To the Editor,
A 55-year-old woman with cytokine-refractory metastatic renal cell cancer (RCC) was admitted to the emergency room with symptoms of respiratory distress, pleuritic pain and dry cough. The symptoms had developed rapidly over the preceding 48 hours. On admission, she was afebrile, had no angina pectoris or peripheral edema.
A history revealed that 8 years earlier she had undergone right nephrectomy for clear-cell RCC. Five years after the nephrectomy, immunotherapy with interferon-alfa was initiated due to occurrence of lung, bone and liver metastases, local relapse and involvement of the contralateral kidney. Within 9 months of starting interferon-alfa, the disease had progressed. Interleukin-2 was subsequently given as salvage treatment but stopped after 3 months due to intolerance of the drug. Treatment with zolendronic acid for hypercalcemia and bone metastases was also initiated at this time.
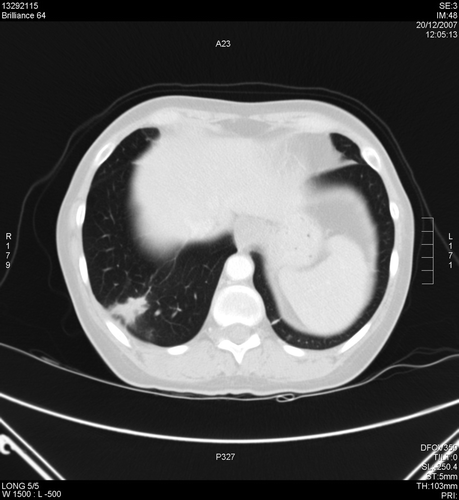
The patient was then switched to treatment with the oral tyrosine kinase inhibitor (TKI) sunitinib (SUTENT®) at a starting dose of 50 mg/day on a 6-week cycle, 4 weeks on treatment followed by 2 weeks off treatment (Schedule 4/2). A partial remission according to Response Evaluation Criteria in Solid Tumors (RECIST) was achieved after four 6-week cycles. Adverse events included grade 2 (National Cancer Institute Common Toxicity Criteria; NCI-CTC) arterial hypertension, hand–foot syndrome, mucositis, diarrhea and hypothyroidism, none of which required dose reduction. Zolendronic acid, initiated 3 months before starting sunitinib, was stopped during cycle 6 due to osteonecrosis of the jaw. During the 2-week off treatment period in cycles 8–11, the patient developed periodic bone pain due to bone metastases. We interpreted this finding as “flare-up” of tumor activity due to interrupted exposure to the TKI, and modified the treatment schedule to continuous dosing of sunitinib 37.5 mg/day. Bone pain resolved and the first two computed tomography (CT) scans after switching to continuous dosing (performed after cycles 14 and 16) confirmed a partial response according to RECIST (). After cycle 16, débridement of the mandibula was planned due to progression of the symptoms of zolendronic acid-related osteonecrosis of the jaw. Sunitinib was stopped 6 days prior to the intervention (with the intention of restarting treatment several days post-surgery) and surgery was performed.
Figure 1. Chest CT scan of the thorax 1 month prior to sunitinib discontinuation after 16 cycles of treatment (20-12-2007).
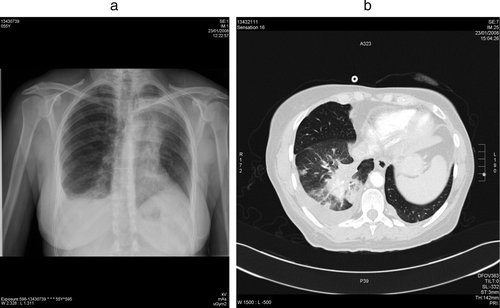
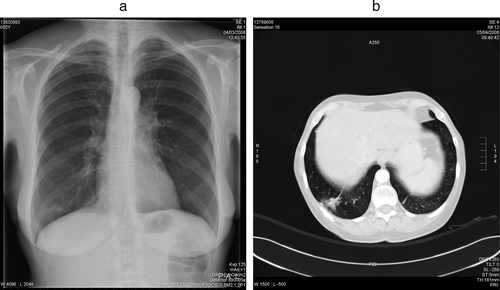
Two days after surgery, respiratory symptoms developed and gradually worsened. The chest radiograph showed a tumoral mass at the left upper hilus, partial opacification of the right lung base and the left upper lobe, and pleural effusion (a). Pulmonary embolism was excluded by spiral CT scanning. Pulmonary infectious syndrome was excluded based on clinical, biochemical and radiographic findings. The scan showed progression of the metastatic lung lesions (b). These findings were again compatible with a “flare-up” due to withdrawal of the TKI. Sunitinib was re-initiated, and within 1 week of treatment resulted in nearly complete resolution of the opacification and pleural effusion seen on the chest radiograph. This was confirmed by a chest x-ray (a) 1 month later and by a CT scan of the chest 10 weeks later (b).
Discussion
Sunitinib is a TKI that targets several kinases, including vascular endothelial growth factor receptors (VEGFRs-1, -2, and -3), stem-cell factor receptor (KIT), platelet-derived growth factor receptors (PDGFRs-α and -β) and FMS-like tyrosine kinase 3 (FLT3) Citation[1–4]. It has both antiproliferative and antiangiogenic properties Citation[5]. This agent has been approved for treatment of imatinib-resistant or -intolerant gastrointestinal tumor (GIST) and for advanced and/or metastatic RCC, and is presently being studied in various other solid tumor types.
The recommended dose of sunitinib is 50 mg taken orally, once daily on an intermittent schedule (Schedule 4/2), an unusual schema for administering TKIs. Schedule 4/2 was selected at the request of the regulatory authorities to allow patients to recover from potential bone marrow and adrenal toxicity observed in animal models Citation[5], Citation[6]. In GIST it has been shown that discontinuing the TKI imatinib may lead to early relapse, which can be salvaged by re-administering the same agent Citation[7]. Most oncologists consider that patients with focal progression on imatinib still benefit from the treatment and that withdrawal might lead to growth stimulation of still-sensitive tumor cell clones. Similarly, some patients seem to experience clinical disease progression during the 2-week off treatment period of the standard sunitinib dosing schedule with a subsequent increase or recurrence of disease-related symptoms. Two phase II studies recently investigated continuous daily dosing of sunitinib 37.5 mg/day in RCC and GIST Citation[8], Citation[9]. Both trials demonstrated promising efficacy and good tolerability, potentially offering a good therapeutic alternative for patients with TKI “flare-up” during intermittent dosing.
Our case is interesting for several reasons. First, we can present radiological evidence for the true existence of “flare-up” in a patient treated with a TKI. Additionally, in a retrospective analysis of 63 metastatic RCC patients treated with sunitinib in our clinical centre, we observed “flare-up” leading to schedule modification and continuous dosing of sunitinib in six patients (9.5%). Our clinical observation is in accordance with pre-clinical studies showing rapid vascular re-growth of tumors after reversal of VEGF inhibition Citation[10]. This implies that it is unlikely that complete eradication of all cancer cells is achievable and that treatment with TKIs should be continued until disease progression and/or intolerance of treatment. Clear criteria for stopping TKIs should be developed, and the recommendation to continue treatment in the event of progression should be evaluated in prospective trials.
Second, our case illustrates that the timing of radiological assessment in patients treated with discontinuous schemes of TKIs is crucial in order to evaluate accurately the quality and quantity of responses.
The case also highlights potential risks when using TKIs in an adjuvant treatment setting, such as completely resected GIST or RCC. It cannot be excluded that stopping TKIs after a predefined period of time in the context of such therapy might lead to “flare-up” of microscopic residual disease, with detrimental treatment outcome.
In conclusion, oncologists should be aware of the possibility of “flare-up” in patients treated with discontinuous dosing of sunitinib and probably other TKIs. Continuous dosing is a possible option in these patients to prevent “pseudo-progression” and early discontinuation of a possibly life-prolonging drug treatment.
Acknowledgements
Editorial support from ACUMED (Tytherington, UK), with funding from Pfizer Inc. The Department of General Medical Oncology at the University Hospitals Leuven has received research grants from Pfizer Inc. to study side-effects of tyrosine kinase inhibitors and has received honoraria for inclusion of patients in Pfizer-related trials. Patrick Schöffski has received honoraria for invited lectures and active participation in scientific events and advisory functions.
References
- Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther 2003; 2: 471–8
- Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 2003; 9: 327–37
- O'Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 2003; 101: 3597–605
- Pfizer Inc., data on file.
- Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future trial development. Nat Rev Drug Discov 2007; 6: 734–45
- Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 2006; 143: 313–4
- Blay JY, Le Cesne A, Ray-Coquard I, Bui B, Duffaud F, Delbaldo C, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. J Clin Oncol 2007; 25: 1107–13
- Blay, JY, George, S, Casali, PG, Le Cesne, A, Morgan, JA, Tyler, A, , et al. Clinical benefit of continuous daily dosing of sunitinib in patients (pts) with advanced gastrointestinal stromal tumor (GIST). Ann Oncol 2006;17(Suppl 9): 508P (Abstract).
- Escudier, B, Roigas, J, Gillessen, S, Srinivas, S, Pisa, P, Vogelzang, N, , et al. Continuous daily administration of sunitinib malate (SU11248)-phase II study in patients (pts) with cytokine-refractory metastatic renal cell carcinoma (MRCC). Ann Oncol 2006, 17(Suppl 9): 436O (Abstract).
- Mancuso MR, Davis R, Norberg SM, O'Brien S, Sennino B, Nakahara Yao VJ, et al. Rapid vascular regrowth in tumours after reversal of VEGF inhibition. J Clin Invest 2006; 116: 2610–21