Abstract
Introduction. About 25% of patients with rectal cancer have incurable disease at the time of diagnosis. In the current study from Western Norway (population of 981 000) we focused on the utilisation of specialist care in patients with primarily incurable rectal cancer. Patients and methods. Between 1997 and 2002, 1 167 patients were diagnosed with rectal cancer, of whom 297 (25%) had incurable disease, according to consecutive and prospective reporting to the Norwegian Colorectal Cancer Registry. Consumption of specialist care facilities was studied with regard to outpatient contacts, hospital admissions, and various treatment modalities. Data were analysed with regard to age, sex, marital status, type of residence, and geographical access to hospital facilities. Data were available for 287 patients (97%). Results. The median age was 77 years. Elderly patients (>77 years) more often lived in nursing homes without a spouse. About 60% of the patients were treated with major surgery, chemotherapy or radiotherapy, either alone or in combination. Of those who did not receive such treatment, 87% were elderly. Oncological treatment, either alone or combined with surgery, predicted increased hospital admissions and outpatient contacts. Age >77 years predicted fewer hospital admissions. Survival varied statistically significantly with the various treatment modalities, and was highest for major resections combined with oncological treatment. The majority of the patients living at home died in hospitals (54%) and only 26% died in their homes, while two-thirds of residents of nursing homes died there. Discussion. Patients with primary incurable rectal cancer are heterogeneous with regard to their needs of treatment. While younger patients receive extensive tumour-related treatment, elderly patients are most commonly treated according to their symptoms. Prospective studies of the effect of various treatment options on the ease of symptoms and improved quality of life in unselected populations are needed.
Colorectal cancer (CRC) is one of the most frequent malignancies in the Western world Citation[1]. Rectal cancer (RC) in particular has traditionally been associated with high morbidity and mortality, with local recurrence encountered in about one-third of patients Citation[2]. Current standards for staging and treatment of RC have improved the prognosis during the past decade Citation[3]. Nevertheless, at the time of diagnosis, 25% of the patients are incurable due to locally advanced disease, distant spread, or both Citation[4]. These patients can only be offered treatment options focusing on prolonged survival, prevention or relief of symptoms. An increasing incidence of RC in Norway together with the aging of the general population make it likely that the need for palliative care for RC patients will increase in the future Citation[5]. Patients with incurable RC are classified as stage IV, but they differ considerably with regard to demographics, functional status, and their individual disease symptoms. The scientific literature dealing with this group of RC patients is sparse, particularly what concerns their needs for specialist care. In addition, patient selection and publication bias, lack of definition of treatments employed, and lack of well-defined end-points make it difficult to compare results Citation[6]. As a consequence, the core knowledge for adequate palliative treatment of RC is hard to retrieve.
In the current population based study, we describe hospital resource consumption in patients with incurable rectal cancer assessed by hospital admissions and out-patient visits. In addition, some treatment aspects, and place of death are addressed.
Patients and methods
The Western Norway region has a population of 981 000, which is one-fifth of the total population of Norway. About half of the population in Western Norway live in urban areas, and the other half live in rural or coastal environments. Due to the geography and infrastructure, including fjords and roads, access to hospitals may involve long journeys. In Western Norway, there are two major referral centres which provide specialist health care; Haukeland University Hospital, Bergen, and Stavanger University Hospital, Stavanger. In addition, five local hospitals add to the general in-hospital palliative care capacity.
The Norwegian Colorectal Cancer Registry contains data on patient demographics, tumour characteristics, treatments, and outcomes based on the mandatory registration and reporting of all malignant diseases to the Norwegian Cancer Registry Citation[7]. All patients in Western Norway diagnosed with RC between 1997 and 2002 were identified, and patients registered with non-curative treatment intent were selected. Data on use of in-hospital resources was retrieved and recorded by retrospective evaluation of hospital records, outpatient charts, and patient administrative data at each hospital in our region (see Appendix).
Definitions
Palliative treatment was defined as treatment employed by patients beyond cure, due to either locally advanced and/or disseminated disease at the time of diagnosis, incomplete resection of the tumour (R2 resection), or when neo-adjuvant preoperative treatment failed to induce curable disease (R0 resection) and the treatment intent was changed from curative to palliative. Accordingly, patients treated with curative intent for local recurrence or distant metastases (e.g. liver or lungs) were excluded from our study. Treatment employed included tumour related modalities, i.e. surgery, radiation and chemotherapy, and was classified with regard to oncologic treatment (i.e. chemotherapy and/or radiotherapy), surgery (i.e. major resection), both oncological and surgery, and none of those. Best supportive care included all other not-tumour-related measures directed to the individual patient's symptoms. The median age was used to stratify age groups of elderly and younger patients. Marital status was defined as living with a spouse (partner) or not.
Statistics
Patient characteristics were analysed by descriptive statistics. Category variables were compared using the ?2 test, and continuous variables with non-parametric distributions by the Mann-Whitney test or Kruskal-Wallis test when appropriate. Time-dependent variables were analysed with Kaplan Meier survival statistics using the Log rank test for comparison of factors, or Cox regression when appropriate. Logistic regression analysis was used to identify possible independent predictors for utilisation of hospital facilities among patient characteristics. The median values for the number of outpatient contacts and number of admissions were used to dichotomise these outcome measures as dependent variables. Probabilities were expressed as Odds ratio (OR) with 95% confidence intervals (CI)
A two-tailed p-value<0.05 was considered statistically significant.
Statistical calculations were done using SPSS statistical software v. 16 for Macintosh (Chicago, Ill.).
Results
Study population
During the study period, 1 167 patients were diagnosed with RC in Western Norway of which 297 patients (25%; 154 males and 143 females) received primary treatment with non-curative intent. Data on hospital resource consumption was available for 287 patients (97%; appropriate data was missing for 10 patients due to the closure of two small local hospitals). Most patients had incurable disease at the time of diagnosis. In some patients (37 patients, 13%; 3% of the entire cohort) the initial curative intent had to be changed to a non-curative treatment aim due to insufficient response of preoperative treatment (). The median age was 77 (range 40-98; interquartiles, 67-84) years, and no statistically significant differences were observed between sexes.
Figure 1. Distribution of 1 167 patients diagnosed with rectal cancer in Western Norway between 1997 and 2002 with regard to intention of treatment. In 3% of the patients, the intent changed from curative to palliative treatment either due to incomplete resection or insufficient effect of the preoperative tumour treatment.
About half of the patients (52%) were resident in urban areas with a short distance to a hospital, 30% resided in areas with a transportation time of up to one hour from the hospital, and 18% had a transportation time of at least one hour and/or requiring ferry transport to reach a hospital. Most patients lived in their own homes, and 13% were permanent residents of various institutions. Half of the patients lived with their spouses. In the elderly patient group (=77 years of age), 28 of 130 patients (22%) lived permanently in nursing homes compared with only 5% of the younger patients (p<0.0001). Significantly more elderly patients (63%) were widows/widowers compared with the younger patients (39%) (p<0.0001).
Primary treatment
Data was analysed with regard to surgical treatment, chemotherapy, and radiotherapy employed.
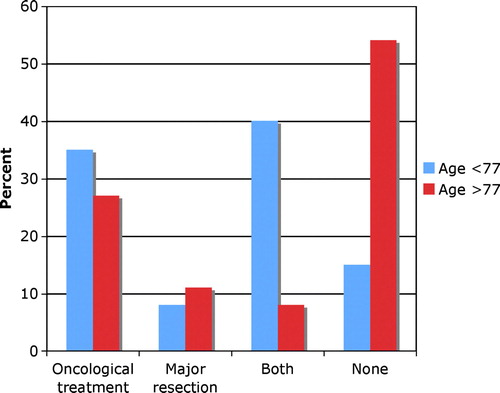
An advanced primary tumour (T4 tumour) was encountered in 177 patients (62%), and distant spread (M1) was diagnosed in 210 patients (73%), and 120 (42%) had both. Tumour-related treatment modalities included oncological treatment, i.e. chemotherapy and/or radiotherapy, and major surgical procedures, which were applied either alone or in various combinations. Treatment choices varied statistically significantly with age, (p<0.000). Of 115 patients who did not receive such treatment, 100 (87%) patients were >77 years of age.
Figure 2. Distribution of various treatment modalities in 287 patients with incurable rectal cancer according to age groups. Oncological treatment includes chemotherapy and/or radiotherapy. Age groups were divided according to median age into younger patients <77 years and older patients >77 years.
One hundred and eighty-five patients (64%) underwent surgery. Surgery was employed significantly more often in the younger patients (74%) compared with the elderly patients (53%) (p<0.001). Major resections (i.e. anterior resection, abdomino-perineal resection, or Hartmann's procedure) were performed in 88 patients (48%), stoma deviation in 89 patients (48%), and local procedures or surgical exploration in 8 patients (4%). Of the remaining 112 patients, surgery was not indicated in 50 patients (45%) due to advanced disease, and 38 patients (34%) were not considered surgical candidates due to significant co-morbidities. Ten patients refused surgical treatment, eight of whom were elderly patients =77 years of age. The reason for non-surgical treatment in 14 patients was unknown. Patients undergoing major resections were statistically significantly different with regard to age, marital status and type of residence compared with the remaining patients (see ).
Table I. Distribution of patients with regard to palliative surgery for rectal cancer. Major resections (i.e. anterior resection, abdomino-perineal resection or Hartmann's procedure) were performed in 88 patients, whilst 97 patients underwent minor procedures (i.e. surgical exploration, stoma, local resection) or non-surgical treatment (n=112).
Eighty-six patients (30%) were offered radiotherapy and 81 patients (28%) received chemotherapy, or a combination of both (n=27; 10% of all palliative patients). The remaining 144 patients (51%) did not receive such treatment. Patients receiving radiotherapy (median age, 74 years) were statistically significantly younger than patients not receiving radiotherapy (median age 77 years) (p=0.015). Fifty-six patients (65%) completed their radiotherapy plan and received a median of 52 Gy (24-66 Gy) compared with a median of 18 Gy (6-44 Gy) in patients who did not complete treatment due to various reasons. No statistically significant differences were evident with regard to distance to hospital, marital status, or type of residence.
Patients treated with chemotherapy were significantly younger compared with those not treated (median age, 64 years versus 80 years; p<0.001). In the age group of patients <77 years, 64% received chemotherapy in contrast to only 11% of those >77 years (p=0.013). About half (53%) received 5-fluorouracil and leucovorin (5-FU/Lv), and the majority of the remaining patients received combinations with either oxaliplatin or irinotecan, or a combination of both. Palliative chemotherapy was given for a median of 6 (range, 1-24; interquartile range, 3-9) months. Patients who did not receive any kind of oncological treatment had significantly older age (median age, 82 years versus 71 years; p<0.0001).
Hospital consumption
Hospital consumption was analysed with regard to the number of visits at outpatient clinics, and number and purpose of admissions (i.e., best supportive care or any kind of tumour-related treatment) (). While outpatient consultations most commonly took place at departments of oncology, hospital admissions were mainly in the departments of surgery. Patients with >1 hour transportation time to the local hospital had statistically significantly more consultations at the surgical outpatient clinic (transportation time >1 hour, 64%; transportation time <1 hour, 52%; patients from urban areas, 40%, p=0.01). Three patients treated at specialised pain clinics lived close to a major centre (University hospital), which offers specialised pain treatment. Hospital consumption with regard to age, marital status, type of residence, distance to hospital facilities and type of treatment modality is shown in . Younger patients had statistically significantly more hospital admissions and stayed at the hospital significantly longer. Moreover, significantly fewer hospital admissions were observed in patients without a spouse. Residing in an institution was associated with fewer admissions to hospital and a shorter hospital stay. In contrast to outpatient clinic visits, distance to hospital was not related to the number of hospital admissions. Patients treated with major resections had significantly more consultations at the surgical outpatient clinics than those treated either non-surgically or with minor procedures (61% versus 41%; p=0.04). There was no statistically significant difference with regard to outpatient contacts at oncological departments (47% versus 34%; p=0.053). Multivariate analysis was performed with age group and various types of treatment as possible independent predictors. Oncological treatment (Odds ratio (OR) 16.8; 95% confidence interval (CI) 4.6 – 61, p<0.000), and major surgical resections combined with oncological treatment (OR 11.3, 95% CI 2.8 – 45.3; p<0.001) were significantly associated with higher frequency of outpatient contacts, as compared to none of these modalities. Multivariate analysis with regard to hospital admissions showed that oncological treatment was associated with higher consumption of hospital admissions (OR 2.8, 95% CI 1.3 – 6.2; p<0.007), while age <77 years was associated with fewer hospital admissions (OR 0.47, 95% CI 0.22 – 0.97; p<0.04).
Table II. Hospital consumption in terms of outpatient visits and hospital admissions for 287 patients palliatively treated for incurable rectal cancer.
Table III. Hospital consumption of 287 patients treated palliatively for incurable rectal cancer with regard to age, marital status, and type of residence, distance to hospital facilities and treatment modalities. Oncological treatment includes chemotherapy and/or radiotherapy.
Survival and place of death
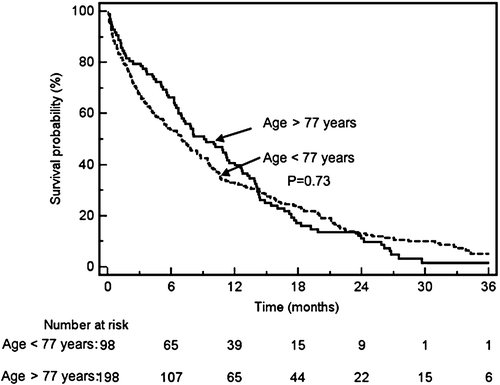
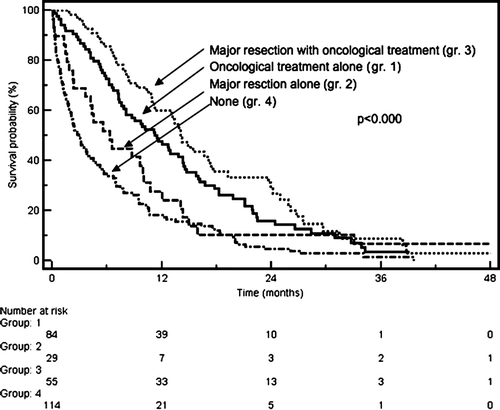
The median survival for all patients treated with palliative intent was 10 months, and was similar for both age groups (). About half of the patients (52%) died in a hospital, 26% in nursing homes, and 22% at home. Two-thirds of patients who stayed permanently in a nursing institution died there, and the remaining one-third died in a hospital. In contrast, only 26% of the patients living in their own homes died there, while 54% died in a hospital, and 20% in a nursing institution (p<0.0001). Most patients who died at a nursing institution were of older age (72%>77 years versus 28%<77 years, p<0.001). There were no differences with regard to distance to hospital. Median time from last hospital admission to death was 36 (IQR, 13-120) days for patients who died at home and 60 (IQR, 15-190) days for those dying at a nursing home (p<0.0001). Survival was longest in patients treated with major resections combined with oncological treatment (median, 14 months, 95% CI 7-20) and oncological treatment (median, 11 months, 95% CI 7-16) as compared to major resections alone (median, 6 months, 95% CI 2-11) or none of these (median, 2 months, 95% CI 0.5-2.5), . A statistically significantly higher proportion of patients with major resections died at the hospital (63% versus 48%; p=0.015). Survival was shorter in patients who died at the hospital compared with patients who died at home or a nursing home (median; 5.7 months versus 10 and 9.5 months, respectively; p=0.05).
Discussion
While several studies have focused on the hospital utilisation for patients with various malignant diseases Citation[8–13], to our knowledge, the present study is the first report on this topic in patients with primarily incurable RC. To improve our knowledge and to enable appropriate palliative care, we believe reliable information on these aspects is of importance.
Our study is based on a general population of almost 1 million inhabitants and, thus, our results can be regarded as valid and applicable to other Western populations. The geographic characteristics of Western Norway did not influence substantially on access to hospital facilities. During the study period, dedicated multidisciplinary palliative teams were not yet established in our region, and specialists in oncology, surgery and medicine delivered in-hospital and outpatient palliative care, both with regard to tumour related treatment and relief of symptoms. It is notable that half of the patients with incurable rectal cancer were older than 77 years. A large number of elderly patients may have had cognitive impairment to varying degrees Citation[14]. In addition, decisions with regard to surgical or oncological treatment differed statistically significantly in the two age groups. Thus, palliative patients with RC apparently comprise two major groups; younger patients who undergo extensive tumour-related treatment, and elderly patients with mainly conservative treatment focusing on symptom-relief. Consequently, hospitals seem to care mainly for younger patients, whilst elderly patients are mostly cared for at nursing institutions. The latter group probably represents a highly vulnerable group of individuals with specific challenges for appropriate palliative care.
Our study shows that departments of surgery and oncology both provide in-hospital palliative care. Generally, surgery for cancer is associated with curative treatment by surgical excision of the tumour. However, surgical approaches are also part of the multidisciplinary care for patients beyond cure. Our study shows that surgical departments were responsible for most of the hospital admissions, with treatment goals evenly distributed between best supportive care and surgical interventions. This observation has important implications for surgical practice and education, but also for the responsible institutions and health care administrations Citation[9], Citation[15].
In modern palliative medicine, home care is the most common patient preference Citation[16]. As a consequence, the care of palliative patients during the course of their disease involves both specialist and primary care including general practitioners, home care facilities and nursing homes. Patients<77 years of age had statistically significantly more hospital admissions and stayed in hospital longer than the elderly. In addition to a more aggressive tumour treatment, this can also be explained by the fact that a majority of younger patients lived in their own homes, and required hospital admission to receive help for their problems. One may hypothesise that access to and delivery of ambulant palliative care was less than most of these patients required. To improve palliative care, close cooperation between the different health care levels has been shown to be effective Citation[17]. Recent studies from Finland and Sweden showed that palliative care was far from optimal in patients discharged from hospital to the primary health care system, particularly due to insufficient transfer of medical information between health care levels Citation[18], Citation[19]. In this respect, a prospective registration and evaluation is warranted to address the content and quality of palliative health care delivered at various levels during the course of disease. At present, such population based data do not exist, as national registries, including the Norwegian Colorectal Cancer Registry, mainly focus on curative treatment Citation[3], Citation[20], Citation[21]. Such prospective, longitudinal studies have been described as difficult, but possible to perform Citation[22].
In-hospital palliative care including surgery, chemotherapy and radiotherapy, was offered either alone or in combinations. Chemotherapy was given for a median of 6 months, and to patients of younger age. Radiotherapy was given to patients of older age. About two-thirds of the patients completed treatment while one-third received only a fraction of the intended radiation. Choice of treatment was significantly associated with survival. While details on treatment effects are of interest and importance in palliative care, the effects of various palliative treatment modalities employed for symptom control in our patient cohort are beyond the scope of the present study.
Surgical procedures were employed either to achieve local tumour control, or to relieve bowel obstruction. Patients undergoing major resections lived statistically significantly longer than patients with exploratory or deviation procedures, as was also shown in a national report Citation[23]. Modern treatment strategies, including endoluminal stenting, add to the minimally invasive approaches to treatment. Hopefully, new health technologies combined with modern imaging modalities will reduce the number of stoma procedures and exploratory laparotomies for an increasing number of sick and fragile patients Citation[24–26].
Patients with advanced RC have limited survival, i.e. a median of 10 months in the present study. This survival compares well with figures from a national study Citation[4]. Many patients and their relatives want to remain at home during the terminal phase and death. Accordingly, modern palliative approaches should aim at providing the best support for this important period of life Citation[16], Citation[27], Citation[28]. Our study revealed that about half of the patients<77 years of age, particularly those with major resections, died in hospital, and only about 25% in their homes. This observation is in concert with others Citation[20]. We could not find a reasonable explanation for this difference. One may speculate that some patients needed treatment that required hospital admission, or even preferred to spend their last time at the place in which they felt most confidence. On the other hand, lack of home care facilities or necessary resources in the primary care system are also plausible explanations Citation[19].
Our study from a large population addresses the use of in-hospital resources in patients with primary incurable RC. However, based on available data, it is hard to tell how this utilisation may translate into ease of symptoms or improved quality of life. National registries are mostly designed for evaluation of curative treatment, and do not provide sufficient data to assess the quality of care in patients beyond cure. Thus, to improve the clinical management of this particular group, well-designed prospective studies from unselected populations, and with focus on individual outcome measures should be conducted.
Acknowledgements
The study was financially supported by the Western Norwegian Regional Health Authorities (Research Fellowship for Helgi Sigurdsson MD, Project # 911158).The authors are grateful to Per Espen Hovde-Hansen MD, Thor Nedrebø MD and Nils Sletteskog MD for their contribution of data. We also appreciate the support from Liv Marit Dørum, secretary of the Norwegian Colorectal Cancer Registry.
References
- Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet 2005; 365: 153–65
- Kapiteijn E, Marijnen CA, Colenbrander AC, Klein Kranenbarg E, Steup WH, van Krieken JH, et al. Local recurrence in patients with rectal cancer diagnosed between 1988 and 1992: A population-based study in the West Netherlands. Eur J Surg Oncol 1998; 24: 528–35
- Wibe A, Carlsen E, Dahl O, Tveit KM, Weedon-Fekjaer H, Hestvik UE, et al. Nationwide quality assurance of rectal cancer treatment. Colorectal Dis 2006; 8: 224–9
- Sigurdsson HK, Kørner H, Dahl O, Skarstein A, Søreide JA. Clinical characteristics and outcomes in patients with advanced rectal cancer: A national prospective cohort study. Dis Colon Rectum 2007; 50: 285–91
- Engeland A, Haldorsen T, Tretli S, Hakulinen T, Horte LG, Luostarinen T, et al. Prediction of cancer incidence in the Nordic countries up to the years 20 00 and 20 10. A collaborative study of the five Nordic Cancer Registries. APMIS Suppl 1993;38:1–124.
- Hofmann B, Håheim LL, Søreide JA. Ethics of palliative surgery in patients with cancer. Br J Surg 2005; 92: 802–9
- Wibe A, Møller B, Norstein J, Carlsen E, Wiig JN, Heald RJ. A national strategic change in treatment policy for rectal cancer–implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum 2002; 45: 857–66
- Back AL, Li YF, Sales AE. Impact of palliative care case management on resource use by patients dying of cancer at a Veterans Affairs medical center. J Palliat Med 2005; 8: 26–35
- Cintron A, Hamel MB, Davis RB, Burns RB, Phillips RS, McCarthy EP. Hospitalization of hospice patients with cancer. J Palliat Med 2003; 6: 757–68
- Cullinane CA, Borneman T, Smith DD, Chu DZ, Ferrell BR, Wagman LD. The surgical treatment of cancer: A comparison of resource utilization following procedures performed with a curative and palliative intent. Cancer 2003; 98: 2266–73
- Gomez-Batiste X, Tuca A, Corrales E, Porta-Sales J, Amor M, Espinosa J, et al. Resource consumption and costs of palliative care services in Spain: A multicenter prospective study. J Pain Symptom Manage 2006; 31: 522–32
- Serra-Prat M, Gallo P, Picaza JM. Home palliative care as a cost-saving alternative: Evidence from Catalonia. Palliat Med 2001; 15: 271–8
- Tibi-Levy Y, Le Vaillant M, de Pouvourville G. Determinants of resource utilization in four palliative care units. Palliat Med 2006; 20: 95–106
- Buchanan RJ, Choi M, Wang S, Huang C. Analyses of nursing home residents in hospice care using the minimum data set. Palliat Med 2002; 16: 465–80
- Bradley CT, Brasel KJ. Core competencies in palliative care for surgeons: interpersonal and communication skills. Am J Hosp Palliat Care 2007; 24: 499–507
- Higginson IJ, Sen-Gupta GJ. Place of care in advanced cancer: A qualitative systematic literature review of patient preferences. J Palliat Med 2000; 3: 287–300
- Johansson B, Holmberg L, Berglund G, Brandberg Y, Hellbom M, Persson C, et al. Reduced utilisation of specialist care among elderly cancer patients: A randomised study of a primary healthcare intervention. Eur J Cancer 2001; 37: 2161–8
- Johansson B, Berglund G, Hoffman K, Glimelius B, Sjoden PO. The role of the general practitioner in cancer care and the effect of an extended information routine. Scand J Prim Health Care 2000; 18: 143–8
- Tasmuth T, Saarto T, Kalso E. How palliative care of cancer patients is organised between a university hospital and primary care in Finland. Acta Oncol 2006; 45: 325–31
- Jones OM, John SK, Horseman N, Lawrance RJ, Fozard JB. Cause and place of death in patients dying with colorectal cancer. Colorectal Dis 2007; 9: 253–7
- Wibe A, Eriksen MT, Syse A, Myrvold HE, Søreide O. Total mesorectal excision for rectal cancer–what can be achieved by a national audit?. Colorectal Dis 2003; 5: 471–7
- Steinhauser KE, Clipp EC, Hays JC, Olsen M, Arnold R, Christakis NA, et al. Identifying, recruiting, and retaining seriously-ill patients and their caregivers in longitudinal research. Palliat Med 2006; 20: 745–54
- Sigurdsson HK, Kørner H, Dahl O, Skarstein A, Søreide JA. Palliative surgery for rectal cancer in a national cohort. Colorectal Dis 2008; 10: 336–43
- Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: Prospective observational study. BMJ 2006;333:779.
- Jost RS, Jost R, Schoch E, Brunner B, Decurtins M, Zollikofer CL. Colorectal stenting: An effective therapy for preoperative and palliative treatment. Cardiovasc Intervent Radiol 2007; 30: 433–40
- Tsurumaru D, Hidaka H, Okada S, Sakoguchi T, Matsuda H, Matsumata T, et al. Self-expandable metallic stents as palliative treatment for malignant colorectal obstruction. Abdom Imaging 2007; 32: 619–23
- Johansson BB, Holmberg L, Berglund IG, Sjoden PO, Glimelius BL. Determinants of cancer patients' utilization of hospital care within two years after diagnosis. Acta Oncol 2004; 43: 536–44
- Yao CA, Hu WY, Lai YF, Cheng SY, Chen CY, Chiu TY. Does dying at home influence the good death of terminal cancer patients?. J Pain Symptom Manage 2007; 34: 497–504
Appendix
The following hospitals provided data to this study: Nordfjordeid Hospital*, Nordfjordeid, Sogn & Fjordane County Lærdal Hospital*, Lærdal, Sogn & Fjordane County Førde Hospital, Førde, Sogn & Fjordane County Voss Hospital, Voss, Hordaland County Haukeland University Hospital, Bergen, Hordaland County Haraldsplass Deaconeal Hospital, Bergen, Hordaland County Haugesund Hospital, Haugesund, Rogaland County Stavanger University Hospital, Stavanger, Rogaland County *Hospital no longer provides care for RC patients