Abstract
Background. Women with high risk for breast cancer due to family history are offered genetic counseling and surveillance. The aim of this cross-sectional study was to characterize women at an oncogenetic counseling clinic in terms of socioeconomic status (SES) and health related quality of life (HRQOL) and to compare data with population based figures. Material and methods. All healthy women who had ever visited the Oncogenetic clinic, Department of Oncology, Sodersjukhuset, 1998–2004 were eligible. A total of 306 women consented to participate (82.5%). SES data were compared with official data for all women (n=277 783), in the same age, living in the same geographical area at the time the study was performed. HRQOL data (SF-36) were compared with Swedish normative data. Results. Significantly more women in the study group were cohabiting (74.2 vs. 43.8%), had the highest education level, (56.7 vs. 39.6%) and had the highest household income (36.9 vs. 12.9%) as compared to the reference population in the same catchment area. Study subjects report significant lower levels of HRQOL for subscales related to mental health and for general health compared to normative data, but similar levels on HRQOL subscales related to physical health. Discussion. Attendees at the oncogenetic clinic appears to have higher socioeconomic status and lower quality of life as compared to women living in the same area, although the genetic predisposition for breast cancer is considered to be evenly distributed in the population. Thus, efforts to reach women in lower socioeconomic groups should be elaborated.
Breast cancer is by far the most frequently diagnosed malignant tumour in females and one Swedish woman in ten will be affected during her lifetime Citation[1]. Although most cases occur late in life and are sporadic, a Scandinavian study on twins has revealed that hereditary factors play an important role in the development of 27% of all breast cancers Citation[2]. Five to ten percent of the cases appear to be the result of autosomal dominant genes Citation[3]. Accordingly, women with a family history of breast cancer may run a risk for the occurrence and earlier onset of this disease that is substantially higher than that of the general population Citation[4].
In Sweden asymptomatic women with a two times higher risk of breast cancer, in virtue of their family history and using the Claus model for risk estimation, are suggested increased mammographic surveillance. Annual screening is advised to start 5–10 years before the earliest age breast cancer was diagnosed in the family. All women are informed of the value of and instructed in breast self-examination. They are encouraged to contact a cancer clinic if they observe any abnormalities in their breasts. Mammography surveillance of women with a family history of breast cancer has been found to be potentially beneficial Citation[5].
Mutation analysis of the BRCA1 and BRCA2 genes is offered to families with clustered breast and/or ovarian cancer suspected to harbour mutations in any of the two genes. Women found to be mutation carriers, are given information that their risk of developing breast cancer is substantially elevated, up to 80% in a lifetime. They are offered annually surveillance including clinical examination, mammography, ultrasound and magnetic resonance imaging, MRI of the breasts. They are also informed of the possibility to have a prophylactic mastectomy with immediate breast reconstruction done. Prophylactic mastectomy has been reported in a number of studies to reduce the risk for breast cancer by more than 90% Citation[6]. Carriers of BRCA1 and BRCA 2 mutations are also at a substantial risk of ovarian cancer (25–40% lifetime risk) Citation[7] and are suggested annual surveillance and to opt for prophylactic salpingo/ooforectomy after childbearing age. Studies have shown that prophylactic salpingo/ooforectomy reduces the risk of ovarian cancer with up 95% Citation[8].
In conclusion, there are several options of surveillance and interventions that can be offered to women found after oncogenetic counselling to have elevated risk of breast and ovarian cancer. In Sweden, however, healthy women with a family history of breast cancer are not referred to oncogenetic counseling unless the subject is brought up in the contact with medical professionals. Consequently, a woman with family history of breast cancer has to be aware of the possibility of counseling to come into question for information, advice and the special surveillance program.
The association between socio-economic factors and breast cancer survival has been explored in many studies, all demonstrating a poorer survival in women from low socio-economic groups Citation[9]. In a Swedish study, Rutqvist and Bern Citation[10] demonstrated a better survival in women with high income, more skilled work and a high level of education but these differences were associated with distribution in clinical stage at diagnosis and no stage-specific survival differences were found according to socio economic variables.
Studies in Sweden and in other western countries have revealed several factors e.g. not having any offspring and/or living without a partner, low or extra-high education as well as low income as predictive of non-attendance at mammography screening Citation[11], Citation[12]. In agreement with such observations, descriptive reports of socioeconomic factors among women attending oncogenetic counselling clinics Citation[13], Citation[14] show that women attending breast cancer risk assessment programs tend to be well-educated, and of middle or upper income status Citation[13]. There is, however, no published data suggesting that family clustering of breast cancer of genetic origin is more prevalent in higher socioeconomic groups. Reports of an association between high socioeconomic status and an elevated risk for breast cancer (with an RR 1.1–3.5) are believed to reflect extrinsic factors rather than genetic factors Citation[15].
Numerous studies have explored psychological factors in women attending onco-genetic counselling and in general they report worse scores for anxiety, distress and depression in women with a family history of breast cancer compared to women in the general population Citation[16], Citation[17]. In contrast, a Swedish study of women going through pre-symptomatic testing for mutations in BRCA 1/2, showed no differences in vitality, mental health, role emotional functioning, social functioning or general health as compared to women in the Swedish population Citation[18]. In addition, in a Norwegian study individuals with hereditary risk for cancer were in better physical and similar mental shape as the general population Citation[19].
Aims
The aim of this cross-sectional study was to characterize women at an oncogenetic counseling clinic in terms of socioeconomic status (SES) and health related quality of life (HRQOL). A second aim was to compare SES in the women at the counseling clinic with population based data for women in the same catchment area. A third aim was to compare HRQOL in the studied sample with normative data from the general population. A fourth aim was to compare different pre-defined objective risk groups in the study sample with respect to SES and HRQOL.
Material and methods
Study group
A consecutive series of 373 women attending genetic counseling at the oncogenetic outpatient clinic at Södersjukhuset, Stockholm, Sweden between 1 April 1998 and 1 June 2004 were eligible for inclusion. All eligible women lived in the catchment area of the clinic and were between 25 and 74 years at the time of first visit. A total of 306 women (82.5%) participated in the study. The women were either self-referred or referred by their doctors (mostly GP or gynaecologist) to the clinic and they all came by virtue of one or several cases of breast cancer in the family. The criteria for attending the clinic and, thus, for inclusion in our study were wide and included having at least one close relative who had developed breast and/or ovarian cancer. The majority (82.3%) of the women had at least two close relatives with breast cancer in their family history and 54% had three or more close relatives with a history of breast cancer. The objective risk groups were pre defined as follows: Lifetime risk of breast cancer; low <21% (n=117), intermediate 21–39% (n=56), high 40–80% (n=124). For nine study subjects the risk was not yet estimated at the time of inclusion.
The consecutive mode of inclusion meant that some of the women had made only one visit to the clinic, while others had visited several times when the questionnaires were sent out.
Women previously treated for breast and/or ovarian cancer or other malignancies were not eligible.
Clinical setting
An outpatient oncogenetic clinic was established at the Oncology Department of Sodersjukhuset, Stockholm in April 1998. The staff of this clinic originally included one nurse trained in oncology and two oncologists trained in oncogenetics but since May of 1999 only one oncologist has been working at the clinic.
Procedure
A letter, explaining the purpose and the procedure of the study was sent to all 373 of the eligible women by June 1, 2004. By answering and returning the questionnaire, enclosed with this letter, the woman agreed to participate.
Questionnaires and reference samples
Socioeconomic status (SES)
The questionnaire addressing socioeconomic characteristics was developed for a study obtaining HRQOL reference values in a large sample of the Swedish population Citation[20]. The questionnaire included three separate SES indicators; marital status (four response categories: “married or cohabiting”, “divorced/separated”, “widowed”, “single”), education (three response categories: “elementary school = 9 years”, “elementary school +2–3 years of education”, “elementary school +4-or more years of education”) and annual household income classified in Swedish crowns, SEK ( three response categories: “ < 300 000”, “300 000–500 000”, “ > 500 000. As of January 2008, 300 000 SEK equals 33 000 Euro or 48 000 US dollars.
The reference population (25–74 years old) included all women living in the same catchment area as the study-population at the time the questionnaire was sent out, i.e., 2004 (n = 277 783). Comparisons between data from study subjects and the population were explored by using for the reference population age specific figures from official statistics from three population based registers. Data on marital status was obtained through the Register of Total Population 2004 that is updated continuously and covers all individuals in Sweden. For reference values on education the National register of Education 2004 was used, which includes the highest level of education achieved for all persons between 16 and 74 living in Sweden and is updated annually. The economic situation in the reference population was obtained from Register of Total Household income 2004 that covers all sources of income subject to taxation including social benefits. Each of these registers includes almost 100% of the reference population.
The Short Form -36 Health Survey (SF-36)
The Short Form -36 Health Survey (SF-36) was used to assess HRQOL Citation[21]. It consists of 36 items constituting eight subscales: physical functioning (PF), role limitations as a result of physical problems (RP), general health perception (GH), pain (BP), role limitations as a result of emotional problems (RE), social functioning (SF), vitality (VT) and emotional well-being (MH). Higher score signifies better HRQOL. For each of the eight scales, the score are summed and transformed to a scale of 0–100. The Swedish version exhibits satisfactory psychometric properties Citation[22]. Normative data for Swedish women are available for SF-36 Citation[21].
Statistical considerations
Descriptive statistics were generated for the study population regarding age at first visit and characteristics of family and family history. The age distribution in the study group and in the general population – from the catchment area – were compared by contrasting the mean age in the general population with the 95% confidence interval for age in the study group. Data on socioeconomic status (SES) rates among women from the study group were adjusted for age using the method of indirect standardization with age in five-age categories and expected age-specific rates from the general population. For each of the studied SES factors observed (O) and expected (E) number of women were calculated. Results are presented as the observed proportion with exact binomial 95% confidence intervals, the expected proportion, and the ratio (O/E) with exact 95% Poisson confidence intervals. The expected mean values for each of the SF-36 subscales were calculated in a similar way by using age-specific mean scale scores from normative Swedish data Citation[23]. Results from these analyses are presented for each subscale as the observed mean and 95% confidence interval together with the calculated expected mean value.
Comparisons of the three defined risk-groups in the study group and each of the SF-36 subscales were made by using linear regression models with group represented by two dummy variables and with age included as a continuous variable.
The Ethics Committee at Karolinska Institute approved the study (02-496).
Results
The characteristics of the study population in terms of age at first visit, description of family, family history and objective risk are shown in . There were no major differences with respect to these variables between participants in the questionnaire study and the 65 non-participants (data not shown).
Table I. Age, number of visits at the clinic and characteristics of family and family history of study subjects at first visit.
Age
Mean age in the study sample was statistically significantly lower, 44.0 (42.8 to 45.1) than in the catchment area population (mean 46.1) within the same age range. In the study group, there was a difference in mean age in the pre-defined risk groups. The low risk group tended to be younger (42.2) than the intermediate (45.5) and the high risk groups (45.3).
Socioeconomic status (SES)
There were statistically significant differences between the study group and women in the same catchment area with respect to all three socioeconomic indicators (). Study subjects were 1.7 times more often married or cohabiting than women from the same catchment area. In study subjects the highest education level was 1.4 times more often university or equal, compared to the reference population. The highest level of household income was seen almost three times more often in the study group. No differences in SES were found between the three objective risk groups (lifetime risk of breast cancer; low <21%, intermediate 21–39%, high 40–80%),
Table II. Observed and expected proportion of socio-economic status (SES) factors in women 25–74 years seeking oncogenetic counseling at the Oncogenetic clinic Södersjukhuset, Stockholm.
HRQOL
shows observed and expected means for the SF-36 subscales in the study group. The study sample scored lower than expected on five of the eight subscales, whereas three subscales related to physical health showed mean scores close to expected (, a).
Figure 1. (a) Observed and expected SF-36 mean scores for all 306 healthy individuals 25–74 years. (b) Mean scores for three pre-defined risk groups; Low lifetime risk <21%, Intermediate life-time risk 21–39%, High lifetime risk 40–80% (Nine study subjects were categorize as “risk estimation not yet completed”, those women are not included in b).
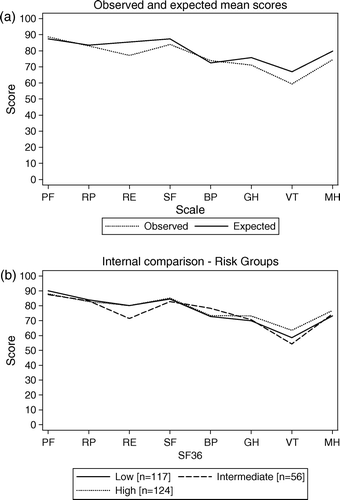
Table III. Mean values for each of the SF-36 scales for all 306 women in the study group answering the survey.
As age differences were found between the risk groups, this variable was accounted for in the analysis of HRQOL (SF-36). No statistical significant differences in HRQOL were found between the risk groups (b).
Discussion
Our results show that women visiting the oncogenetic counseling clinic were higher educated, more often married or cohabiting and had a better economic situation as compared to women from the same catchment area. In addition they reported lower levels of health related quality of life for all four subscales (VT, SF, RE, MH) related to mental health. Scoring for general health (GH) were also lower for study subjects but equal to normative data considering the other variables valuating physical health.
Although, previous studies have described the socioeconomic status of women who attend risk assessment programs Citation[13], Citation[14], to our knowledge, the present study is the first that examines possible differences in socioeconomic status between women with a family history of breast cancer who seek oncogenetic counselling and the general population in the same geographical area. We would like to emphasize the quality and almost complete coverage of the three national registers from which SES data concerning the reference group were obtained.
Our study group included a substantially higher proportion of women living together with another adult than the reference population, which could, at least in part, explain the difference in household income between these two groups. At the same time, it is possible that a more favorable economic situation in the household influences attitudes towards risk management, even though this socioeconomic indicator does not reflect only the contribution of the woman to the families economy. Another possible explanation for the observed difference in household income could be the fact that a higher level of education often leads to better paid employment. The proportion of higher level of education was larger than in the reference population. The total household income of the reference population includes social benefits. In contrast, we cannot rule out the possibility that this income has been left out by our study subjects as we did not specify this source of income in the SES questionnaire. However, that would have underestimated and not exaggerated the difference in total household income between the two groups.
Several reports describe a significant relationship between parental level of education and the academic career of offspring Citation[24], Citation[25]. Consequently, it is not impossible that the family history of breast cancer of some of our subjects was due to extrinsic risk factors that are more prevalent among more highly educated women and assumed from one generation of women to the next (e.g., early menarche, few children, use of oral contraceptives) rather than to genetic predisposition Citation[15]. In addition, there were no differences in SES between the objective risk groups, indicating that socioeconomic factors may play a more important role than actual cancer risk for attending oncogenetic counselling.
The lower -than-normal mean scores for HRQOL in our study group on all sub-scales of the SF-36 reflecting mental health as well as general health remained even when the subjects were divided into three sub-groups on the basis of highest education achieved and compared to women from the general population with a corresponding level of education (data not shown).
The normative values for the Swedish SF-36 Health Survey reveal a positive association between a high level of education and high scores for both mental and physical health Citation[21]. Furthermore, married or cohabiting women in general report higher scores than women living alone Citation[21]. These observations indicate that the differences observed here are actually even more pronounced. One explanation could be that attendance to the clinic, including counseling and surveillance, causes distress that result in decrease in areas of HRQOL reflecting mental health. This explanation is, however, less likely as the clinic aims at helping the women to cope with their objective and perceived breast cancer risk. However, data from the SF-36 reflects a cross-sectional picture of HRQOL in women attending oncogenetic counseling and surveillance program. Thus, no conclusions can be drawn whether the observed “lower-than-normal” scores for all subscales related to mental health and general health are due to anxiety and distress secondary to perceived risk and or due to personality in these women.
However, other factors may also be involved here. Only 15% of the population on which the Swedish normative values are based live in urban or suburban areas Citation[21], whereas nearly all of our subjects live in such areas. Since populations living in the rural areas or small towns report higher SF-36 scores than populations in cities and their surroundings Citation[21], the most appropriate reference sample would have been women living in the same kind of environment.
In summary our findings reveal differences between socioeconomic groups in attendance to a genetic counseling clinic. The genetic predisposition for breast cancer is considered to be evenly distributed in the population. Thus, these differences are likely explained by lack of knowledge about the possibility of genetic counseling among women with lower SES. Efforts should be elaborated to increase the knowledge among women in these groups with a family history for breast cancer. More information concerning the effects of oncogenetic counseling on HRQL in women with a family history of breast cancer is required and this will be explored in future studies.
Acknowledgements
To every woman who decided to participate in this study and thus provided us with the data. The present study was supported by grants from Stockholm County Council and the Swedish Cancer Society.
References
- Cancer incidence in Sweden 1999: Swedish National Board of Health and Welfare.
- Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 2000; 343: 78–85
- Claus EB, Risch N, Thompson WD. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet 1991; 48: 232–42
- Claus EB, Risch NJ, Thompson WD. Age at onset as an indicator of familial risk of breast cancer. Am J Epidemiol 1990; 131: 961–72
- Maurice A, Evans DG, Shenton A, Ashcroft L, Baildam A, Barr L, et al. Screening younger women with a family history of breast cancer–does early detection improve outcome?. Eur J Cancer 2006; 42: 1385–90
- Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van′t Veer L, Garber JE, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004; 22: 1055–62
- Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet. 2003; 72: 1117–30
- Finch A, Beiner M, Lubinski J, Lynch HT, Moller P, Rosen B, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA 2006; 296: 185–92
- Kaffashian F, Godward S, Davies T, Solomon L, McCann J, Duffy SW. Socioeconomic effects on breast cancer survival: Proportion attributable to stage and morphology. Br J Cancer 2003; 89: 1693–6
- Rutqvist LE, Bern A; Stockholm Breast Cancer Study Group. Socioeconomic gradients in clinical stage at presentation and survival among breast cancer patients in the Stockholm area 1977–1997. Int J Cancer 2006;119:1433–9.
- Lagerlund M, Maxwell AE, Bastani R, Thurfjell E, Ekbom A, Lambe M. Sociodemographic predictors of non-attendance at invitational mammography screening–a population-based register study (Sweden). Cancer Causes Control 2002; 13: 73–82
- Katz SJ, Zemencuk JK, Hofer TP. Breast cancer screening in the United States and Canada, 1994: Socioeconomic gradients persist. Am J Public Health 2000; 90: 799–803
- Bastani R, Maxwell AE, Bradford C, Das IP, Yan KX. Tailored risk notification for women with a family history of breast cancer. Prev Med 1999; 29: 355–64
- Watson M, Kash KM, Homewood J, Ebbs S, Murday V, Eeles R. Does genetic counseling have any impact on management of breast cancer risk?. Genet Test 2005; 9: 167–74
- Braaten T, Weiderpass E, Kumle M, Adami HO, Lund E. Education and risk of breast cancer in the Norwegian-Swedish women's lifestyle and health cohort study. Int J Cancer 2004; 110: 579–83
- Cull A, Anderson ED, Campbell S, Mackay J, Smyth E, Steel M. The impact of genetic counselling about breast cancer risk on women's risk perceptions and levels of distress. Br J Cancer 1999; 79: 501–8
- Martin W, Degner L. Perception of risk and surveillance practices of women with a family history of breast cancer. Cancer Nurs 2006; 29: 227–35
- Arver B, Haegermark A, Platten U, Lindblom A, Brandberg Y. Evaluation of psychosocial effects of pre-symptomatic testing for breast/ovarian and colon cancer pre-disposing genes: A 12-month follow-up. Fam Cancer 2004; 3: 109–16
- Carlsson AH, Bjorvatn C, Engebretsen LF, Berglung G, Natviq GK. Psychosocial factors associated with quality of life among individuals attending genetic counseling for hereditary cancer. J Genet Couns 2004; 13: 425–45
- Michelson H, Bolund C, Nilsson B, Brandberg Y. Health-related quality of life measured by the EORTC QLQ-C30–reference values from a large sample of Swedish population. Acta Oncol 2000; 39: 477–84
- Sullivan M, Karlsson J, Taft C. SF-36 Swedish Manual and Interpretation Guide2nd ed. Sahlgrenska University Hospital, Gothenburg 2002
- Sullivan M, Karlsson J, Ware J., Jr. The Swedish SF-36 Survey I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general population in Sweden. Soc Sci Med 1995; 41: 1349–58
- Fayers PM, Machin D. Quality of life. Assessment, analysis and interpretation. Wiley, Chichester 2000
- Hagquist CE. Health inequalities among adolescents: The impact of academic orientation and parents' education. Eur J Public Health 2007; 17: 21–6
- Swedish National Agency for Higher Education, Statistics Sweden. Higher education. Social background among university entrants 2005/2006 and first time postgraduate students 2004/2005. Serie Utbildning och forskning. Statistics Sweden Dec 20 2006.
