To the Editor
We report the case of a 63-year-old South-east Asian (Indian) man who presented to his family physician with fever, headache, fatigue, nausea, a pruritic macular rash on his shoulders and arms, arthralgias and myalgias. The patient and his family recently had returned from a trip to India, where the patient's 12-year-old son contracted chikungunya. The boy's symptoms—fever, headache, fatigue, nausea, vomiting, rash, myalgia, and arthralgia—resolved within a week. Because the patient's symptoms were similar to his son's, the patient thought he also may have acquired chikungunya. Chikungunya is an acute viral disease transmitted by infected mosquitoes of the genus Aedes and generally encountered in West Africa and South-east Asia Citation[1]. Chikungunya generally manifests with nonspecific symptoms such as fever, fatigue, arthralgias, and a macular rash. The fever typically lasts about 2 weeks, but some patients experience prolonged fatigue that lasts several weeks. No specific antiviral treatment exists for chikungunya virus infection. The arthralgias are treated with analgesics and anti-inflammatory medication.
In the case here reported, the physical examination revealed a resolving macular rash, a tender 3-cm left axillary lymph node, without other palpable lymphadenopathy, and splenomegaly. Laboratory evaluation revealed microcytic anemia, eosinophilia, and elevated lactate dehydrogenase and beta-2 microglobulin levels. There was evidence of a previous cytomegalovirus infection. Serum protein electrophoresis showed two very small, relatively indistinct protein bands in the mid-gamma region. Specific serological testing for chikungunya was sent to reference laboratory and eventually the anti-chikungunya immunoglobulin M (IgM) assay came back negative. There was persistence of lymphadenopathy and the Computed Tomography (CT) scan of the patient's neck and chest showed enlarged bilateral cervical and axillary lymph nodes. Positron emission tomography (PET) revealed extensive 18F-fluorodeoxyglucose (FDG) activity above and below the diaphragm, in the lymph nodes, spleen and numerous subcutaneous nodules (, Panel A and ). An excisional biopsy of an axillary lymph node revealed peripheral T-cell lymphoma (PTCL) with features of angioimmunoblastic T-cell lymphoma (AITL). There was positive staining for CD3, CD4 (dim), and dim CD10 co-expression in some T-cells, but negative T-cell-restricted intracellular antigen-1 and Leu-7. In situ hybridization for Epstein-Barr virus (EBV)-encoded small RNA was negative. A bone marrow biopsy revealed atypical lymphohistiocytic aggregates with eosinophilia consistent with AITL, occupying less than 5% of the medullary space. Cytogenetic analysis showed a pseudodiploid karyotype, 6,XY,del(11)(q23),add(21q22). A fluorescence in situ hybridization assay ruled out chromosomal translocation t(11;21). Polymerase chain reaction analysis did not reveal any monoclonal T-cell receptor beta- or gamma-chain gene rearrangements.
Figure 1. A. Initial 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) reveals mild FDG activity in bilateral jugulodigastric (standardized uptake value [SUV] of 3.5) and axillary lymph nodes (SUV of 2.7) with additional uptake noted in the right supraclavicular, right hilar, subcarinal, portocaval, mesenteric, retroperitoneal, iliac (SUV of 13.8), and inguinal lymph nodes. There is an enlargement of the spleen with increased activity (SUV of 7.1) as well. B. Compared to the examination obtained at diagnosis, the repeat PET scan obtained a year later shows complete remission of the disease, with no enlarged FDG-avid nodes in the neck, chest, abdomen, or the pelvis.
![Figure 1. A. Initial 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) reveals mild FDG activity in bilateral jugulodigastric (standardized uptake value [SUV] of 3.5) and axillary lymph nodes (SUV of 2.7) with additional uptake noted in the right supraclavicular, right hilar, subcarinal, portocaval, mesenteric, retroperitoneal, iliac (SUV of 13.8), and inguinal lymph nodes. There is an enlargement of the spleen with increased activity (SUV of 7.1) as well. B. Compared to the examination obtained at diagnosis, the repeat PET scan obtained a year later shows complete remission of the disease, with no enlarged FDG-avid nodes in the neck, chest, abdomen, or the pelvis.](/cms/asset/0d91ed0e-e5f5-426a-aa9c-ea92920be09b/ionc_a_363947_f0001_b.gif)
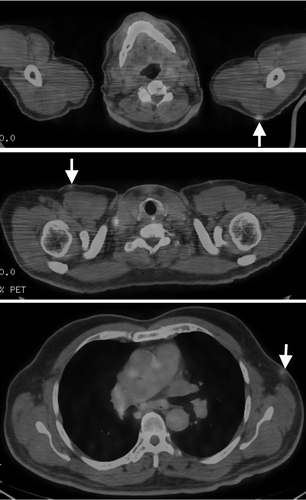
Figure 2. PET/CT scan shows numerous subcutaneous FDG-avid nodules (arrows) of cutaneous involvement by the lymphoma in the shoulder, chest wall, and axilla.
Angioimmunoblastic T-cell lymphoma (AITL) is a subtype of peripheral T-cell lymphomas (PTCL). It is an aggressive lymphoma with a higher incidence in East and South-east Asia Citation[2]. AITL commonly affects older adults, who may present with acute onset of nonspecific symptoms that include fever, fatigue, weight loss, generalized lymphadenopathy, and splenomegaly. Pleural effusion, ascites, and pruritic macular rash affect one quarter of the patients with AITL Citation[3]. The rash is felt to result from lymphohistiocytic vasculitis. Of note, polyclonal hypergammaglobulinemia is common in patients with AITL. For the AITL, the patient was treated with a chemotherapy regimen of dose-intense cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD), to which the disease was refractory. There was only partial response after two cycles of ifosfamide, carboplatin, and etoposide (ICE). After stem cell mobilization with ifosfamide and etoposide, and conditioning with a myeloablative regimen of cytarabine, etoposide, carmustine, and melphalan (BEAM), the patient underwent autologous hematopoietic stem cell transplantation (HSCT). Engraftment was successful, and the patient attained complete remission for over a year after HSCT (, Panel B). The patient ultimately had recurrent disease and did undergo an allogeneic transplant and is currently alive with no evidence of disease a month later.
In this case, the disease presentation was initially consistent with the natural history of chikungunya, but enlarged lymph nodes and constitutional symptoms prompted further investigation which revealed AITL. AITL is the second most common type of PTCL after unspecified PTCL. In the Kiel Registry, AITL accounts for 20% of T-cell lymphomas but only 4% of all lymphomas Citation[4]. AITL is histologically characterized by polymorphous infiltration of the lymph nodes with major proliferation of endothelial venules and follicular dendritic cells. AITL has considerable histomorphologic diversity, variable immunophenotypes, and inconsistent T-cell receptor gene rearrangement. It is believed to derive from follicular helper T cells Citation[5]. Positive flow cytometry for CD10 and the presence of EBV-encoded RNA on in situ hybridization are common histological features of AITL Citation[6]. Bystander B cells including plasma cells are often present, and they probably correlate with the hypergammaglobulinemia that is commonly seen in AITL. The common expression in AITL of CXCL13, a chemokine that mediates B-cell migration, is likely related to the B-cell manifestations of AITL Citation[7]. Roles of interleukin-6 and herpes simplex virus-8 in the etiology of AITL have been suggested but not well established Citation[8].
The onset of symptoms of AITL may be linked with infectious diseases, antibiotic therapy, or systemic diseases such as Crohn's disease, Sjogren's syndrome, rheumatoid arthritis, drug eruption, dermatitis herpetiformis, sclerosing cholangitis, or other systemic diseases Citation[9–11]. AITL is often detected following the administration of drugs such as sulfasalazine, salazosulfapyridine, ciprofloxacin, doxycycline, and macrolides Citation[12], Citation[13]. The apparent association between AITL and antibiotic therapy may be coincidental, related to the empirical use of antibiotics to treat nonspecific infections when the patients’ symptoms are in fact due to AITL. As reflected in the case presented, AITL's relationship with certain infectious diseases is of interest particularly in South-east Asian patients. AITL has been diagnosed at the same time as tuberculosis, hepatitis C, Yersinia enterocolitica infection, scabies, Strongyloides stercoralis hyperinfection, and endocarditis Citation[14], Citation[15]. To our knowledge, no cases of AITL and chikungunya diagnosed concurrently have been reported. However, chikungunya has been reported to precede cases of EBV-related Burkitt's lymphoma in Africa, which suggests that populations there are exposed to multiple viruses Citation[16].
The prognosis of AITL is poor. Traditionally, anthracycline-based chemotherapy has been used to treat AITL. Data on the management of AITL is found largely in retrospective series and case reports (). Numerous treatments have some efficacy in the management of AITL, but to date there is no consensus about which regimen is best. Because of unsatisfactory results with standard therapy, numerous small studies with mixed results have explored treating AITL with alternative agents such as cyclosporine Citation[17], fludarabine Citation[18], cladribine Citation[19], alemtuzumab Citation[20], methotrexate Citation[21], and thalidomide Citation[22]. Some studies have shown that AITL can be effectively treated with high-dose chemotherapy followed by autologous HSCT Citation[23]. Intensive or investigational therapy is warranted for AITL. The patient in our case had a complete remission for more than a year in response to autologous HSCT.
Table I. Selected published studies and reports on the treatment of AITL.
An acute onset of symptoms of fever, fatigue, arthralgias is often part of a viral syndrome. However, malignancies such as AITL should be part of the differential diagnosis, especially when body cavity effusions, atypical pruritic rash, and persistent constitutional symptoms dominate the clinical picture.
References
- Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis 2007; 7: 319–27
- The World Health Organization classification of malignant lymphomas in Japan: Incidence of recently recognized entities. Lymphoma Study Group of Japanese Pathologists. Pathol Int 2000;50:696–702.
- Park BB, Ryoo BY, Lee JH, Kwon HC, Yang SH, Kang HJ, et al. Clinical features and treatment outcomes of angioimmunoblastic T-cell lymphoma. Leuk Lymphoma 2007; 48(4)716–22
- Lennert K, Feller A. Histopathology of non-Hodgkin's lymphomas. Springer-Verlag, New York 1992
- Dunleavy K, Wilson WH, Jaffe ES. Angioimmunoblastic T cell lymphoma: Pathobiological insights and clinical implications. Curr Opin Hematol 2007; 14: 348–53
- Attygalle AD, Chuang SS, Diss TC, Du MQ, Isaacson PG, Dogan A. Distinguishing angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified, using morphology, immunophenotype and molecular genetics. Histopathology 2007; 50: 498–508
- Dupuis, J, Boye, K, Martin, N, Copie-Bergman, C, Plonquet, A, Fabiani, B, , et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): A new diagnostic marker providing evidence that AITL derives from follicular helper T cells. Am J Surg Pathol 2006;30:490–4.
- Luppi M, Barozzi P, Maiorana A, Artusi T, Trovato R, Marasca R, et al. Human herpesvirus-8 DNA sequences in human immunodeficiency virus-negative angioimmunoblastic lymphadenopathy and benign lymphadenopathy with giant germinal center hyperplasia and increased vascularity. Blood 1996; 87(9)3903–9
- Kang HY, Hwang JH, Park YS, Bang SM, Lee JS, Chung JH, et al. Angioimmunoblastic T-cell lymphoma mimicking Crohn's disease. Dig Dis Sci 2007; 52(10)2743–7
- Saito M, Fukuda T, Shiohara T, Homori M. Angioimmunoblastic T-cell lymphoma: A relatively common type of T-cell lymphoma in Sjogren's syndrome. Clin Exp Rheumatol 2005; 23: 888–90
- Tsochatzis E, Vassilopoulos D, Deutsch M, Filiotou A, Tasidou A, Archimandritis AJ. Angioimmunoblastic T-cell lymphoma-associated arthritis: Case report and literature review. J Clin Rheumatol 2005; 11: 326–8
- Batinac T, Zamolo G, Jonjic N, Gruber F, Nacinovic A, Seili-Bekafigo I, et al. Angioimmunoblastic lymphadenopathy with dysproteinemia following doxycycline administration. Tumori 2003; 89(1)91–5
- Pay S, Dinc A, Simsek I, Can C, Erdem H. Sulfasalazine-induced angioimmunoblastic lymphadenopathy developing in a patient with juvenile chronic arthritis. Rheumatol Int 2000; 20: 25–7
- Ozyilkan O, Ozyilkan E, Karagoz F, Ozdamar S, Kandemir B. Hepatitis C virus infection and angioimmunoblastic lymphadenopathy. Am J Gastroenterol 1995; 90: 1029–30
- Rho R, Laddis T, McQuain C, Selves J, Woda B, Knecht H. Miliary tuberculosis in a patient with Epstein-Barr virus-associated angioimmunoblastic lymphadenopathy. Ann Hematol 1996; 72: 333–5
- van den BC, Lloyd G. Chikungunya fever as a risk factor for endemic Burkitt's lymphoma in Malawi. Trans R Soc Trop Med Hyg 2000; 94: 704–5
- Advani R, Horwitz S, Zelenetz A, Horning SJ. Angioimmunoblastic T cell lymphoma: Treatment experience with cyclosporine. Leuk Lymphoma 2007; 48: 521–5
- Tsatalas C, Margaritis D, Pantelidou D, Spanudakis E, Kaloutsi V, Bourikas G. Treatment of angioimmunoblastic lymphadenopathy with dysproteinemia-type T-cell lymphoma with fludarabine. Acta Haematol 2003; 109: 110
- Sallah S, Wehbie R, Lepera P, Sallah W, Bobzien W. The role of 2-chlorodeoxyadenosine in the treatment of patients with refractory angioimmunoblastic lymphadenopathy with dysproteinemia. Br J Haematol 1999; 104: 163–5
- Halene S, Zieske A, Berliner N. Sustained remission from angioimmunoblastic T-cell lymphoma induced by alemtuzumab. Nat Clin Pract Oncol 2006; 3: 165–8
- Quintini G, Iannitto E, Barbera V, Turri D, Franco V, Florena AM, et al. Response to low-dose oral methotrexate and prednisone in two patients with angio-immunoblastic lymphadenopathy-type T-cell lymphoma. Hematol J 2001; 2: 393–5
- Strupp C, Aivado M, Germing U, Gattermann N, Haas R. Angioimmunoblastic lymphadenopathy (AILD) may respond to thalidomide treatment: Two case reports. Leuk Lymphoma 2002; 43: 133–7
- Kyriakou C, Canals C, Goldstone A, Caballero D, Metzner B, Kobbe G, et al. High-dose therapy and autologous stem-cell transplantation in angioimmunoblastic lymphoma: Complete remission at transplantation is the major determinant of Outcome-Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 2008; 26(2)218–24
- Rodriguez J, Conde E, Gutierrez A, Arranz R, Gandarillas M, Leon A, et al. Prolonged survival of patients with angioimmunoblastic T-cell lymphoma after high-dose chemotherapy and autologous stem cell transplantation: The GELTAMO experience. Eur J Haematol 2007; 78(4)290–6
- Schetelig J, Fetscher S, Reichle A, Berdel WE, Beguin Y, Brunet S, et al. Long-term disease-free survival in patients with angioimmunoblastic T-cell lymphoma after high-dose chemotherapy and autologous stem cell transplantation. Haematologica 2003; 88(11)1272–8
- Gerlando Q, Barbera V, Ammatuna E, Franco V, Florena AM, Mariani G. Successful treatment of angioimmunoblastic lymphadenopathy with dysproteinemia-type T-cell lymphoma by combined methotrexate and prednisone. Haematologica 2000; 85: 880–1
- Bourgeois E, Auxenfants E, Jouffrey C, Dubest C, Mahieu M, Rose C. Efficacy of fludarabine in the treatment of angioimmunoblastic lymphoma (AIL)]. Ann Med Interne (Paris) 2000; 151: 230–1
- Hast R, Jacobsson B, Petrescu A, Hjalmar V. Successful treatment with fludarabine in two cases of angioimmunoblastic lymphadenopathy with dysproteinemia. Leuk Lymphoma 1999; 34: 597–601
- Pautier P, Devidas A, Delmer A, Dombret H, Sutton L, Zini JM, et al. Angioimmunoblastic-like T-cell non Hodgkin's lymphoma: Outcome after chemotherapy in 33 patients and review of the literature. Leuk Lymphoma 1999; 32((5–6))545–52
