progression and to the relatively poorer outcomes of somatostatin analog treatment in some clinical trials. Therefore, gene transfer of SSTR2 has been studied extensively in those SSTR2-negative tumors. Material and methods. In this research, the anti-proliferation effects of overexpressed SSTR2 were studied in our experimental cancer xenografts with different profiles of endogenous SSTRs expression. An adenoviral vector was used to express full length human SSTR2 in capan-2 and A549 xenografts. The potential pathways involved in SSTR2 signaling were then studied using immunoassays. Results. Our results showed that overexpression of SSTR2 inhibited the growth of both SSTR2-positive and SSTR2-negative cancer xenografts. The SSTR2-mediated anti-proliferation involved both cytostatic (growth arrest) and cytotoxic (apoptotic) actions by affecting the cellular levels of signaling molecules in apoptotic pathway, MAPK pathway and angiogenesis. Conclusion. SSTR2 inhibits cancer growth via multiple pathways and is a potential candidate for gene therapy for both SSTR2-positive and SSTR2-negative tumors.
Somatostatin receptors (SSTRs) are G-protein coupled plasma membrane receptors with two forms of somatostatin (SST) peptides, SS-14 and SS-28, as their natural ligands. The two peptides produced by SST cells act as neurotransmitters or paracrine/autocrine regulators via five different subtypes of human SSTRs (SSTR1-5), encoded by five distinct SSTR genes segregated on chromosome 14, 16, 17, 20, 22 Citation[1]. The highest homology of hSSTRs have been found in their transmembrane domains (TM), with a highly conserved sequence, YANSCANPI/VLY, in the seventh TM, found in all human SSTRs as well as in other species Citation[2].
In general, activation of SSTRs results in inhibition of cell proliferation and secretion, for example, pituitary growth hormone and thyroid-stimulating hormone releasing Citation[2]. Although five receptors have all been shown involved in this variety of biological actions, some effects display relative subtype selectivity. For instance, growth hormone secretion from pituitary somatotrophs is preferentially modulated by SSTR2 and SSTR5 Citation[3]. Nonetheless, given that multiple SSTRs are usually expressed in the same cell, it is suggested that SSTRs are redundant and function in orchestra, instead of as individual functional molecules.
The expression of SSTRs during development is time-specific and tissue-specific. High levels of SSTR2 have been identified in pituitary, pancreatic islet cells, adrenals and thymus. Expression of SSTRs has also been demonstrated in most tumors of neuroendocrine origin whereas they have been found expressed with much lower frequencies in nonendocrine tumors Citation[4], Citation[5]. Activation of SSTRs in these SSTR-expressing tumors usually resulted in remarkable inhibition of tumor cell proliferation via both indirect activities of inhibiting growth hormone secretion and direct activity through SSTR signaling pathways Citation[6]. Therefore, given the short half-life of SST, SST analogues with much greater metabolic stability and subtype-selectivity have been developed and have been commonly used in the treatment of SSTR-positive tumors Citation[7], Citation[8]. However, poor therapeutic results of the treatments with SST analogues have been frequently observed with the loss-of-expression of SSTRs, particularly SSTR2, in some of the tumors Citation[9]. Furthermore, the loss-of-expression of SSTR2 is believed to correlate with the metastatic progression of most of these tumors Citation[9]. Therefore, gene transfer of SSTR2, as a potential treatment for those SSTR2-negative cancers, has been subjected to intensive investigation.
Overexpressing SSTR2 displayed anti-tumor effect and significantly increased the sensitivity of SST treatment in a number of experimental SSTR-negative cancer cell lines and xenografts Citation[10–13]. Nonetheless, the detailed mechanisms of the SSTR-mediated inhibition of tumor growth remain to be further investigated. Also, intracellular pathways involved in SSTR signaling may need to be studied in each individual case since subtype- and cell type-specific signaling components have been reported Citation[1], Citation[14]. However, despite of the contradictory information obtained in different cell types using different SST analogs, it is generally accepted that all five SSTR subtypes are coupled to inhibition of the adenylyl cyclase-cAMP pathway and stimulate phosphotyrosyl protein phosphatase (PTP) Citation[2]. All five subtypes also interfere with the MAPK pathway to modulate cell proliferation, although the precise mechanisms seem to be subtype- and cell type- specific Citation[14], Citation[15].
In this study, we evaluated the anti-tumor effect of exogenous SSTR2 in our experimental cancers. Remarkably decreased tumor growth was observed with SSTR2 overexpression in both SSTR2-positive capan-2 xenografts and SSTR2-negative A549 xenografts. The components of the potential pathways involved in regulating tumor proliferation, including components of MAPK pathway, apoptotic pathway and angiogenesis, were further investigated in this research. The data presented in this research suggested that SSTR2 could be a very promising candidate for tumor gene therapy for both SSTR2-positive and SSTR2-negative cancers.
Material and methods
Materials
Capan-2 (a human pancreatic cancer cell line) was a gift from Sun Yat-sen university (Guangzhou, China) and A549 (a human lung cancer cell line) was a gift from Guangzhou Biomedicine Research & Development Centre (Jinan University, Guangzhou, China). The HEK-293T cells were purchased from Microbix Co. RPMI1640, MEM and fetal calf serum (FCS) used in tissue culture were purchased from Invitrogen. 3,3-diaminobenzidine tetrachloride (DAB), 1,4-dithiothreitol (DTT), 3-[4, 5-dimehyl-2-thiazolyl]-2, 5-diphenyl-2H-tetrazolium bromide (MTT), Dimethyl Sulfoxide (DMSO), Penicillin,streptomycin, 5-Bromo-4-chloro-3-indoxyl-beta-D-galactopyranoside (X-gal), hematoxylin, formalin and glutaraldehyde were purchased from Sigma. Blue RangeTM Prestained protein Molecular Marker was purchased from Pierce. Goat polyclonal anti-PARP-1, goat polyclonal anti-hSSTR2, rabbit polyclonal anti-VEGF, mouse monoclonal anti-p16, mouse monoclonal anti-p21, mouse monoclonal anti-Bax, mouse monoclonal anti-Bcl-2, mouse monoclonal anti-caspase-3, mouse monoclonal anti-p53, mouse monoclonal anti-TIMP-2, rabbit polyclonal anti-survivin, rabbit polyclonal anti-ERK-2 and goat polyclonal anti-β-actin were purchased from Santa Cruz Co. Mouse monoclonal anti-ras was purchased from abcam. The horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG, rabbit anti-goat IgG and goat anti-mouse IgG were purchased from QED Bioscience.
Propagation of the adenoviral vectors
The recombinated adenoviral vectors encoding full length human SSTR2 (Adv-SSTR2) or Escherichia coli β-galactosidase (Adv-LacZ) were propagated in 293T cells and were purified using Adeno-X™ Virus Purification Kit (Clontech) according to the manufacturer's instruction. The recombinant adenoviruses were stored at -70°C before use. Viral titers [PFU] were determined using a modified plaque assay, where 293T cells were infected overnight to ensure entry of all functional virions and to avoid confounding by receptor density. The resulting titers for Adv-SSTR2 and Adv-LacZ were 6.3×109 pfu/ml and 3.2×109 pfu/ml, respectively. These PFU titers (functional virions) were used for dosing in cell transfection.
Cell culture and evaluation of gene transduction
To evaluate the transfection efficacy of the adenoviral vectors, ∼3×104 cells were grown in 1ml MEM (supplemented with 100 IU/mL penicillin, 25g/mL streptomycin, and 10% FCS) in 24-well microtiter plates and maintained overnight (∼80% confluency) at 37°C in a humidified atomosphere containing 5% CO2. Briefly washed with phosphate-buffered saline (PBS), the cells were transfected with Adv-SSTR2 or Adv-LacZ in 200 µl serum free MEM at the density of a multiplicity of infection (MOI) of 100 for 3h in a humidified 37°C atmosphere containing 5% CO2. The cells were cultured for additional 48 h and the LacZ gene expression was visualized by staining for β-galactosidase. For β-galactosidase staining, briefly, the cells were washed with 37°C prewarmed PBS and fixed with 1 ml 0.05% glutaraldehyde for 15 minutes at room temperature. The cells were again rinsed three times with PBS, followed by incubation with 1 mg/mL X-Gal, 1 mmol/LMgCl2 and 5 mmol/L K4Fe (CN)6/K3Fe(CN)6 in 200 µl PBS for 12 hours at 37°C. The cells stained for β-galactosidase were observed under an inverted phase contrast microscope (Nikon Eclipse TE2000-S).
RNA isolation and reverse transcription-PCR
Total RNA was extracted from capan-2 and A549 cells with TRIZOL (Invitrogen) according to the manufacturer's instructions. 2µg of the total RNA in each reaction was reverse-transcribed into complementary DNA (cDNA) with oiligo(dT) primers using Moloney murine leukemia virus reverse transcriptase (Promega). The cDNAs were subjected to polymerase chain reaction under the condition of 94°C for 30 seconds, 55°C for 30 s and 72°C for 30 s for 30 cycles. Aliquots of 10 µl samples from the PCR reactions were analyzed by electrophoresis on 1.0% agarose gel containing ethidium bromide and the images were taken with Alpha ImagerTM2200 (Alpha Innotech). The sequences of the SSTR1-5 specific primers and the lengths of each fragments amplified were shown in .
Table I. Sequences of the primers used in amplifying SSTR1-5 cDNA and the length of each fragment amplified.
Immunoblotting analysis
Cultured cells were collected and resuspended in lysis buffer containing 50mM Tris-HCl (pH7.4), 100 mM KCl, 10% glycerol, 1mM EDTA, 1% TritonX-100 and 1mM DTT protease inhibitors (Roche). Tumor tissues were excised and washed with PBS. The tissues were then homogenized in homogenization buffer (1ml per 100mg tumor tissue) containing 50 mmol/L Tris-HCL (pH 8.5), 150 mmol/L NaCL, 0.2 g/L NaNO3, 0.1 g/L SDS, 100 µg /mL PMSF, 1 µg/mL aprotinin, 10 g/L NP-40 and 5 g/L sodium deoxycholate, followed by sonication. Cells/tissues were lysed for 20 min at 4°C and the supernatants were collected by centrifugation at 12 000 × g for 15 min at 4°C. A total of 70 µg proteins were separated by electrophoresis on 12% SDS-polyacrylamide gels and transferred to PVDF membrane (Whatman). Specific primary antibodies and horseradish peroxidase-conjugated secondary antibodies were then used for probing. The immunoblots were visualized by chemiluminescence with the ECL kit (Amersham Pharmacia) and the results were further analyzed using Alpha part II Ease (Alpha Innotech).
Histology and immunohistochemistry
The excised tumor tissues were fixed in formalin for 48 hours, followed by dehydration in 20% and then in 30% sucrose solutions. 10-mm thick serial cross sections were obtained using a microtome and the sections were seared at 37°C for 24 h. For X-Gal staining, the sections were incubated in X-Gal substrate (1 mg/mL X-Gal, 1 mmol/L MgCl2, 5 mmol/L K4Fe (CN)6/K3Fe(CN)6 in PBS) for 12 hours at 37°C and were observed under a inverted phase contrast microscope.
For immuno-staining of SSTR2, the sections were treated with 3% H2O2 for 10 minutes at room temperature and were then blocked with 1%BSA plus 0.1%Triton X-100 in PBS for 30 minutes. The sections were incubated with anti-SSTR2 for 12 hours at 4°C and then washed with 3XPBS. Followed by incubation with the horseradish peroxidase conjugated secondary antibody for 30 minutes at 30°C, the excess antibody was washed off with PBS and 250µl DAB per section was then added for color reaction. After intensive wash with PBS, the slides were counterstained with hematoxylin for 8 min.
Animals and tumor models
4-week-old athymic male nude mice (BALB/C nu/nu, obtained from the Medical College of Nan Fang University, Guangzhou, China), weighing 13 – 15g, were inoculated with exponentially growing cells (107 cells in 0.2 mL MEM/xenograft) subcutaneously into the left and right flanks. Mice were bred and maintained under pathogen-free conditions.
Experiment 1
A group of four mice were inoculated with Adv-LacZ and Adv-SSTR2 transfected capan-2 cells (MOI=100) in the left and right flanks, respectively. A second group of four mice were inoculated with Adv-LacZ and Adv-SSTR2 transfected A549 cells (MOI=100) into the left and right flanks, respectively. The tumor growth was observed 8 weeks after inoculation.
Experiment 2
A group of four mice were inoculated with capan-2 cells into the left and right flanks. After 8 weeks of the inoculation, the mice received intratumoral injections of 1.0 × 109 pfu/xenograft Adv-SSTR2 and Adv-LacZ in the left and right capan-2 xenografts, respectively. The injections were performed every 10 days, for a total of three injections. Four weeks after the last injection, the mice were sacrificed. The tumor tissues were excised and weighed immediately. The inhibition of tumor growth was determined using the following formula: rate of inhibition=(weight of the Adv-LacZ transfected tumor-weight of the Adv-SSTR2 transfected tumor/weight of the Adv-LacZ transfected tumor) × 100%. The tissues were then stored in liquid nitrogen for further studies. All the animal experiments were carried out in accordance with institutional guidelines for animal care.
Results
Expression of SSTRs in Capan-2 and A549 cells
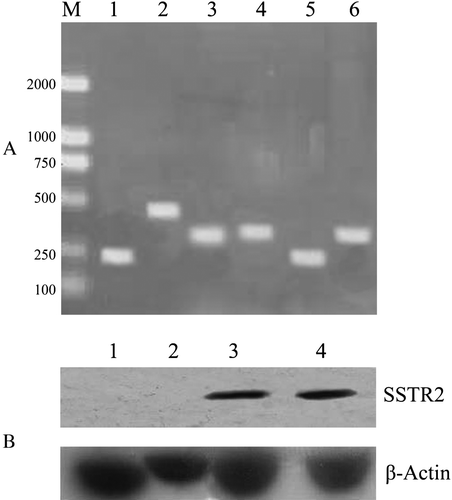
The mRNA expressions of five SSTRs in cultured capan-2 cells and A549 cells were analyzed by RT-PCR. As expected, all five SSTRs except SSTR3 were expressed in capan-2 cells while only SSTR1 and SSTR4 were expressed in A549 cells (A). However, the endogenous SSTR2 protein was not detected in either cell lines using western blot assay (results not shown). It suggested that the protein expression of SSTR2 in these cells should be further determined or that the level of the endogenous protein was below the threshold of the western blot assay in this study. Elevated SSTR2 protein expression was observed in both capan-2 and A549 cells transfected with Adv-SSTR2, but not in the cells transfected with control adenovirus expressing LacZ (B). The results suggested the successful adenoviral infection and the expression of the exogenous SSTR2 gene in the in vitro transfection of the cultured cells.
Figure 1. The expression of SSTRs in capan-2 and A549 cells. A: The RNA expressions of all five SSTRs were analyzed by RT-PCR. SSTR1 (lane 1), SSTR2 (lane 2), SSTR4 (lane 3) and SSTR5 (lane 4) were expressed in capan-2 cells while SSTR1 (lane 5) and SSTR3 (lane 6) were expressed in A549 cells. DNA ladders were loaded in lane M.B: The protein expression of SSTR2 was detected in capan-2 (3) and A549 cells (4) transfected with Adv-SSTR2, shown by western blotting with anti-SSTR2. No SSTR2 proteins were detected in control capan-2 (1) cells or A549 (2) cells transfected with Adv-LacZ. The cellular β-actin was shown as an internal control.
The transfection efficacy of the adenovirus vectors
Purified Adv-LacZ (at the density of MOI of 100) was used to infect cultured capan-2 and A549 cells, respectively. The efficacy of transfection was analyzed 48 hrs post-transfection. The expression of LacZ gene was visualized by β-gal staining. It was shown that nearly 100% transfection was obtained at MOI of 100, without showing obvious cytotoxity (). This optimized amount of adenoviral vectors was used in the in vitro transfections in this research. The transfection efficacies of Adv-LacZ and Adv-SSTR2 were much lower when introduced by intratumoral injection in the experimental capan-2 xenografts, as shown by the X-gal assay and immunohistochemistry, respectively ().
Figure 2. The efficacy of transfection of the constructed adenovirus vector in cultured cells. The transfection efficacy of the adenovirus vectors was demonstrated with Adv-LacZ using the β-galactosidase staining. A: untransfected capan-2 cells. B: untransfected A549 cells. C: Adv-LacZ transfected capan-2 cells. D: Adv-LacZ transfected A549 cells. The cells showed positive β-galactosidase staining were visualized under microscope (x 200) and nearly 100% transfection was shown when MOI was up to 100. Untransfected cells showed negative β-galactosidase staining.
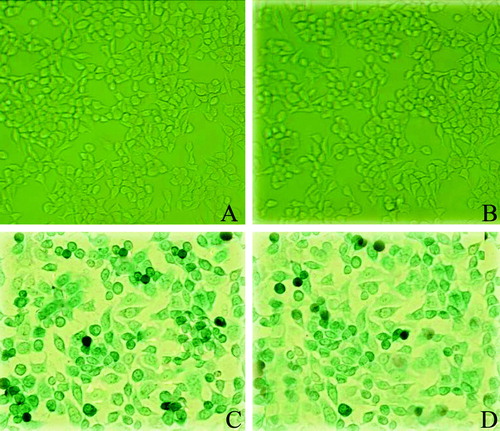
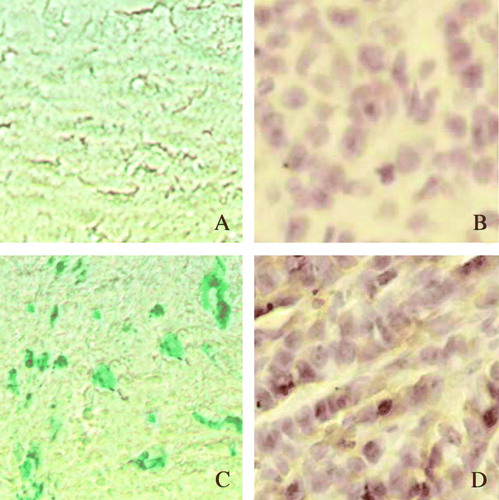
Figure 3. The transfection efficacy of intratumoral injection of the adenovirus. The cells that were successfully transfected by intratumoral injection of Adv-LacZ (C) or Adv-SSTR2 (D) in capan-2 xenografts were shown by β-galactosidase staining and histrochemistry, respectively. The results were observed under light microscope (x 400). A: untransfected conrol of capan-2 xenograft, stained for β-galactosidase; B: untransfected control of capan-2 xenograft stained with anti-SSTR2 and HRP-conjugated secondary antibody; C: capan-2 xenograft transfected with Adv-LacZ, stained for β-galactosidase; D: capan-2 xenograft transfected with Adv-SSTR2 was stained with anti-SSTR2 and HRP-conjugated secondary antibody.
Overexpression of SSTR2 inhibited the growth of both SSTR2-positive and SSTR2-negative tumor xenografts
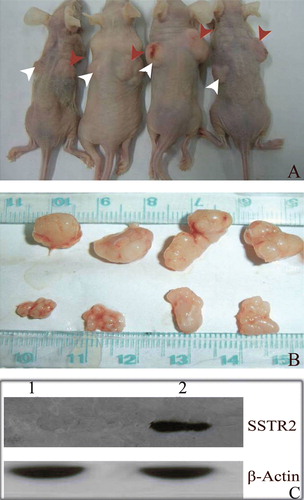
In experiment 1, we found that inoculation of the Adv-LacZ transfected capan-2 cells and A549 cells in nude mice resulted in successful tumor formation. However, inoculation of the Adv-SSTR2 transfected capan-2 cells and A549 cells failed to form any visible tumor in our mouse models (). In experiment 2, the intratumoral injection with Adv-SSTR2 resulted in dramatic inhibition of the capan-2 xenografts (A). The average tumor weight of the Adv-SSTR2 transfected xenografts was 0.378g ± 0.004 while the average tumor weight of the Adv-LacZ transfected xenografts was 0 .764g ± 0.030 (p<0.01) (B). The inhibition rate was 50.47%. The overexpression of SSTR2 in the capan-2 xenografts injected with Adv-SSTR2 was confirmed by western blot assay (C).
Figure 4. Cancer cells overexpressing exogenous hSSTR2 failed in tumor formation. Capan-2 cells and A549 cells overexpressing exogenous human SSTR2 failed in tumor formation in all our experimental nude mice. MOI=100 was used in in vitro transfection of cultured cells. A: No discernable tumors were observed with the injected capan-2 cells transfected with Adv-SSTR2 (indicated with white arrows), whereas successful tumor formations were observed with the injected capan-2 cells transfected with Adv-LacZ (indicated with red arrows). B: A: No discernable tumors were observed with the injected A549 cells transfected with Adv-SSTR2 (indicated with white arrows), whereas successful tumor formations were observed with the injected A549 cells transfected with Adv-LacZ (indicated with red arrows).
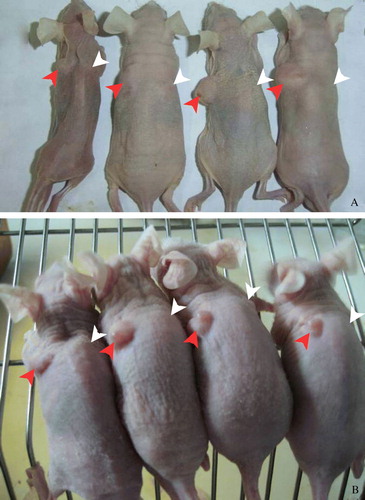
Figure 5. Overexpression of hSSTR2 inhibited the growth of human pancreatic tumors. The intratumoral injection of the Adv-SSTR2 in the capan-2 xenografts resulted in marked inhibition of the tumor growth. A: Adv-SSTR2 were injected into the capan-2 xenografts (indicated with white arrows) while Adv-LacZ was injected into the controls (indicated with red arrows). B: The tumor tissues, Adv-LacZ transfected (upper panel) and Adv-SSTR2 transfected (lower panel), were excised and measured four weeks after the last injection. C: The overexpressed SSTR2 was detected in capan-2 xenografts transfected with Adv-SSTR2 (lane 2) but not in Adv-LacZ transfected controls (lane 1), shown by Western Blot assay (lane 2) while it was using anti-SSTR2 and HRP-conjugated secondary antibody. The endogenous β-actin was used as a control.
Inhibition of growth of pancreatic carcinoma xenografts involved multiple pathways
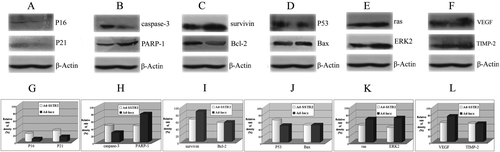
Cell-type specific and subtype-specific pathways have been implicated in SSTR signaling. Potential pathways involved in SSTR2 signaling in regulating cancer cell growth, including molecules regulating cell cycle, apoptosis and angiogenesis were studied in this research. The expressions of the cyclin-dependent kinase inhibitors p21 and p16 were markedly upregulated in the Adv-SSTR2 transfected capan-2 xenografts (A,G). The expression of the critical effector of both extrinsic and intrinsic apoptotic pathway, caspase-3, was increased while its downstream substrate, PARP-1, was decreased in the capan-2 xenografts overexpressing SSTR2 (B,H). However, the expression of the members of the BCL2 family of proteins, BAX and BCL2, remained unaffected by SSTR2 overexpression (C,D,I,J). Remarkably increased expression was also observed with the tumor associated antigen, Survivin suppressor gene, P53 and decreased expression was seen with the tumor-associated antigen, suvivin (C,D,I,J). The expressions of the oncogenic ras and ERK were downregulated in Adv-SSTR2 transfected capan-2 xenografts, comparing with the Adv-LacZ transfected controls (E,K). A decrease in the expression of the vascular endothelial growth factor (VEGF), which played a critical role in tumor blood vessel formation, was also demonstrated in capan-2 xenografts transfected with Adv-SSTR2 (F,L). No discernable changes in TIMP-2 expression were observed, although downregulation of MMP-2 by SSTR2 overexpression was reported in a previous study Citation[12].
Figure 6. Overexpression of SSTR2 affected multiple components in different pathways. The influences of SSTR2 overexpression on components in a number of different pathways were analyzed in Adv-SSTR2 transfected capan-2 xenografts using western blotting. The control capan-2 xenografts were transfected with Adv-LacZ and the expression of β-Actin was used as a sample loading control. The overexpression of SSTR2 was confirmed in the Adv-SSTR2 transfected capan-2 xenograft by western (Lane 2) while was not observed in the Adv-LacZ transfected control (Lane 1), as shown in . The expressions of p16, p21, caspase-3, PARP-1, p53, Bax, survivin, Bcl-2, ras, ERK2, VEGF and TIMP-2 in both Adv-SSTR2 transfected capan-2 xenografts and Adv-LacZ transfected controls were monitored by western blot assay (A, B, C, D, E and F). The results of the western blot assay were analyzed by AlphaEase FC StandAlone and the cellular levels of the above molecules were compared between the Adv-SSTR2 transfected tumors and the Adv-LacZ transfected controls (G, H, I, J, K and L).
Discussion
High levels of SSTR2 expression have been seen in normal tissues as well as in most human tumors including pituitary tumors, insulinomas, breast cancers and pancreatic cancers. These tissues usually feature SSTR isotypes other than SSTR2 with relatively lower levels Citation[6]. The SSTR-positive tumors are sensitive to the treatment with somatostatin and its analogs Citation[16]. However, treatment with somatostatin/analogs has poor outcomes in SSTR-negative tumors and gene transfer of SSTRs, particularly SSTR2, significantly increased the sensitivity of SSTR2-negative tumors to somatostatin treatment Citation[17]. Therefore, intensive studies have been done in gene transfer of SSTR2 as a potential treatment for SSTR2-negative tumors and have achieved encouraging outcomes.
In this study, we showed that overexpression of SSTR2 inhibited the growth of both SSTR2-positive and SSTR2-negative tumors in a somatostatin-independent manner. The pancreatic carcinoma derived capan-2 cells were used to establish SSTR2-positive while the lung cancer derived A549 cells were used to establish SSTR2-negative experimental cancer xenografts. The SSTR2 mRNA expression was confirmed in capan-2 cells but not in A549. However, the correspondent functional proteins of SSTR2 might not be simply assumed in capan-2 cells since SSTR2 proteins were not detected in either cell lines using immunoassays. Given that most of the expression analyses used in the clinical screening of SSTR2-positive tumors were based on mRNA expressions, the capan-2 cells were considered as SSTR2-positive while A549 cells were considered as SSTR2-negative cancer cells in this study. Intratumoral injection of Adv-SSTR2 significantly reduced the growth of capan-2 xenografts to a comparable level of the reduced growth of A549 xenografts overexpressing SSTR2. No discernable tumor was formed with Adv-SSTR2 transfected capan-2 cells or A549 cells. These observations suggested that the tumor inhibition is SSTR2-dependent and the tumors with endogenous SSTR2 expression may also be suitable for SSTR2 gene therapy.
We further investigated the possible mechanisms of the anti-tumor effects of SSTR2. All five SSTRs have been indicated in positive (SSTR2, SSTR3 and SSTR5) or negative coupling (SSTR1 and SSTR4) to MAPK signaling pathway. SSTR1 was shown to increase the expression of the cyclin-dependent protein kinase inhibitors in a MAPK-dependent manner and inhibited cell proliferation Citation[1]. Here in this research, Western Blot studies revealed that the overexpression of SSTR2 resulted in up-regulation of the cyclin-dependent kinase (CDK2, CDK4 and CDK6) inhibitors p21 and p16, which in turn, could cease the G1/S phase transition and stop the cell cycle progression. Also, we suggested that the apoptotic pathway might play a pivotal role in the SSTR2 mediated inhibition of cell proliferation by showing that the expression of Caspase-3 was increased while its downstream target, poly ADP-ribose polymerase (PARP) was decreased in SSTR2 transfected tumors. Similar results have been demonstrated in different cancer cells Citation[17]. Although controversial observations on the influence of SSTR2 activation on the p53 induced apoptosis have been reported, the findings in this study showed that the cellular level of p53 was significantly increased in SSTR2 transfected tumors Citation[18], Citation[19]. The differences in p53 accumulation upon SSTR2 activation could be consequences of the different experimental cancer cells used in these studies. Restoration of p53 expression in our SSTR2 transfected tumors could inhibit cell proliferation via cell cycle arresting and inducing apoptosis. Survivin, a member of the inhibitors of apoptosis (IAP) gene family, was down regulated in the SSTR2 overexpressing tumors. Downregulation of survivin could inhibit cell proliferation via the direct influence on cell cycle completion or via promoting apoptosis by inhibiting caspase-9/caspase-3 processing Citation[20]. However, on the contrary to the findings in the previous study, in which overexpression of SSTR2 significantly decreased Bcl-2 and increased BAX expression in PC-3 pancreatic cancer cells, overexpression of SSTR2 was shown to have minimal influence on the expression of Bcl-2 and Bax in our study Citation[10]. It suggested that SSTR2 functions downstream of these pro-apoptotic members of Bcl2 family of proteins or there are cell-type specific cellular targets for SSTR2.
The small GTPase, Ras, is another signaling molecule that has usually been found overexpressed in human tumors. Activated Ras activates Raf and in turn, activates MEK and contributes to substantial tumor cell growth Citation[21]. ERK promotes cell growth by enhancing the RAS-MEK pathway as well as by accelerating beta-catenin accumulation in Wnt pathway. The expression of both RAS and ERK was downregulated in our SSTR2 transfected tumors, suggesting SSTR2 interferes with the essential oncogenic RAS-ERK signaling cascade.
New blood vessel formation is fundamental to tumor growth and the vascular endothelial growth factor (VEGF), which has been found expressed by lots of tumor cells, is critical for these events. Encouraging results have been achieved using anti-VEGF treatment in clinical trails of cancers Citation[22]. Here, we found that the expression of VEGF was dramatically decreased in the SSTR2 overexpressing tumors. The matrix metalloproteinases (MMPs) and the tissue inhibitor of metalloproteinase (TIMPs) regulate the remodeling of the extracellular matrix, which is critical to the invasion and metastatic dissemination of cancer cells Citation[23]. Although, overexpression of SSTR2 was reported to decrease the expression of MMP-2 in a previous study Citation[12], no influence on the expression of TIMP-2 was observed in this research.
Collectively, the findings in this research extended the spectrum of tumors that could be considered for SSTR2 gene therapy by showing that overexpressing SSTR2 significantly inhibited the growth of both SSTR2-positive and SSTR2-negative experimental tumor xenografts. The inhibitory effects of SSTR2 involved multiple signaling molecules in both cytostatic and cytotoxic pathways. Previous research also suggested that the inhibitory action observed with SSTR2 could be indirect effects mediated by the other SSTR subtypes since heteromeric interactions between SSTR subtypes have been reported for SSTR2/SSTR5 Citation[24]. However, given the similar degree of inhibition observed in Adv-SSTR2 transfected capan-2 xenografts and A549 xenografts, we suggested that the heterodimerization of SSTR2/SSTR5 might not be necessary in SSTR2 mediated anti-proliferation since A549 cells were negative of endogenous SSTR5 expression. Nonetheless, given the limitations of the overexpression assay, further investigations in tumors with different endogenous SSTR expression utilizing subtype-selective somatostatin analogs/antagonists will lead to a better understanding of the mechanisms of the anti-tumor effects of SSTR2.
Acknowledgements
This study was sponsored by National Natural Science Foundation of China (90608024).
References
- Patel YC, Greenwood MT, Panetta R, Demchyshyn H, Niznik H, Srikant CB. The somatostatin receptor family: A minireview. Life Sci 1995; 57: 1249–65
- Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 1999; 20: 157–98
- Kimura N, Tomizawa S, Arai KN, Kimura N. Chronic treatment with estrogen up-regulatesexpression of sst2 messenger ribonucleic acid (mRNA) but down-regulates expression of sst5 mRNA in rat pituitaries. Endocrinology 1998; 139: 1573–80
- Hofland LJ, Lamberts SW. Somatostatin receptors and disease: Role of receptor subtypes. Baillieres Clin Endocrinol Metab 1996; 10: 163–76
- Taboada GF, Luque RM, Bastos W, Guimarães RF, Marcondes JB, Chimelli LM, et al. Quantitative analysis of somatostatin receptor subtype (SSTR1-5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur J Endocrinol 2007; 156: 65–74
- Guillermet-Guibert J, Lahlou H, Cordelier P, Bousquet C, Pyronnet S, Susini C. Physiology of somatostatin receptors. Endocrinol Invest 2005; 28: 5
- Hofland LJ, van der Hoek J, Feelders R, van der Lely AJ, de Herder W, Lamberts SW. Pre-clinical and clinical experiences with novel somatostatin ligands: Advantages, disadvantages and new prospects. Endocrinol Invest 2005; 28: 36–42
- Petersenn S. Efficacy and limits of somatostatin analogs. Endocrinol Invest 2005; 28: 53–7
- Janson ET, Gobl A, Kälkner KM, Oberg K. A comparison between the efficacy of somatostatin receptor scintigraphy and that of in situ hybridization for somatostatin receptor subtype 2 messenger RNA to predict therapeutic outcome in carcinoid patients. Cancer Res 1996; 56: 2561–5
- Kumar M, Liu ZR, Thapa L, Wang DY, Tian R, Qin RY. Mechanisms of inhibition of growth of human pancreatic carcinoma implanted in nude mice by somatostatin receptor subtype2. Pancreas 2004; 29: 141–51
- Szepeshazi K, Schally AV, Halmos G, Sun B, Hebert F, Csernus B, et al. Targeting of cytotoxic somatostatin analog AN-238 to somatostatin receptor subtypes 5 and/or 3 in experimental pancreatic cancers. Clin Cancer Res 2001; 7: 2854–61
- Kumar M, Liu ZR, Thapa L, Qin RY. Anti-angiogenic effects of somatostatin receptor subtype2 on human pancreatic cancer xenografts. Carcinogenesis 2004; 25: 2075–81
- Celinski SA, Fisher WE, Amaya F, Wu YQ, Yao Q, Youker KA, et al. Somatostatin receptor gene transfer inhibits established pancreatic cancer xenografts. J Surg Res 2003; 115: 41–7
- Cattaneo AG, Taylor JE, Culler MD, Nisoli E, Vicentini LM. Selective stimulation of somatostatin receptor subtypes: Differential effects on Ras/MAP kinase pathway and cell proliferation in human neuroblastoma cells. FEBS Lett 2000; 481: 271–6
- Liu AM, Wong YH. Activation of nuclear factor {kappa}B by somatostatin type 2 receptor in pancreatic acinar AR42J cells involves G{alpha}14 and multiple signaling components: A mechanism requiring protein kinase C, calmodulin-dependent kinase II, ERK, and c-Src. J Biol Chem 2005; 280: 34617–25
- Taboada GF, Luque RM, Neto LV, Machado Ede O, Sbaffi BC, Domingues RC, et al. Quantitative analysis of somatostatin receptor subtypes (1-5) gene expression levels in somatotropinomas and correlation to in vivo hormonal and tumor volume responses to treatment with octreotide LAR. Eur J Endocrinol 2008; 158: 295–303
- Vernejoul F, Faure P, Benali N, Calise D, Tiraby G, Pradayrol L, et al. Antitumor effect of in vivo somatostatin receptor subtype 2 gene transfer in primary and metastatic pancreatic cancer models. Cancer Res 2002; 62: 6124–31
- Teijeiro R, Rios R, Costoya JA, Castro R, Bello JL, Devesa J, et al. Activation of human somatostatin receptor 2 promotes apoptosis through a mechanism that is independent from induction of p53. Cell Physiol Biochem 2002; 12: 31–8
- Ferrante E, Pellegrini C, Bondioni S, Peverelli E, Locatelli M, Gelmini P, et al. Octreotide promotes apoptosis in human somatotroph tumor cells by activating somatostatin receptor type 2. Endocr Relat Cancer 2006; 13: 955–62
- Andersen MH, Svane IM, Becker JC, Straten PT. The universal character of the tumor-associated antigen survivin. Clin Cancer Res 2007; 13: 5991–3
- Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol 2008; 10: 138–47
- Veeravagu A, Hsu AR, Cai W, Hou LC, Tse VC, Chen X. Vascular endothelial growth factor and vascular endothelial growth factor receptor inhibitors as anti-angiogenic agents in cancer therapy. Recent Patents Anticancer Drug Discov 2007; 2: 59–71
- Murate T, Hayakawa T. Multiple functions of tissue inhibitors of metalloproteinases (TIMPs): New aspects in hematopoiesis. Platelets 1999; 10: 5–16
- Ren SG, Taylor J, Dong J, Yu R, Culler MD, Melmed S. Functional association of somatostatin receptor subtypes 2 and 5 in inhibiting human growth hormone secretion. J Clin Endocrinol Metab 2003; 88: 4239–45
