To the Editor
Uveal melanoma is remarkable for its initially purely hematogenous dissemination and its tendency to metastasize to the liver Citation[1]. At least 40% of patients develop metastases during the first decade Citation[2] and the liver, a “sentinel node” for uveal melanoma, is involved in about 90% of them Citation[3], Citation[4]. Micrometastasis may take place several years before diagnosis of the primary tumor Citation[5], whereas development of clinically detectable metastasis can be delayed for many years or even decades after the primary tumor has been definitively cured Citation[2].
Systemic chemotherapy results in an objective response rate in the range of 5 – 15%, without proof of prolonging survival Citation[6]. The longest overall survival times after resection of liver metastases combined with intra-arterial chemotherapy have varied from 60 to 110 months Citation[7], Citation[8]; as the only treatment, they range from 11 to 86 months Citation[9]. Longest survival after two most common chemotherapy regimens, hepatic intra-arterial fotemustine and intravenous gemcitabine and treosulfan has been 50 months Citation[10] and 18 – 58 months Citation[11–13], respectively. We report a patient whose progression-free survival now exceeds 72 months after chemoimmunotherapy and consolidation with thermoablation.
Case report
A 52-year-old male patient with recent metamorphopsia was referred at the age of 42 years to the Ocular Oncology Service because of a pigmented tumor in his right fundus. Ophthalmoscopy and ultrasonography (US) showed a solid, low reflective brown mass measuring 6.2 mm in thickness and 9×12 mm in diameter within 2 mm of the fovea, and an exudative retinal detachment. Uveal melanoma was diagnosed and iodine-125 brachytherapy with a 20 mm plaque was applied (100 Gy to the tumor apex). The retinal detachment resolved in 2 months and tumor thickness reduced to 3.8 mm during the first year. Radiation retinopathy was diagnosed at 18 months, optic neuropathy at 24 months, and cataract at 5 years. By 10 years, tumor thickness was 1.9 mm.
Liver US and liver function tests were reviewed annually Citation[14]. In August 2001, 9 years 10 months after treatment, a solitary low reflective 24 mm target lesion was seen in the right lobe of the liver. CT showed a 20 mm contrast-enhancing hypodense nodule, which was T1 hyperintense (A) and T2 isointense by MRI. A core needle biopsy showed tumor cells with melanin pigment, prominent nucleoli, 1-2 mitoses per 10 high power fields, and some secondary inflammatory reaction, confirming metastasis (B). Liver function tests were normal. The patient staged to the Helsinki University Central Hospital (HUCH) working formulation stage IVa, a group with predicted median overall survival of over 12 months Citation[4].
Figure 1. Liver metastasis from uveal melanoma. Magnetic resonance imaging (MRI) shows a solitary 20 mm target lesion (arrowhead) in the right lobe of the liver (A). Core needle biopsy confirms metastatic melanoma (B; hematoxylin-eosin). MRI documents partial response after the 3rd (C) cycle, maintained after the 9th cycle and subsequent interferon monotherapy (D). Same lesion seen with ultrasonography before (E; arrowhead) and after (F) adjuvant thermoablation (small crosses) with the ablated needle track (arrowheads).
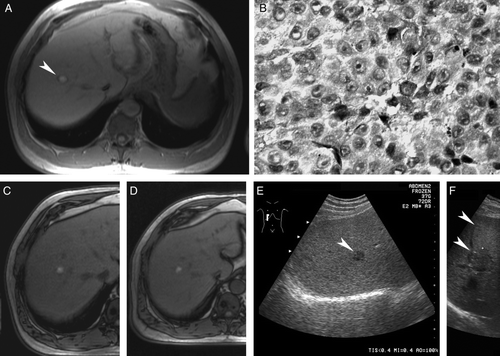
Laparoscopy with an aim to resect the metastasis was done, but showed several black spots on both lobes of the liver not seen by US, and no resection was performed. Instead, systemic chemoimmunotherapy with bleomycin, vincristine, lomustine, dacarbazine (BOLD) and recombinant interferon (Introna,® Schering Corporation, Kenilworth, NJ) was started Citation[15] and eventually given for 9 cycles at a 4 week interval. MRI revealed a partial response (PR) after the 3rd cycle (tumor diameter 10 mm; C). Dacarbazine was subsequently reduced because of neutropenia and vincristine was omitted because of neuropathy. From the 5th cycle on, interferon was omitted. PR was maintained after the 9th cycle, and interferon 6 million IU 3 times weekly for 3 weeks with one week rest period was subsequently given for 3 months.
In November 2004, MRI (D) and US (E) showed no growth and the surgeon was reconsulted. He proposed consolidating the treatment with percutaneous thermoablation. A radiofrequency thermoablation needle was guided to the metastasis under US control, and both the metastasis and the needle track were ablated. Subsequently, control CT and US (F) showed only a scar from the thermoablation. The patient was last seen in February 2008 without signs of recurrence. Visual acuity was counting fingers, a moderate cataract was present, thickness of the irradiated primary tumor was 1.2 mm, and radiation retinopathy had resolved.
Discussion
BOLD chemotherapy combined with interferon produces a partial response rate of 6 to 14% in metastatic uveal melanoma, according to the overlap of confidence intervals of three nonrandomized studies Citation[6]. No long-term progression-free survival has been reported. Our patient also achieved a partial response that was maintained through the 6 subsequent cycles and beyond. The response was consolidated with thermal ablation. Because the smaller metastases detected at laparoscopy were not visible by clinical imaging, we can not be sure that they are eradicated.
A factor contributing to this outcome might have been a less than average aggressiveness of the metastases, which appeared 10 years after treatment of the primary tumor. Indeed, estimated doubling times (DT) of untreated metastases from uveal melanoma range from 34 to 220 days, with a median of 63 days, and these times lengthen during chemotherapy Citation[5]. However, no significant association between DT and observed disease-free interval was detected Citation[5].
This report documents that it is possible to achieve long-term progression-free survival without resorting to liver resection. In our patient, BOLD chemoimmunotherapy also produced a meaningful objective response, like it does in a minority of patients with metastatic uveal melanoma Citation[15–17]. There is little evidence to suggest, however, that any of the more frequently used chemotherapy regimens would be more effective than the other in this group of patients Citation[6].
Acknowledgements
Supported by grants from the Helsinki University Central Hospital Research Fund (TYH2008203), The Sigrid Jusélius Foundation, The Eye Foundation, The Instrumentarium Research Foundation, and Evald and Hilda Nissi Foundation, Finland. No conflict of interest.
References
- McLean IW. The biology of haematogenous metastasis in human uveal malignant melanoma. Virchows Arch A 1993; 422: 433–7
- Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci 2003; 44: 4651–9
- Lorigan JG, Wallace S, Mavligit GM. The prevalence and location of metastases from ocular melanoma: Imaging study in 110 patients. Am J Roentgenol 1991; 157: 1279–81
- Eskelin S, Pyrhönen S, Hahka-Kemppinen M, Tuomaala S, Kivelä T. A prognostic model and staging for metastatic uveal melanoma. Cancer 2003; 97: 465–75
- Eskelin S, Pyrhönen S, Summanen P, Hahka-Kemppinen M, Kivelä T. Tumor doubling times in metastatic malignant melanoma of the uvea: Tumor progression before and after treatment. Ophthalmology 2000; 107: 1443–9
- Kivelä T, Eskelin S, Kujala E. Metastatic uveal melanoma. Int Ophthalmol Clin 2006; 46: 133–49
- Kodjikian L, Grange JD, Baldo S, Baillif S, Garweg JG, Rivoire M. Prognostic factors of liver metastases from uveal melanoma. Graefes Arch Clin Exp Ophthalmol 2005; 243: 985–93
- Peters S, Voelter V, Pampallona S, Popescu R, Gillet M, Bosshard W. Intra-arterial hepatic fotemustine for the treatment of liver metastases from uveal melanoma: Experience in 101 patients. Ann Oncol 2006; 17: 578–83
- Aoyama T, Mastrangelo MJ, Berd D, Nathan FE, Shields CL, Shields JA, et al. Protracted survival after resection of metastatic uveal melanoma. Cancer 2000; 89: 1561–8
- Egerer G, Lehnert T, Max R, Naeher H, Keilholz U, Ho AD. Pilot study of hepatic intraarterial fotemustine chemotherapy for liver metastases from uveal melanoma: A single-center experience with seven patients. Int J Clin Oncol 2001; 6: 25–8
- Schmittel A, Schmidt-Hieber M, Martus P, Bechrakis NE, Schuster R, Siehl JM, et al. A randomized phase II trial of gemcitabine plus treosulfan versus treosulfan alone in patients with metastatic uveal melanoma. Ann Oncol 2006; 17: 1826–9
- Schmittel A, Schuster R, Bechrakis NE, Siehl JM, Foerster MH, Thiel E, et al. A two-cohort phase II clinical trial of gemcitabine plus treosulfan in patients with metastatic uveal melanoma. Melanoma Res 2005; 15: 447–51
- Pföhler C, Cree IA, Ugurel S, Kuwert C, Haass N, Neuber K, et al. Treosulfan and gemcitabine in metastatic uveal melanoma patients: Results of a multicenter feasibility study. Anticancer Drugs 2003; 14: 337–40
- Eskelin S, Summanen P, Pyrhönen S, Tarkkanen A, Kivelä T. Screening for metastatic uveal melanoma revisited. Cancer 1999; 85: 1151–9
- Kivelä T, Suciu S, Hansson J, Kruit WH, Vuoristo MS, Kloke O, et al. Bleomycin, vincristine, lomustine and dacarbazine (BOLD) in combination with recombinant interferon alpha-2b for metastatic uveal melanoma. Eur J Cancer 2003; 39: 1115–20
- Nathan FE, Berd D, Sato T, Shield JA, Shields CL, De PP, et al. BOLD+interferon in the treatment of metastatic uveal melanoma: First report of active systemic therapy. J Exp Clin Cancer Res 1997; 16: 201–8
- Pyrhönen S, Hahka-Kemppinen M, Muhonen T, Nikkanen V, Eskelin S, Summanen P, et al. Chemoimmunotherapy with bleomycin, vincristine, lomustine, dacarbazine (BOLD), and human leukocyte interferon for metastatic uveal melanoma. Cancer 2002; 95: 2366–72
