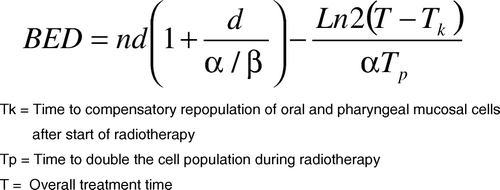Abstract
Introduction. Altered fractionation has demonstrated clinical benefits compared to the conventional 2 Gy/day standard of 70 Gy. When using synchronous chemotherapy, there is uncertainty about optimum fractionation. IMRT with its potential for Simultaneous Integrated Boost (SIB) adds further to this uncertainty. This survey will examine international practice of IMRT fractionation and suggest possible reasons for diversity in approach. Material and methods. Fourteen international cancer centres were surveyed for IMRT dose/fractionation practised in each centre. Results. Twelve different types of dose fractionation were reported. Conventional 70–72 Gy (daily 2 Gy/fraction) was used in 3/14 centres with concurrent chemotherapy while 11/14 centres used altered fractionation. Two centres used >1 schedule. Reported schedules and number of centres included 6 fractions/week DAHANCA regime (3), modest hypofractionation (≤2.2 Gy/fraction) (3), dose-escalated hypofractionation (≥2.3 Gy/fraction) (4), hyperfractionation (1), continuous acceleration (1) and concomitant boost (1). Reasons for dose fractionation variability include (i) dose escalation; (ii) total irradiated volume; (iii) number of target volumes; (iv) synchronous systemic treatment; (v) shorter overall treatment time; (vi) resources availability; (vii) longer time on treatment couch; (viii) variable GTV margins; (ix) confidence in treatment setup; (x) late tissue toxicity and (xi) use of lower neck anterior fields. Conclusions. This variability in IMRT fractionation makes any meaningful comparison of treatment results difficult. Some standardization is needed particularly for design of multi-centre randomized clinical trials.
Dose fractionation radiotherapy trials in head and neck cancer over the past 20 years have established clinical benefits for a variety of modified fractionation schedules compared to the conventional 2 Gy per day to a total of 70 Gy over 7 weeks. When concurrent chemoradiation is used, there is further variation over the choice of optimum fractionation schedule. IMRT, with its potential for Simultaneous Integrated Boost (SIB), further adds to this uncertainty Citation[1] such that there is an increasing lack of uniformity of practice with no single ‘standard of care’.
In SIB, each target volume is treated to the same number of fractions and therefore receives a different dose per fraction. Planning studies suggest that this method produces the most conformal dose distribution compared with using sequential IMRT plans Citation[2]. Although this offers the distinct advantage of tailoring the appropriate dose to each target volume according to risk, it is a departure from the uniform fraction size conventionally employed.
There are now several published series of patients treated with head and neck IMRT which together demonstrate a plethora of fractionation regimes. It is apparent that single institutions are developing not only protocols that are ‘in-house’ but which are also evolving and changing with experience. We aim to examine the international practice of IMRT used for head and neck cancer through a survey of IMRT dose fractionation currently used in cancer centres around the world. A radiobiological comparison of the schedules is made. The variability of IMRT practice that currently exists is discussed with suggestions of possible reasons for this diversity in approach.
Material and methods
A postal survey was sent out in April 2006 to enquire about the IMRT dose fractionation used for the definitive treatment of head and neck cancer in 18 international cancer centres. Clinicians that were approached were those felt to be from centres which had contributed significantly to recent published literature on the radiotherapy practice for head and neck cancer. In the questionnaire, each centre was asked about the number of target volumes employed, mean dose to CTVs, number of fractions, Gross Target Volume (GTV) expansion to Clinical Target Volume (CTV) and use of concurrent systemic agents. We defined the target volumes as follows: CTV1=GTV with margin for microscopic spread, CTV2=organs and nodes at high risk of microscopic spread (e.g. 1st station lymph nodes or post operative node +ve regions), CTV3=surgically unperturbed or electively treated nodes at modest risk of microscopic disease.
The aim of radiotherapy is to deliver a high dose to the tumour sufficient to eradicate gross disease whilst limiting the total dose to mucosa to ensure that patients complete treatment without unplanned interruptions. Furthermore, normal tissue at risk of late effects must be protected from exceeding radiation tolerance. These factors were all evaluated and compared for each radiotherapy schedule according to three radiobiological criteria Citation[3]:
Acute Mucosal Biologically Effective Doses (BED)
Tumour BED
Late Tissue BED
Fowler et al. estimated an acute mucosal BED threshold level of 61 Gy10 (grey zone of 59-61 Gy10) for intolerable acute oral and pharyngeal mucosal reactions from radiotherapy only treatments. This threshold was developed from analysing clinical data from conventional radiotherapy trials and therefore is provisional for any new radiotherapy modality such as IMRT Citation[4]. His suggested parameters and BED formula () were applied to calculate the acute mucosal BED for comparing between schedules: α=0.35 Gy−1, α/β=10 Gy, Tk=7days, Tp=2.5days Citation[4]. The first treatment is on day 0 rather than on day 1 onwards when calculating overall treatment time. All treatments were assumed to commence on a Monday.
For calculation of tumour BED, the following parameters were applied to the linear quadratic formula: α/β=10 Gy, Tk=21days, k=0.66 Gy/day. When calculating late tissue BED, α/β was 3 Gy and the threshold was taken to be 117 Gy3 based on rates of late toxicity when using conventional 70 Gy delivered in 2 Gy/fraction. Both tumour and acute mucosal BED were corrected for overall treatment time but late tissue BED was not. Log10 cell kill is calculated using calculated tumour BED multiplied by α (0.35 Gy−1) and this number divided by 2.303 Citation[3].
Results
A total of 14 head and neck clinicians (including our centre), responded to the survey and returned the questionnaire (). Schedules were available for comparison from nine European cancer centres four from North America and one from Japan.
Table I. IMRT dose fractionation reported in survey.
Dose fractionation
A total of 12 different schedules were reported from the survey (). More than one schedule was used in two centres, either because of trials or according to departmental treatment protocol tailored to different indications. Conventional fractionation of 2 Gy per fraction daily for a total of 7 weeks, considered by many as the standard of care, was only used in three centres. The majority of the other centres surveyed used altered fractionation
In centres that used altered fractionation, three centres selected an accelerated schedule of 6 fractions/ week delivering 66–70 Gy based on the DAHANCA regime Citation[5]. In another three centres, dose per fraction was increased up to 2.2 Gy per fraction. An even higher dose per fraction was used for four centres, including our own, for phase I radiotherapy dose escalation trials. The concomitant boost schedule employing a 2 phase treatment was used for IMRT in 1 centre surveyed. In the one centre where hyperfractionation schedule was used, patients are treated continuously twice daily for 10 days followed by a week's break and then another 10 days of twice daily fractions to complete the treatment.
The dose escalated hypofractionated schedules are all being used within a phase I study design in each of the four centres to investigate its safety and tolerability. The MD Anderson Cancer Centre is using the accelerated schedule of 6 fractions per week as part of the RTOG 0522 protocol. The other dose fractionation reported as part of clinical trials were by Shirato, De Neve Citation[6], Nutting Citation[7], Citation[8] and Slevin.
The median total overall treatment time for all the schedules used for primary radiotherapy is 39 days (range 25 – 47 days) or 6 weeks. Dose per fraction to the PTV1, PTV2 and PTV3 ranged between 1.5 to 3.0 Gy (median 2.0 Gy), 1.4 to 2.3 Gy (median 1.8 Gy) and 1.15 to 2 Gy (median 1.8 Gy) respectively.
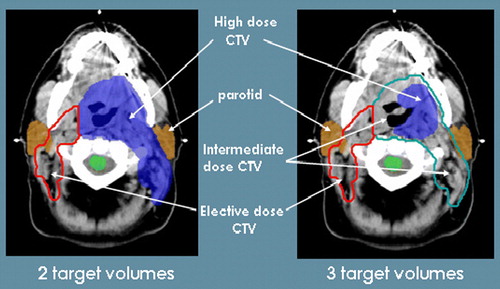
Nine of the fourteen centres incorporated an intermediate dose to a moderate risk target volume for a primary radiotherapy treatment plan to make a total of 3 dose levels (). Eight centres reported using the same number of fractions for all target volumes. The other six centres reported using different number of fractions and dose per fraction mainly for prophylactic PTV3 where an anterior neck field can be matched on to the IMRT plan.
Biologically Equivalent Dose (BED) comparison
ranks the schedules according to the tumour BED delivered. At least half of the centres were using schedules that delivered a high tumour dose of above 11 log10 cell kill. The dose escalated fractionation delivered the highest tumour BED and unsurprisingly, also delivered a high late tissue BED, acute mucosal dose or both. The top tumour cell kill was achieved by De Neve through a dose escalation study treating the high dose volume to 72.5–77.5 Gy in 32 fractions delivering a very high log10 cell kill of 12.5 or tumour BED of 82.2 Gy. The first phase consisted of dose escalation to a focal FDG-PET defined region to a dose of 25 Gy (dose level 1) and 30 Gy (dose level 2) in 10 fractions. The second phase delivered 47.5 Gy in 22 fractions (2.16 Gy per fraction) using a sequential IMRT plan Citation[6]. The potential of the top 3 schedules for tumour cell kill in this survey has already exceeded (>11.25 log10 cell kill) the tumour cell kill ability from the top head and neck schedules previously ranked by Fowler Citation[3].
Table II. Radiobiological comparison of schedules.
Four of the schedules lay in the ‘grey zone’ (59–61 Gy10) for intolerable acute oral and pharyngeal mucosal reactions with 2 schedules markedly (by ∼10%) above this provisional limit of 61 Gy10. If a contribution of 3–5 Gy10 by systemic chemotherapy toward this threshold was taken into account, the majority of the schedules will either be in or exceeding this provisional limit Citation[4].
The majority of the schedules were used within a multi-modality treatment strategy for locally advanced stage disease. Centres that used synchronous chemotherapy all preferred platinum based chemotherapy, most commonly Cisplatin single agent while 2 other centres reported the use of combination doublet chemotherapy. All the 5 centres using conventional dose fractionation of 70–72 Gy in 2 Gy per fraction treating 5 times a week used synchronous chemotherapy. One centre combined nimorazole and Cisplatin with their modestly accelerated radiotherapy schedule. Two centres were using synchronous cetuximab including one centre participating in a chemoradiation trial randomised to with or without cetuximab (RTOG 0522). Only 2 centres were deploying IMRT as a single modality primary treatment with both using altered fractionation.
ranks the schedules according to the calculated acute mucosal dose and looks at any correlation between how ‘hot’ a schedule is with the decision to deliver concurrent treatment, number of target volumes and margins around CTV. Only the top dose level is displayed if there is a 2 dose level escalation study. For comparison, schedules are divided into those that lie above or within the ‘grey zone’ of acute mucosal BED as proposed by Fowler et al. Citation[4].
Table III. Acute mucosal tolerance factors.
Six centres were using schedules that lay in or above the acute mucosal BED ‘grey zone’ (59–61 Gy10) including 2 dose-escalated schedules, delivering 66.9 Gy10 and 68.3 Gy10, that were well beyond this provisional limit. Four of these six centres were also using systemic chemotherapy with their schedule. Eight of ten centres below this provisional limit listed systemic chemotherapy as part of the treatment. Late tissue BED exceeds 117 Gy3 in 7 of these schedules. When comparing dose to late tissues, despite the relatively high late tissue BED (>125Gy3) for 3 of the schedules, Cisplatin synchronous chemotherapy was used in these 3 schedules.
Minimum margins placed around a GTV to form the CTV as per ICRU 50 and 62 varied considerably, mainly ranging between 0.3 – 1 cm with a maximum of 2 cm stipulated by two centres. Four respondents did not specify any figures and indicated that their margins were either variable according to the clinical case or expanded to the anatomical compartment within which the GTV is contained. For the respondents who defined a minimum margin, half (5 centres) selected <1 cm with the other half (5 centres) preferred a minimum margin of 1 cm. Margins used for CTV expansion to PTV were not surveyed.
Discussion
The implementation of IMRT not only involves delivering highly conformal non-uniform beams but demands a whole new approach to the treatment planning process, right from initial patient immobilisation to accurate beam delivery and verification. Factors at each stage will influence the selection of a preferred dose fractionation.
We wish to emphasise that the radiotherapy schedules, systemic agents used and margins applied to target volumes reported are not intended to justify or support any one particular approach for IMRT. Furthermore, the institutions surveyed may not have fully specified the entire IMRT protocol used due to specific indications for individual dose fractionation. We recognise that any survey, however comprehensive, may not fully represent the entire picture of what is ultimately a complex treatment. However, we feel that a comparison and discussion of the radiotherapy schedules kindly provided by the respondents can provide important and informative views of the variability of the IMRT dose fractionation currently in use.
Dose escalation
There is a well established steep dose response relationship in head and neck carcinoma for local tumour control which translates into improved survival. It is well accepted that the increase (or decrease) of loco-regional control in head and neck cancer (near the middle of a dose-response curve) is approximately 1.7% of loco-regional control per 1% change in total dose (expressed as Normalised Tumour Dose in 2 Gy fraction equivalents; and this means 1.2% of tumour BED), assuming that α/β is 10 Gy for tumours Citation[9]. However at higher doses, increased levels of acute normal tissue toxicity become dose limiting with conventional radiotherapy techniques. From the survey, half of the centres were already attempting to deliver more than 11 log10 cell kill using IMRT despite some schedules delivering a high acute mucosal BED beyond the provisional limit (61 Gy10) estimated by Fowler et al. Citation[4]. This acute mucosal BED constraint is by no means definitive but can be useful to provide a safety threshold beyond which any higher mucosal BED needs to be considered with caution and to exceed only gradually.
In the three centres investigating a 2 dose level escalation, the schedules selected have followed a strategy of increasing dose per fraction (mean 2.6 Gy/fraction) and reducing overall treatment time (mean 37.5 days). On the other hand, there were different approaches to the use of synchronous systemic treatment. De Neve and Nutting are both using synchronous Cisplatin chemotherapy, especially for patients with locally advanced disease. Although chemoradiation may be the standard treatment for locally advanced disease, local control may be improved with IMRT dose escalation rather than adding chemotherapy to radiotherapy with its unpredictable enhanced toxicities. Combination of cetuximab with radiotherapy is reported not to be associated with increase in acute radiation toxicity (e.g. mucositis) compared with radiotherapy alone (in contrast to synchronous chemotherapy) and is therefore ideal for study in a radiotherapy dose escalation study Citation[10].
Total volume
It is well accepted that acute radiotherapy toxicity correlates with total irradiated volume. Mucositis has always been a crucial dose limiting factor for radiotherapy and Fowler et al. estimated a provisional limit (61 Gy10) beyond which acute mucosal reaction becomes intolerable Citation[4]. However, this limit was based on conventional treatment planning and does not take into account field size or treatment volume which was frequently relatively large. IMRT is superior to conventional planning and delivery in terms of the closeness of fit to the desired dose map potentially leading to smaller target volumes and therefore, as Fowler predicted, IMRT is already testing this mucosal tolerance threshold.
However, there has been little research into the actual impact of IMRT on acute mucositis and no randomised study comparing conventional conformal radiotherapy and IMRT in sparing mucositis. A comparison of 2 series of patients, found that regardless of treatment technique, mucositis remained a severe acute reaction for any patient having to receive head and neck radiotherapy Citation[11].
Total treatment volume can an important consideration when selecting an appropriate dose fractionation although a comparison of treatment volumes between each centre is beyond the scope of this survey. Ultimately, many head and neck patients need to have extensive PTV coverage and hence spare relatively little mucosa compared to conventional treatment. Bernier and Grau will vary the total dose slightly (+/−2 GyGy) based on the total treatment volume. Another centre (Maciejewski) used a continuous accelerated (CAIR) schedule of 72 Gy in 40 fractions for small to average treatment volume but preferred to offer patients hyperfractionated radiotherapy for irradiating large treatment volumes. However, radiobiological modelling () shows that the hyperfractionation regime in fact delivers a higher mucosal dose (59.9 Gy10) compared to the CAIR schedule (58.8 Gy10). The PET guided radiotherapy used in the dose escalation study by De Neve recognizes the pitfalls of irradiating too large a high dose volume and therefore limits the dose escalated PTV to 10 cm3 Citation[6].
Number of target volumes
SIB fully utilizes the potential of IMRT by tailoring the dose to each tissue volume according to risk. Theoretically, there can be any number of target volumes although in practice, most radiotherapists will delineate not more than 3 target volumes. Regions at high risk of microscopic spread, traditionally treated to a radical dose, can be encompassed within an intermediate dose target volume which reduces the total volume receiving a high dose. By restricting the high dose volume, acute radiotherapy toxicity can be reduced and GTV can potentially be dose escalated.
From the survey, however, clinicians seem to be divided on the benefit of an intermediate target volume in primary IMRT with many opting for the traditional 2 target volumes approach. The type of schedule used did not seem to influence the decision of whether each centre chose 2 or 3 dose levels. This may be due to the absence of any randomized IMRT studies comparing patterns of recurrence between the use of 2 or 3 target volumes. Secondly, although a sub-radical dose is acceptable and commonly used for post-operative regions at high risk of microscopic disease, there is limited evidence to indicate that this dose is sufficient to eradicate microscopic disease around a GTV or in a first station lymph node level. To date, in published case series of patients treated with 3 target volumes IMRT, the area of recurrence remains mainly in the high dose region but further follow-up is awaited to show that an intermediate dose does not compromise tumour control Citation[12], Citation[13]. Additionally, the definition of an intermediate target volume is unclear. One respondent found it difficult to define an intermediate zone where an elective dose of 50 Gy is inadequate and where a definitive dose of 70 Gy potentially over treats; and hence preferred to continue using just 2 target volumes. Another respondent remarked that CTV1 is difficult enough to delineate without even considering an intermediate target volume yet. This is likely due to a lack of confidence in the accurate localization of GTV and consequently is compensated by a larger high dose volume. It remains to be seen if promising imaging modalities like FDG-PET can help to localize gross disease more accurately and make an impact on target volume delineation. Early work suggests that GTV drawn with FDG-PET guidance is smaller than with CT or MR and where there are surgical specimens for comparison, perhaps more accurate Citation[14].
Systemic synchronous treatment
Chemoradiation is now widely adopted for treating locally advanced disease but the optimal radiotherapy schedule to be used with synchronous chemotherapy remains unclear. Although altered fractionation remains an attractive option due to its superior tumour control rates, its role in chemoradiation is even more uncertain as this combination is regarded by some as intolerable for routine use and may be the reason why some of these schedules were delivered without chemotherapy.
Fowler et al. estimates that concurrent chemotherapy adds an equivalent of 3–5 Gy10 to the acute mucosal dose Citation[4]. Centres that were using schedules delivering mucosal dose at or above the threshold for mucosal tolerance seemed to be less likely to use systemic chemoradiation compared to those centres using less intensive schedules in our survey (4/6 vs 8/10) although this did not approach statistical significance due to the small numbers (p=0.60, Fisher's Exact Test). Centres that offered hyperfractionation did not use synchronous chemotherapy although the addition of chemotherapy to hyperfractionation has shown evidence of improved local control compared with hyperfractionation alone Citation[15–17]. However, adding in synchronous chemotherapy into hyperfractionation, which already appears to fall into the threshold grey zone of uncertainty (59–61 Gy10), can potentially push the acute mucosal dose beyond this threshold resulting in intolerable acute mucositis, treatment gaps or consequential normal tissue damage.
Overall treatment time
Shorter overall treatment time is advantageous for tumour control in head and neck carcinoma to overcome accelerated repopulation of tumour clonogens Citation[18], In SIB, the approach of using a higher dose/fraction conveniently adds the advantage of a shorter course of treatment. Overall, IMRT schedules surveyed showed a considerably shorter overall treatment time (median 39 days) than the conventional 70 Gy delivered over 7 weeks (46 days).
Treatment planning and delivery
Planning of IMRT demands considerable resources in planning and verification which restricts the choice of schedules requiring two or more plans such as the concomitant boost technique where a separate plan is required for the second phase boost. The dose escalation schedule by De Neve also requires a 2 phase IMRT treatment plan which may make this type of schedule more difficult to implement.
IMRT plans require multiple beams, sometimes in excess of 10 beams, which means a prolonged immobilization time for the patient on the treatment couch. Furthermore, clinicians may employ complex patient positioning systems that requires patient immobilization up to 35 minutes Citation[19]. This may increase patient discomfort leading to increased intra-fraction movement and reduced treatment accuracy. A longer treatment couch time may favour schedules using less number of fractions which is probably reflected in the RTOG-0522 study where a 6 fractions/week schedule (35 fractions) was preferred for IMRT while centres using 3D-conformal radiotherapy were recommended to use the concomitant boost schedule (42 fractions) Citation[20]. Prolonged treatment time may result in loss of efficiency in tumour cell kill due to purported intracellular repair in 1/2 hour or more Citation[21].
GTV to CTV margins
There is undoubtedly a wide variation in margins applied around GTV to form the CTV in this survey reflecting the lack of research into what defines an optimal GTV margin. An analysis of the extent of microscopic disease around an involved lymph node has been reported and a minimum of 1 cm was proposed to account for extracapsular spread Citation[22]. However, there is a lack of corresponding data to make any recommendations when delineating the primary CTV. Some respondents believe that entire tissue volumes bounded by natural barriers (or compartments) such as fascial planes should be treated while others may employ a larger margin to avoid local recurrence. If a larger head and neck total volume is irradiated, a less intense dose fractionation is sometimes used ostensibly to enable patients to complete treatment safely. In our survey, however, there do not seem to be any association between margins applied around the GTV and BED doses to the mucosa or late tissues.
Geometric margins
Implementation of IMRT, especially when escalating treatment dose, demands highly accurate imaging for verifying treatment position. Lack of confidence in consistent reproducibility of patient setup may result in wrapping a larger margin around the CTV for expansion to PTV. This is increasingly an important issue when the use of intensive altered fractionation combined with chemotherapy is expanding. This increases the risk of significant weight loss and rapid tumour shrinkage, leading to a change in planned dose distributions where at present, guidance on whether and when re-planning should be carried out is lacking Citation[23]. In our survey, Shirato reports using 3 gold fiducial markers in a precise mouthpiece with 2 sets of fluoroscopy for real time tumour tracking to enable treatment with a markedly dose escalated IMRT schedule in his study Citation[19]. Harari (University of Wisconsin) reports using optical guidance in his centre with continuous monitoring and thereby justifying a small geometric expansion margin of 3 mm.
Late tissue toxicity
The SIB delivery of a higher than 2 Gy/fraction to the CTV1 is a concern for radiotherapists worried about late tissue toxicity. This is especially important in IMRT where relatively more inhomogeneity within the target volume will result in higher dose/fraction than prescribed Citation[24]. However, this survey indicated that nearly half of the centres selected a schedule with a higher than conventional dose/fraction, especially for 2 level dose escalation studies. Keeping high dose target volumes small with IMRT and utilizing 3 target volumes may be a factor why this strategy was considered. In addition, we look forward to learning whether schedules that deliver a high late tissue BED, especially the top 4 schedules () delivering >125 Gy3, can be maintained after longer follow-up.
Although the use of synchronous chemotherapy is widely recognised to increase late tissue toxicity, relatively little is known of its actual consequences. Hence, the addition of chemotherapy to already markedly dose escalated schedules delivering a high late tissue BED merits careful scrutiny. Even if IMRT can reduce dose to tissues and organs outside of target volumes, normal tissue embedded within the target volume will inevitably risk long-term damage.
Lower neck irradiation
Conventional elective dose to the lower neck is 50 Gy/25 fractions or 40Gy/15 fractions using a matched-on anterior field. One of the key advantages of IMRT is the ability to treat an entire volume in a single plan which reduces the uncertainty of matching on an anterior neck field or a posterior neck electron beam. To enable this approach using a conventional schedule of 35 fractions, at least 56–60 Gy will have to be delivered to the lower neck over the same number of fractions. This dose has not been validated to be an adequate prophylactic dose especially when the dose per fraction is much lower (1.6–1.7 Gy/fraction). Concerns have been expressed about this low dose/fraction; in order to maintain a daily dose/fraction of at least 1.8 Gy, either a smaller than conventional number of fractions (≤30 fractions) or separate sequential IMRT plans will have to be considered Citation[25].
Conclusions
There is wide variation in the dose and fractionation used internationally in head and neck IMRT. There is perhaps a larger variation in the results reported due to the sampling of more research-oriented centres and therefore more likely to modify dose fractionation.
Based on this survey, we have surmised that the variables include (i) the desire to dose escalate; (ii) total irradiated volume; (iii) number of target volumes delineated; (iv) synchronous use of systemic treatment; (v) shortening of overall treatment time; (vi) availability of resources for IMRT planning; (vii) longer time on treatment couch; (viii) variable GTV margins; (ix) confidence in treatment position setup; (x) late tissue toxicity concerns and (xi) use of lower neck anterior fields.
Together with a remarkably heterogeneous variation in head and neck IMRT target delineation Citation[26] and substantial discrepancy in dose prescribing during planning Citation[27], it becomes even more difficult to make any meaningful comparison of IMRT treatment results. Some standardization is needed particularly for design of randomized multi-centre clinical trials.
Acknowledgements
We thank the respondents () who kindly returned our survey questionnaires and helped to answer any subsequent queries. There are no conflicts of interest.
References
- Allen AM, Tishler RB. Commentary: IMRT for head and neck cancer: Many chapters left to write. Oncologist 2007; 12: 565–8
- Mohan R, Wu Q, Manning M, Schmidt-Ullrich R. Radiobiological considerations in the design of fractionation strategies for intensity-modulated radiation therapy of head and neck cancers. Int J Radiat Oncol Biol Phys 2000; 46: 619
- Fowler JF. Is there an optimum overall time for head and neck radiotherapy? A review, with new modelling. Clin Oncol 2007; 19: 8
- Fowler JF, Harari PM, Leborgne F, Leborgne JH. Acute radiation reactions in oral and pharyngeal mucosa: Tolerable levels in altered fractionation schedules. Radiother Oncol 2003; 69: 161
- Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet 2003; 362(9388)933–40
- Madani I, Duthoy W, Derie C, De Gersem W, Boterberg T, Saerens M, et al. Positron emission tomography-guided, focal-dose escalation using intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2007; 68: 126–35
- Guerrero Urbano T, Clark CH, Hansen VN, Adams EJ, A'Hern R, Miles EA, et al. A phase I study of dose-escalated chemoradiation with accelerated intensity modulated radiotherapy in locally advanced head and neck cancer. Radiother Oncol 2007; 85: 36–41
- Guerrero Urbano MT, Clark CH, Kong C, Miles E, Dearnaley DP, Harrington KJ, et al. Target volume definition for head and neck intensity modulated radiotherapy: Pre-clinical evaluation of PARSPORT trial guidelines. Clin Oncol 2007; 19: 604
- Okunieff P, Morgan D, Niemierko A, Suit HD. Radiation dose-response of human tumors. Int J Radiat Oncol Biol Phys 1995; 32: 1227–37
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354: 567–78
- Elting LS, Isitt J, Murphy BA, Garden AS, Gwede CK, Trotti A, et al. 2428: Retrospective and prospective studies of the severity of Oral Mucositis (OM) in Intensity Modulated Radiation Therapy (IMRT) compared to conventional radiation therapy in head and neck cancer (HNC) patients. Int J Radiat Oncol Biol Phys 2006; 66(3 Suppl 1)S447
- Garden AS, Morrison WH, Wong PF, Tung SS, Rosenthal DI, Dong L, et al. Disease-control rates following intensity-modulated radiation therapy for small primary oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2007; 67: 438–44
- Yao M, Chang K, Funk GF, Lu H, Tan H, Wacha J, et al. The failure patterns of oral cavity squamous cell carcinoma after intensity-modulated radiotherapy-the University of Iowa experience. Int J Radiat Oncol Biol Phys 2007; 67: 1332–41
- Daisne JF, Duprez T, Weynand B, Lonneux M, Hamoir M, Reychler H, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: Comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology 2004; 233: 93–100
- Brizel DM, Albers ME, Fisher SR, Scher RL, Richtsmeier WJ, Hars V, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med 1998; 338: 1798–804
- Jeremic B, Shibamoto Y, Milicic B, Nikolic N, Dagovic A, Aleksandrovic J, et al. Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: A prospective randomized trial. J Clin Oncol 2000; 18: 1458–64
- Huguenin P, Beer KT, Allal A, Rufibach K, Friedli C, Davis JB, et al. Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J Clin Oncol 2004; 22: 4665–73
- Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol 1988; 27: 131–46
- Oita M, Ohmori K, Obinata K, Kinoshita R, Onimaru R, Tsuchiya K, et al. Uncertainty in treatment of head-and-neck tumors by use of intraoral mouthpiece and embedded fiducials. Int J Radiat Oncol Biol Phys 2006; 64: 1581–8
- http://www.rtog.org/members/protocols/0522/0522.pdf.
- Fowler JF, Welsh JS, Howard SP. Loss of biological effect in prolonged fraction delivery. Int J Radiat Oncol Biol Phys 2004; 59: 242–9
- Apisarnthanarax S, Elliott DD, El-Naggar AK, Asper JA, Blanco A, Ang KK, et al. Determining optimal clinical target volume margins in head-and-neck cancer based on microscopic extracapsular extension of metastatic neck nodes. Int J Radiat Oncol Biol Phys 2006; 64: 678–83
- Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: Promises and pitfalls. J Clin Oncol 2006; 24: 2618–23
- Eisbruch A. Intensity-modulated radiation therapy in the treatment of head and neck cancer. Nat Clin Pract Oncol 2005; 2: 34–9
- Yao M, Dornfeld KJ, Buatti JM, Skwarchuk M, Tan H, Nguyen T, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma–the University of Iowa experience. Int J Radiat Oncol Biol Phys 2005; 63: 410
- Hong TS, Tome WA, Chappell RJ, Harari PM. Variations in target delineation for head and neck IMRT: An international multi-institutional study. Int J Radiat Oncol Biol Phys 2004; 60: S157
- Das IJ, Cheng CW, Chopra KL, Mitra RK, Srivastava SP, Glatstein E. Intensity-modulated radiation therapy dose prescription, recording, and delivery: Patterns of variability among institutions and treatment planning systems. J Natl Cancer Inst 2008; 100: 300–7