To the Editor
The epidermal growth factor receptor (EGFR) is a promising target for anticancer therapy because it is expressed in a variety of tumors including non-small-cell lung cancer (NSCLC), and elevation of serum levels of EGFR expression has been associated with a poor prognosis. Gefitinib is an orally active anilinoquinazoline compound which inhibits EGFR tyrosine kinase activity. Previous studies of the gefitinib monotherapy for NSCLC patients showed a significant antitumor activity with the response rate of around 20% and acceptable toxicities even for elderly patients Citation[1–5]. Recently, EGFR-mutant tumors were found to yield a high sensitivity to gefitinib as observed in preclinical and clinical studies Citation[6], Citation[7]. Surprisingly, most responders to gefitinib monotherpay showed rapid tumor regression, with 68% meeting the criteria for objective response by the first postbaseline assessment and the remaining patients met the criteria in the second, third, or fourth month following randomization Citation[8]. Indeed, there have been no reports showing an objective response to gefitinib after long-term treatment. In this report, we showed a case of advanced NSCLC in whom a partial response to gefitinib was finally identified 14 months after achieving disease stabilization.
Case report
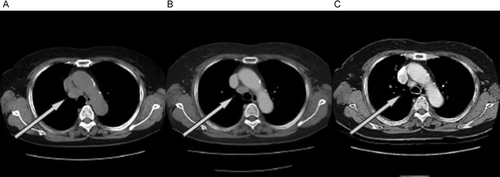
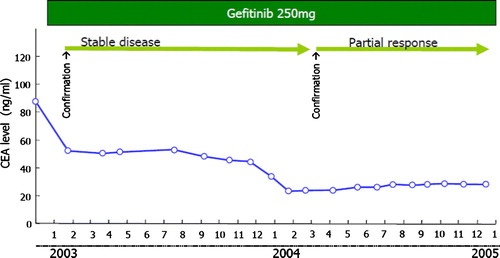
A 76-year-old woman was referred to our department in November 2002 because of an abnormal chest shadow (A) and was diagnosed with stage IV adenocarcinoma of the lung (T1N2M1) with pulmonary metastasis. The mutation analysis by enriched polymerase chain reaction method revealed that the tumor possessed the deletion mutation in the exon 19 of EGFR gene. Treatment with gefitinib 250 mg/day was initiated in January 2003 as first-line therapy because she was considered unfit for platinum-based chemotherapy. The patient obtained stable disease (20% regression) as an overall response 2 months after the start of gefitinib treatment, as defined by standard Response Evaluation Criteria In Solid Tumors (RECIST) guidelines (B). The patient continued to receive gefitinib because of this favorable disease control coupled with symptom improvement and the absence of severe adverse events. Around January 2003, serum level of carcinoembryonic antigen (CEA) gradually decreased (). Unexpectedly, after this reduction in serum CEA level, radiological tumor regression was observed in March 2004, which was defined as a partial response by RECIST (32% regression; C). In July 2006, the primary and medistinal tumors progressed with the elevation of the CEA level with 43.42 ng/ml. The gefitinib treatment was withdrawn and switched to the vinorelbine monotherapy.
Discussion
We experienced a very exceptional case; a partial response was achieved 14 months after the initiation of gefitinib treatment in our patient, in contrast to the majority of responses to gefitinib (250 mg/day), which occurred within the first 4 weeks of treatment Citation[8]. As potential reasons explaining this observation, (i) sensitivity to gefitinib might naturally change over time Citation[9] although no definitive mechanisms were shown so far, and (ii) the tumor might have originally possessed somewhat different biological characters from other NSCLC tumors. Further investigation will be warranted to elucidate the mechanism underlying this phenomenon.
We previously reported that in NSCLC patients obtaining disease stabilization, those who continued gefitinib treatment until disease progression tended to have longer overall and progression-free survival compared with those discontinuing gefitinib treatment (1-year survival rate 52.1% versus 36.6%, p=0.08; 1-year progression-free survival rate 31.8% versus 5.2%, p<0.01) Citation[5]; the pattern of sensitivity to gefitinib in the case presented here might give us one of the potential clues to understanding of this advantage of the continued gefitinib treatment.
Monitoring of serum tumor marker levels were reported to predict tumor response in previous papers. Massacesi et al. demonstrated that response of serum CEA level to chemotherapy correlated well with subsequent radiological response in patients with metastatic cancer including lung cancer Citation[10]. In our case, the decline of serum CEA level was also observed 2 months before radiological response. Usefulness of tumor markers as predictive factors of tumor response should be reappraised in future studies Citation[11].
In conclusion, this patient obtained an unprecedented response to gefitinib as first-line treatment. It suggests that physicians should be aware of potential objective responses to gefitinib even after the tumor seems to be no more reduced in size with the best overall response of stable disease.
References
- Hotta K, Kiura K, Takigawa N, Fujiwara Y, Tabata M, Ueoka H, et al Association of the benefit from gefitinib monotherapy with smoking status in Japanese patients with non-small-cell lung cancer. Lung Cancer ( in press).
- Hotta K, Kiura K, Ueoka H, Tabata M, Fujiwara K, Kozuki T, et al Effect of gefitinib ('Iressa’, ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer 2004;46:255–61.
- Hotta K, Ueoka H, Kiura K, Tabata M, Ogino A, Umemura S, et al. Safety and efficacy of gefitinib treatment in elderly patients with non-small-cell lung cancer: Okayama Lung Cancer Study Group experience. Acta Oncol 2005; 44: 717–22
- Hotta K, Kiura K, Takigawa N, Kuyama S, Segawa Y, Yonei T, et al Sex difference in the influence of smoking status on the responsiveness to gefitinib monotherapy in adenocarcinoma of the lung: Okayama Lung Cancer Study Group experience. J Cancer Res Clin Oncol ( in press).
- Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Harita S, et al. Continued gefitinib treatment after disease stabilisation prolongs survival of patients with non-small-cell lung cancer: Okayama Lung Cancer Study Group Experience. Ann Oncol 2005; 16: 1817–23
- Hotta K, Kiura K, Toyooka S, Takigawa N, Soh J, Fujiwara Y, et al. Clinical significance of epidermal growth factor receptor gene mutations on treatment outcome after first-line cytotoxic chemotherapy in Japanese patients with non-small-cell lung cancer. J Thorac Oncol 2007; 2: 632–7
- Hotta K, Tabata M, Kiura K, Kozuki T, Hisamoto A, Katayama H, et al. Gefitinib induces premature senescence in non-small-cell lung cancer cells with or without EGFR gene mutation. Oncol Rep 2007; 17: 313–7
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol 2003; 21: 2237–46
- Kurata T, Tamura K, Kaneda H, Nogami T, Uejima H, Asai Go, et al Effect of re-treatment with gefitinib (‘Iressa’, ZD1839) after acquisition of resistance. Ann Oncol 2004;15:173–4.
- Massacesi C, Rocchi MB, Marcucci F, Pilone A, Galeazzi M, Bonsignori M. Serum tumor markers may precede instrumental response to chemotherapy in patients with metastatic cancer. Int J Biol Markers 2003; 18: 295–300
- Hotta K, Kiura K, Tabata M, Takigawa N, Fujiwara Y, Umemura S, et al. Role of early serial change in serum carcinoembryonic antigen levels as a predictive marker for radiological response to gefitinib in Japanese patients with non-small cell lung cancer. Anticancer Res 2007; 27: 1737–41