Abstract
Background. Extrapulmonary small cell carcinomas (EPSCC) can clinically progress differently depending on the primary site of disease involvement. This review is focused on patients with small cell carcinoma (SmCC) exclusively localized in a lymph node or in multiple lymph nodes without any evidence of a primary tumor in any other organ. Methods. We searched the period 1980 to 2007 in the PubMed database and idendified 11 publications in the English language presenting at least one case of SmCC. In total 28 individual patients were included in the present study. They were scrutinized in terms of epidemiology, clinical presentation, staging, pathology, etiology, treatment and prognosis. Results. Characteristics such as age, gender and smoking were similar to those seen in other EPSCCs. Median survival was not reached (42+, range, 9.1 to 100 months). The survival rate was found to be 79% at 3 years. Seventy-seven percent of the patients had limited stage disease. These patients completely responded to surgical therapy, chemotherapy, radiotherapy or to a combination of these treatments. Seventy-one percent of the patients with limited stage SmCC localized in lymph nodes were recurrence-free during the study periods. Discussion. Our review patient group with SmCC localized in lymph nodes exhibited an excellent clinical behavior and survival results when compared to other patients with pulmonary and non-pulmonary SmCCs. SmCCs localized in lymph nodes may be a separate clinical entity.
Extrapulmonary small cell carcinoma (EPSCC) has been increasingly recognized as a clinical/pathological entity with biological behavior and prognosis distinct from small cell lung carcinoma (SCLC). Primary EPSCCs have since been described in all sites of the body, except for in the central nervous system. However, EPSCCs are uncommon malignant neoplasms with a reported incidence of 0.1 to 0.4% in the United States and they account for 2.5 to 5.0% of all small cell carcinomas (SmCC). Little information regarding the therapeutic approach to patients with SmCC in extrapulmonary sites is available Citation[1], Citation[2]. Unlike SCLC, much remains to be uncovered about the natural history and definition of optimal therapy of EPSCCs. Early data have indicated that EPSCCs share many features of SCLC, including its histological appearance, aggressive clinical behavior and frequent, yet almost invariably short lasting, response to either chemotherapy or radiotherapy Citation[3]. Moreover, prognosis has varied according to the primary site of the disease Citation[4]. Due to our own experience with an unexpectedly long surviving extensive-stage case of EPSCC with unknown primary of lymph nodes (SmCC localized in lymph nodes), we made a literature search on extrapulmonary SmCCs localized in the lymph nodes solely in order to determine whether this group of SmCCs behaves differently than the other areas. This review is focused on patients with SmCC exclusively localized in a lymph node or multiple lymph nodes without any evidence of a primary tumor in any other organ.
Methods
We searched the PubMed database of the U.S. National Library of Medicine (http://www.nlm.nih.gov/) from 1980 until December 2007 to identify articles on SmCC localized in lymph nodes. We used the terms “small cell carcinoma”, “oat cell carcinoma”, “extrapulmonary small cell carcinoma”, and “undifferentiated neuroendocrine carcinoma” combined with “lymph node’’. The reference lists of the articles retrieved were checked. Totally 649 publications were identified. Only patients diagnosed with SmCC were assessed. Almost all studies reported that carcinoid tumors were excluded. The condition of undifferentiated neuroendocrine carcinoma was not assessed as SmCC and these patients were not included in the group so as not to disrupt homogeneity. Primary retroperitoneal and mediastinal case reports that could be associated with SmCC localized in the lymph node were evaluated, but reports without any clear evidence about lymph node involvement were not evaluated. Finally, 11 publications in the English language reviewed. Twenty-eight individual patients from these 11 publications were described in this study. Reports were reviewed with special attention to the epidemiology, clinical presentation, staging, pathology, etiology, treatment, and prognosis. In total 28 individual patients have been described. Unfortunately, much information about cases to be evaluated was not reported. Assessments could only be performed in a restricted manner within the group formed by the 28 patients as reported by the authors of various studies based on their own criteria.
Survival estimates were obtained via the Kaplan-Meier method according to survival data in these reports.
Results
shows the clinical and pathological characteristics and the clinical course in each of the 28 patients. The patients were identified from nine retrospective EPSCC case series Citation[2], Citation[4–11], one clinical study Citation[12] and one case report Citation[13].
Table I. Clinicopathological characteristics and clinical course in patients with extrapulmonary small cell localized in lymph nodes.
Demographic characteristics
There were 18 males (72%) and seven females (28%) in the study. Gender was not reported in three patients. The median age of 12 patients who underwent an evaluation of age was 56 (range, 20–77 years). Eleven patients could be assessed in terms of smoking. Seven of 11 assessed patients (64%) had a smoking history. Paraneoplastic syndrome was not reported in any of the patients. Of 16 patients with reported localizations, six (37%) were submandibular lymph nodes. The second most common location was cervical lymph nodes. The frequency of SmCC localized in the lymph nodes ranges between 0% and 20% in presented EPSCC series Citation[2], Citation[4–16].
Clinical presentation and staging
Clinical presentation was only reported in four patients. Three patients with submandibular and cervical nodal localization had painless fixed mass and hoarseness Citation[2], Citation[13]. One patient suffered from abdominal discomfort Citation[5]. These patients did not experience systemic symptoms. Similar to other cases of EPSCC, it is probable that to have site-specific symptoms playing an important role during diagnosis.
According to the TNM system, these tumors could be staged as Tx or T0, N+ (single or multiple region), M0. However, in the review, all reports have been staged by using the staging system of the Veterans’ Administration Lung Study Group Citation[2], Citation[4–13]. The staging system consists of two categories: a) limited disease, defined as a tumor being localized to the organ of origin and the locoregional lymph nodes, easily encompassable within one radiation therapy treatment portal, and, b) extensive disease, defined as spread of disease beyond locoregional boundaries Citation[17]. Stage information was available for 27 patients. Twenty-one (77%) of these had limited stage disease.
Treatment and survival
Information about the treatment of 17 patients could be accessed (). Modalities that have been used to treat SmCC localized in lymph nodes include surgery, chemotherapy, and radiotherapy.
Table II. Results of patients with small cell carcinoma localized in lymph nodes.
a) Limited Disease
Surgical resection of lymph node can result in long-term disease-free survival. Galanis et al. described three patients with SmCC localized in lymph nodes (2 submandibular and 1 inguinal), who remained disease-free more than 42 months after only radical surgery Citation[6]. Median time to recurrence was not reached in their study. Hainsworth et al. reported survival of more than 12 months with only curative surgery in a patient with SmCC localized in inguinal lymph nodes Citation[12]. In a study conducted by Kasimis et al., recurrence free survival more than 40 months was observed after excisional biopsy with tumor-free margin in a patient with limited stage SmCC localized in submandibular lymph nodes Citation[13]. No relapse occurred during the study period in the five patients who underwent primary surgery Citation[6], Citation[12], Citation[13].
Remmick et al. established recurrence-free survival of more than 84 months in a limited stage patient with SmCC localized in submandibular lymph nodes who had 46 Gy radiotherapy treatment applied to bilateral necks Citation[2]. Kasimis et al. applied 59 Gy radiotherapy to a patient with limited stage SmCC localized in submandibular lymph nodes following radical neck dissection Citation[13]. The patient experienced relapse with metastases in the brain and other areas, and underwent radiotherapy and surgical intervention on the 24th month. After a complete response, the patient died of the recurrent disease after 36 months. Levenson et al. carried out radical radiotherapy on a limited stage patient with SmCC localized in the cervical nodes Citation[10]. After achieving complete response, the physicians applied adjuvant chemotherapy. The patient was reported to be relapse-free and healthy after 15 months.
In five separate studies, six patients with limited stage disease underwent various combined chemotherapy regimens or adjuvant radiotherapy following chemotherapy Citation[7–10], Citation[15]. Complete response was achieved in all patients. A patient who was treated only by chemotherapy was alive with a local relapse after 22 months Citation[10]. Two patients, who underwent sequential treatment with chemotherapy and radiotherapy, had relapse with distant metastases after 13 and 42 months and they died of progressive disease after 14.5 and 44 months, respectively Citation[7]. The remaining three patients were relapse-free and healthy on months 12, 48 and 100, respectively Citation[8], Citation[9], Citation[12]. Complete response was obtained with all treatment modalities (surgery, radiotherapy, chemotherapy and combined modalities) in all limited-stage patients. Only four of 14 limited stage patients experienced relapses. Three patients died of the disease during the study periods. The median survival rate was not reached (42 months +). The three-year survival rate was 81%.
b) Extensive disease
Galanis et al. reported a median survival of longer than 42 months for two patients with extensive stage disease in their study, containing ambiguous data about treatment modalities and outcome Citation[6]. In two other studies, two of three patients with extensive stage disease, for whom treatment results are available, underwent chemotherapy initially whereas one patient received radiotherapy following chemotherapy Citation[5], Citation[9]. Contrary to limited stage patients, none of the patients exhibited an objective response. Two stable responses were observed. Radiotherapy and complete surgery were performed in the patient with stable response to chemotherapy and a complete response was observed. Regional relapse developed on the 10th month. Stable disease was observed in the patient who received salvage chemotherapy Citation[9]. Another patient with extensive stage reported by us exhibited stable disease after chemotherapy. The patient is still being followed up on the 80th month without progression Citation[5]. Median survival was not reached (42 months +).
The patients were given various chemotherapy regimens including CDE: cyclophosphamide, doxorubicin, etoposide; CAP: cyclophosphamide, adriamycin, cisplatin; CE: cisplatin, etoposide; CEV: Cyclophosphamide, epidoxorubicin, vincristine; CAV: cyclophosphamide, doxorubicin, vincristine; PEI: cisplatin, etoposide, ifosfamide Citation[2], Citation[4–13]. Totally nine patients, six of them with limited stage disease, underwent primary chemotherapy. All of six patients with limited stage showed complete response while stable response was observed in two patients with advanced stage disease. The response rates to the therapies with or without platinum were similar. No median survival was reached (42 months +) in all patients. The three-year survival rate was 81%.
Brain metastasis was reported in only one of the patients and complete response was obtained with radiotherapy Citation[13].
Haider et al. reported eight patients with SmCC localized in lymph nodes in their series involving 101 patients with EPSCC Citation[4]. The treatment modalities administered for these patients were not clearly defined. Unfortunately, the largest patient series with SmCC localized in the lymph nodes was not assessed as a separate sub-group. Although one patient with SmCC localized in the lymph nodes was reported in a Korean study, no separate data about the treatment and the results were not obtained Citation[11].
Discussion and conclusions
The limited number of cases and insufficient patient characteristics of the reported cases caused demographical findings to be far from clear. Characteristics such as smoking, gender, age etc. seem to be similar to other EPSCC's Citation[2], Citation[4–16]. The clinical presentation is dominated by the limited stage in SmCCs localized in lymph nodes. The most important difference of SmCCs localized in lymph nodes from other EPSCC's is that SmCCs localized in lymph nodes seem to be highly curable diseases. In the EPSCC series, the overall survival varied between 5 and 50 months in patients with limited stage, and 5 and 12 months in patients with extensive stage while median survival was not reached for SmCC localized in lymph nodes Citation[2], Citation[4–16].
SmCCs localized in lymph nodes were efficiently treated with surgery, radiotherapy, chemotherapy or a combination of these modalities. Thus, although there is no standard treatment, any one or a combination of these treatment modalities can be preferred in the limited stage disease according to the site of tumor and the options of the physician and the patient. We suggested that local curative treatment (surgery or radiotherapy) should not be omitted in this curable disease. EPSCC studies showed that radiation frequently induced clinical response, but complete response was rare, advanced disease appeared rapidly in almost all patients. It was suggested that radiotherapy should not be used as a single treatment option by itself Citation[5], Citation[18]. We believe that complete surgery is the cornerstone of treatment in limited stage disease. Adjuvant or neoadjuvant platinum-based chemotherapy is a reasonable choice. In limited stage disease, patients who do not undergo surgery, concurrent radiochemotherapy should be preferred over sequential treatment as concurrent radiochemotherapy can be as efficient as surgery. Although response to chemotherapy seems to be insufficient in advanced stage patients, experience gained from other EPSCC's and the efficiency of chemotherapy in the limited stage disease suggest that this mode of treatment should be the main component of therapy. On the other hand, curative surgery or radiotherapy can also be considered in advanced stage patients by taking into account the distention and localization of the disease. Brain metastasis is not common and there is no need for prophylactic radiotherapy.
It should be asked whether SmCC localized in lymph nodes is a metastatic carcinoma with an unknown primary or a primary disease of the lymph nodes. If it is a tumor with an unknown primary, there are two probable explanations: the primary tumor have disappeared via spontaneous regression and the second possibility is that the primary tumor possesses a phenotype and a genotype favoring early metastases. Consequently, metastatic tumor cells proliferate more significantly than primary tumor cell in both conditions Citation[19]. However, the fact that SmCC localized in lymph nodes have longer survival rates and higher curability than other pulmonary and non-pulmonary SmCCs, and carcinomas with unknown primary, is discordant with these. Furthermore, it is possible that SmCCs localized in the lymph nodes can be a primary disease of the lymph nodes which can appear via tumorogenesis from a multipotential stem cell in the lymph node Citation[19]. Therefore, it can be speculated that SmCCs localized in lymph nodes may be a slow-progressing primary lymph node disease.
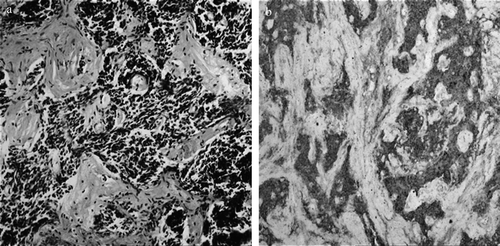
The histological criteria for SmCC localized in the lymph nodes is identical with those for SmCC in the lung; namely, round to spindle-shaped small cells with hyperchromatic dense nuclei, inconspicuous nucleoli, and sparse cytoplasm. Immunohistochemistry may be helpful in the diagnosis, since these neoplasms usually stain positively for chromogranin A, neuron-specific enolase, and/or synaptophysin () Citation[20]. The light microscopy features of SmCC localized in lymph nodes are indistinguishable from those of SCLC. However, pulmonary SmCC and EPSCCs are not cytogenetically identical. For example, loss of chromosome 3p, 10q and deletion in chromosome 13 are rare in EPSCC, while mutations involving deletion of 5q or alterations in chromosome 17 are common in both SCLC and EPSCCs Citation[20], Citation[21].
Figure 1. (a) Tumor cells haphazardly arranged in-between fibrous tissue, with scant cytoplasm and round to oval hyperchromatic nuclei. The crashing artifact of the tumor cells is prominent (HEx100). (b) Diffuse intense staining of the tumor cells for chromogranin-A (Chromogranin-Ax100).
The histogenesis of these tumors both in the lung and extrapulmonary sites is controversial. In view of the presence of neurosecretory granules in the tumor cells in a significant number of cases, and the production of ectopic hormones by some of these tumors, it is likely that EPSCCs are derived from the neuroendocrine amine precursor uptake and decarboxylation (APUD) cells, based upon a common ancestral cell derived from the neural crest, which then migrates to various epithelial tissues and sites within the body. The attractiveness of the APUD system hypothesis is that it may explain the diversity of sites in which SmCC has been reported, but embryologic evidence for neural crest migration is lacking Citation[2], Citation[3]. However, this theory offers little explanation for the common occurrence of tumors showing an admixture of SmCC and various other epithelial cell types. Some authors have hypothesized that small cell elements may arise as a late-stage phenomenon in the genetic progression of more organ-typical carcinomas Citation[22], Citation[23]. Recent molecular studies have added evidence for this hypothesis. These primarily include studies of tumors with mixed cellular composition, which show identical molecular alterations in both small cell and non-small cell components, with additional alterations unique only to the small cell component Citation[24], Citation[25]. Currently, the most widely accepted theory, which does account for mixed morphology, is that EPSCCs arise from a multipotential stem cell capable of divergent differentiation Citation[2], Citation[3].
Our study has a number of limitations. In most cases, follow-up was not complete. A small number of patients were studied. As with many rare tumors, it is difficult to define the clinical characteristics and prognostic factors for SmCCs localized in the lymph nodes with clear evidence. As it takes long periods of time for patients with in the series of patients with EPSSC to accumulate, clinically relevant conclusions are limited due to changes in diagnosis, staging and treatment procedures. On the other hand, in patients with SmCCs localized in the lymph nodes, more favourable results from new staging and treatment procedures should be expected. In addition, as the vast majority of the patients were identified from retrospective EPSCC series, it cannot be thought that only patients with long survival were reported.
In conclusion, SmCCs localized in the lymph nodes may be a separate clinic entity. Until now, little is known about this topic and the present modest review may be a guide for physicians.
References
- Remick SC, Hafez GR, Carbone PP. Extrapulmonary small-cell carcinoma. A review of the literature with emphasis on therapy and outcome. Medicine (Baltimore) 1987; 66: 457–71
- Remick SC, Ruckdeschel JC. Extrapulmonary and pulmonary small-cell carcinoma: tumor biology, therapy, and outcome. Med Pediatr Oncol 1992; 20: 89–99
- Vrouvas J, Ash DV. Extrapulmonary small cell cancer. Clin Oncol (R Coll Radiol) 1995; 7: 377–81
- Haider K, Shahid RK, Finch D, Sami A, Ahmad I, Yadav S, et al. Extrapulmonary small cell cancer: A Canadian province's experience. Cancer 2006; 107: 2262–9
- Cicin I, Karagol H, Uzunoglu S, Uygun K, Usta U, Kocak Z, et al. Extrapulmonary small cell carcinoma compared with small cell lung carcinoma: A retrospective single-center study. Cancer 2007; 110: 1068–76
- Galanis E, Frytak S, Lloyd RV. Extrapulmonary small cell carcinoma. Cancer 1997; 79: 1729–36
- Kurt E, Sezgin C, Evrensel T, Yalcinkaya U, Kanat O, Veral A, et al. Therapy, outcome and analysis of c-kit expression in patients with extrapulmonary small cell carcinoma. Int J Clin Pract 2005; 59: 537–43
- Van Der Gaast A, Verwey J, Prins E, Splinter TA. Chemotherapy as treatment of choice in extrapulmonary undifferentiated small cell carcinomas. Cancer 1990; 65: 422–4
- Lo Re G, Canzonieri V, Veronesi A, Dal Bo V, Barzan L, Zancanaro C, et al. Extrapulmonary small cell carcinoma: A single-institution experience and review of the literature. Ann Oncol 1994; 5: 909–13
- Levenson RM, Jr, Ihde DC, Matthews MJ, Cohen MH, Gazdar AF, Bunn PA, Jr, et al. Small cell carcinoma presenting as an extrapulmonary neoplasm: Sites of origin and response to chemotherapy. J Natl Cancer Inst 1981; 67: 607–12
- Kim KO, Lee HY, Chun SH, Shin SJ, Kim MK, Lee KH, et al. Clinical overview of extrapulmonary small cell carcinoma. J Korean Med Sci 2006; 21: 833–7
- Hainsworth JD, Johnson DH, Greco A. Poorly differentiated neuroendocrine carcinoma of unknown primary site. Ann Intern Med 1988; 109: 364–71
- Kasimis BS, Wuerker RB, Malefatto JP, Moran EM. Prolonged survival of patients with extrapulmonary small cell carcinoma arising in the neck. Med Pediatr Oncol 1983; 11: 27–32
- Kim JH, Lee SH, Park J, Kim HY, Lee SI, Nam EM, et al. Extrapulmonary small-cell carcinoma: A single institution experience. Jpn J Clin Oncol 2004; 34: 250–4
- Sengoz M, Abacioglu U, Salepci T, Eren F, Yumuk F, Turhal S. Extrapulmonary small cell carcinoma: Multimodality treatment results. Tumori 2003; 89: 274–7
- Lee SS, Lee JL, Ryu MH, Chang HM, Kim TW, Kim WK, et al. Extrapulmonary small cell carcinoma: Single center experience with 61 patients. Acta Oncol 2007; 46: 846–51
- Micke P, Faldum A, Metz T, Beeh KM, Bittinger F, Hengstler JG, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer–what limits limited disease?. Lung Cancer 2002; 37: 271–6
- Brenner B, Tang LH, Klimstra DS, Kelsen DP. Small-cell carcinomas of the gastrointestinal tract: A review. J Clin Oncol 2004; 22: 2730–9
- Abbruzzese JL, Lenzi R, Raber MN, Pathak S, Frost P. The biology of unknown primary tumors. Semin Oncol 1993; 20: 238–43
- World Health Organization. The World Health Organization histologic typing of lung tumor Am J Clin Pathol 1982;77:123–36.
- Welborn J, Jenks H, Taplett J, Walling P. High-grade neuroendocrine carcinomas display unique cytogenetic aberratons. Cancer Genet Cytogenet 2004; 155: 33–41
- Testa JR, Liu Z, Feder M, Bell DW, Balsara B, Cheng JQ. Advances in analysis of chromosome alterations in human lung carcinomas. Cancer Genet Cytogenet 1997; 95: 20–32
- Tanaka M, Suzuki Y, Takaoka K, Suzuki N, Murakami S, Matsuzaki O, et al. Progression of prostate cancer to neuroendocrine cell tumor. Int J Urol 2001; 8: 431–7
- Rossi G, Bertolini F, Sartori G, Bigiani N, Cavazza A, Foroni M, et al. Primary mixed adenocarcinoma and small cell carcinoma of the appendix: A clinicopathologic, immunohistochemical, and molecular study of a hitherto unreported tumor. Am J Surg Pathol 2004; 28: 1233–9
- Hoang MP, Maitra A, Gazdar AF, Albores-Saavedra J. Primary mammary small-cell carcinoma: A molecular analysis of 2 cases. Hum Pathol 2001; 32: 753–7
