Abstract
Background. Adult T-cell leukemia/lymphoma (ATL) is a severe disease caused by HTLV-I. This paper describes the clinicopathological and immunohistochemical findings of 52 cases of ATL with skin involvement and investigates whether there is any relationship between median survival time (MST) and histological patterns, primary cutaneous involvement and CD8 positivity.Material and methods. All cases were HTLV-I+ and HIV- and were clinically classified. HTLV-I proviral integration was investigated in atypical cases. Immunohistochemistry was performed using CD3, CD4, CD5, CD7, CD8, CD20, CD25, CD30 and CD45RO markers. Ki-67 was used to evaluate the proliferative index. Results. Twenty-seven cases were primary, while 25 were secondary. Monoclonal viral integration was demonstrated in all atypical cases. Patterns resembling mycosis fungoides (MF) were found in 19 cases and anaplastic large-cell lymphoma (ALCL) in two cases. Fifteen cases had an atypical immunophenotype and expressed CD8. Primary cutaneous ATL had a longer MST (48 months) than the secondary cutaneous ATL (7 months) and the difference was statistically significant, but no statistically significant difference was found between the MST of CD8-positive and negative cases.Conclusions. It is important to differentiate between primary and secondary cutaneous ATL and classify the cases histologically in order to better evaluate the prognosis. The two forms of primary cutaneous ATL, primary cutaneous smoldering and primary cutaneous tumoral (PCT), should also be identified. The smoldering type presented a longer survival (58 months) and histological aspects suggestive of better prognosis in contrast to the PCT type that had a shorter survival (20 months) and histological characteristics suggestive of worse outcome.
The human T-cell lymphotropic virus type I (HTLV-I) is a retrovirus that was first isolated in 1981 from T-cells of a patient with cutaneous lymphoma, and was soon after linked to a type of leukemia/lymphoma that had been previously described in Japan, adult T-cell leukemia/lymphoma (ATL) Citation[1].
Infection by HTLV-I is endemic in many parts of the world, including southwestern Japan, the Caribbean basin, and parts of Central Africa and South America Citation[2], Citation[3]. In Brazil, the highest seroprevalence rate of HTLV-I in healthy subjects (1.8%) was observed in the state of Bahia Citation[4], situated on the north-eastern coast where the population is largely of African descent. Estimates of the number of people infected with HTLV-I worldwide range from 10 to 20 million, the majority of whom are asymptomatic carriers; however, around 5% of the infected individuals may develop associated diseases including ATL, HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) and infective dermatitis associated with HTLV-I (IDH) Citation[5], Citation[6].
The action of HTLV-I within the organism is very slow, and the majority of the diseases caused by the virus are of late onset, emerging in adulthood Citation[7]. However IDH is a severe and infected dermatitis that occurs in childhood. Besides there is a juvenile form of HAM/TSP and even ATL may occur earlier, in adolescence or in early adulthood Citation[8].ATL is an aggressive mature T-cell malignancy that affects the skin in 43–72% of cases Citation[9].
ATL has been classified into four clinical types: acute, chronic, lymphoma and smoldering Citation[10]. In the smoldering type lymphocytosis is not observed and when more than 5% of abnormal T-lymphocytes are present in peripheral blood, it has been defined as leukemic smoldering ATL Citation[3], Citation[11]. Smoldering cases showing less than 5% of abnormal T-lymphocytes in peripheral blood should have a diagnosis of lymphoma in skin or lung biopsy Citation[10]. We have recently proposed to include another clinical type, primary cutaneous tumoral (PCT) in the Shimoyama's classification Citation[12]. This clinical form is characterized by the presence of cutaneous tumors, and absence of lymphadenomegaly, lymphocytosis, hypercalcemia, and involvement of internal organs Citation[12].
A previous study, carried out in Bahia, Brazil, which included 70 cases of ATL, found that certain variables, such as the smoldering and chronic clinical forms, mycosis fungoides (MF)-like histological pattern, small and medium-sized cell morphology and a low proliferative index, were correlated with a better prognosis Citation[12]. Prognosis was correlated with immunophenotype by some authors who found an association between CD8 positivity and poor prognosis Citation[13].
In this study we present the clinicopathological and immunopathological features of 52 cases of ATL with histologically-proven primary or secondary cutaneous involvement. In addition, we correlated the ATL patients’ survival with clinical and histological types, primary vs. secondary cutaneous involvement and CD8 positivity.
Material and methods
Fifty-two cases of ATL with histologically proven cutaneous involvement identified among 88 cases of ATL diagnosed or referred to the Pathology Department of the Federal University of Bahia's Teaching Hospital were included in this study. All patients were HTLV-I-positive (positive ELISA confirmed by Western blot or PCR) and HIV-negative. An epidemiological questionnaire was applied to the patients and a follow-up study was undertaken. The patients were submitted to staging Citation[12] and skin biopsy. Survival intervals were calculated from the date of diagnosis until last follow-up or death (cut-off date was February, 2008). Clinical types were classified as acute, chronic, lymphomatous, smoldering or PCT Citation[10], Citation[12]. Primary cutaneous ATL was defined as the presence of cutaneous lymphoma without extracutaneous disease within the first 6 months after the diagnosis Citation[14]. In primary cutaneous ATL are included the smoldering subtype with only skin lesions and with less than 5% of abnormal lymphocytes in peripheral blood (primary cutaneous smoldering) and the PCT type. Diagnosis was based on both positive serology and a histologically-proven diagnosis of cutaneous lymphoma of peripheral T-cell origin. Cases with more than 5 years of survival and with less than 19 years of age were considered as atypical cases and in all of them HTLV-I proviral integration was investigated using Southern blot or long-inverse PCR Citation[15]. Patients with smoldering disease were submitted to conservative therapy only, while those with disseminated lesions received Psoralen-UV-A treatment in addition to conservative therapy. In the PCT type, radiotherapy associated with chemotherapy and/or interferon-alpha (IFN-α) plus zidovudine (AZT) were used to reduce the size of the tumors. The chronic, lymphoma and the acute cases were treated with chemotherapy alone or associated with IFN-α plus AZT. Most of the patients in the present series had been included in previous publications Citation[12], Citation[16–18]. The protocol of the present study was approved by the Institutional Review Board of the Teaching Hospital of the Federal University of Bahia. Informed consent was obtained in all cases.
Histopathologic and immunohistochemical studies
The cases were classified morphologically according to the WHO/EORTC (World Health Organization/European Organization for Research and Treatment of Cancer) classification of cutaneous lymphomas Citation[19]. The immunohistochemical study was performed on formalin-fixed, paraffin-embedded sections using a standard streptavidin-biotin-peroxidase technique Citation[20] and the following immunohistochemical markers: CD45R0 (clones OPD4 or UCHL-1), CD3, CD4, CD5, CD7, CD8, CD20, CD25, CD30 and CD79a. ALK-1 was performed in cases with a morphological appearance of anaplastic large cell lymphoma (ALCL) and CD68 in two cases in which there was a granulomatous reaction. The proliferative index (PI) was evaluated using Ki-67. With the exception of CD4 and CD25, which were purchased from Novocastra (Newcastle, UK), all other antibodies were obtained from DakoCytomation, Glostrup, Denmark. The immunophosphatase technique (Streptavidin Biotin System) for identification of cytotoxic granules was performed in 11/15 CD8+ cases using the anti-granzyme B, anti-perforin (Novocastra) and anti-TIA-1 (Immunotech, Marseille, France) antibodies.
Statistical analysis
Univariate analysis was carried out to examine the association between each variable and progression to death. The variables analyzed for their prognostic value were: clinical and histological types, primary cutaneous ATL versus secondary ATL, and CD8-positive cases versus CD8-negative cases. The Kaplan-Meier method was used to estimate the cumulative probability of patient survival over time. Comparison of different curves according to subgroups was carried out using the generalized log-rank test.
Results
Clinical features
All the patients were born in Bahia, Brazil with exception of one case born in São Paulo, Brazil. Forty-six patients (88.5%) were of African descent and six were white (6.5%). The male:female ratio was 1.3:1. The mean age was 50.4 years (range 9–86 years). Twenty-two cases were classified as smoldering, 12 as chronic, eight as acute, five as lymphomatous and five as primary cutaneous tumoral types (). In 13 smoldering cases and in all PCT cases no abnormal T-cells were seen in peripheral blood; nine smoldering cases had between 1 to 4% of abnormal cells in peripheral blood. Twenty-seven cases (smoldering and PCT) were primary of the skin and 25 secondary (chronic, lymphomatous and acute). The skin lesions were always multiple, being generalized in half of the cases. Erythroderma was found in 20 cases (), infiltrated plaques in 19, papules in 18, tumors in eight, nodules in six, and erythematous patches in two cases. Rarely, purpuric lesions or vesicles were also found. Erythroderma was found in all clinical types except the PCT type and was the only skin lesion in 15 cases; nodules were not found in the smoldering type. Tumors were not found in smoldering or chronic types. Of the 32 cases in which information concerning childhood eczema was available, 12 patients (37.5%) had had a severe eczema affecting the scalp and other areas of the body early in childhood. One of these patients, an adolescent, presented concurrent HAM/TSP, IDH and ATL Citation[17]. HAM/TSP was observed in 10/52 (19%) cases of ATL, six of the smoldering type, three of the chronic type and one of the acute type. The duration of follow-up varied from 1 to 210 months (mean of 33.80 months). During follow-up four smoldering cases evolved to the acute, chronic or PCT types and two chronic cases to the acute type. Eleven of the 52 patients (21%) are still alive, eight of whom have the smoldering type and three the chronic type. Three of the living patients, two with the smoldering type and one the chronic type, are free of disease. Monoclonal integration of the HTLV-I was confirmed in eight cases with more than five years of follow-up, including one child, and in two cases of adolescents (≤18 years). Twenty-four deaths occurred as a result of the disease, 13 due to infection and four of causes unrelated to ATL. Two smoldering cases died of cardiac disease and another patient with associated HAM/TSP, of mesenteric thrombosis. One chronic case died of uncontrolled diabetes.
Table I. Demographic, clinical and histological features of the 52 cases of adult T-cell leukemia/lymphoma with cutaneous presentation.
Histopathologic and immunohistochemical features
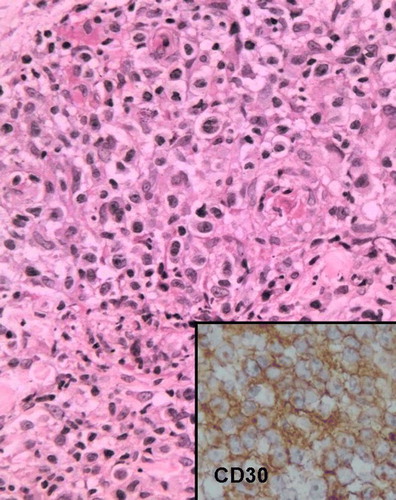
Thirty-one cases were histologically classified as peripheral T-cell lymphoma, unspecified (PTCL-U), 19 as MF, and two as ALCL. Half of the cases with erythroderma had morphology similar to MF. No case of MF had nodules. Tumors were observed in eight cases of PTCL-U and in only one case of MF. The diagnosis of MF corresponded to 59% of the smoldering cases, to 50% of the chronic cases, to 20% of the lymphomatous and PCT cases and to no acute case. All but one case of MF presented epidermotropism, and eight had small Pautrier microabscesses (). Eighteen cases of PTCL-U presented epidermotropism. Pautrier abscesses were found in 16 cases of PTCL-U (), and vasculotropism and folliculotropism in two cases. The two cases of ALCL corresponded to the smoldering and chronic clinical types respectively and survival time exceeded four years in both cases. Histologically, an infiltration of large CD30+ lymphoid cells with nuclear pleomorphism was seen in the upper dermis ().
Figure 2. Mycosis fungoides pattern. Infiltration of atypical lymphocytes in the upper dermis with epidermotropism and Pautrier abscesses (HE, ×160).
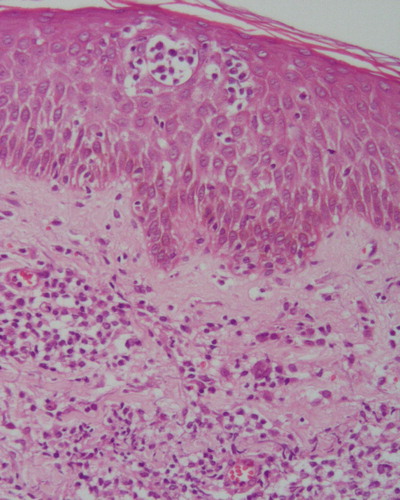
Figure 3. Peripheral Tcell lymphoma, unspecified pattern. Patient with primary cutaneous tumoral ATL. Medium and large cells infiltrating the dermis with Pautrier's abscesses (HE, ×100). Insert: Pleomorphic cells in greater magnification (HE, ×650).
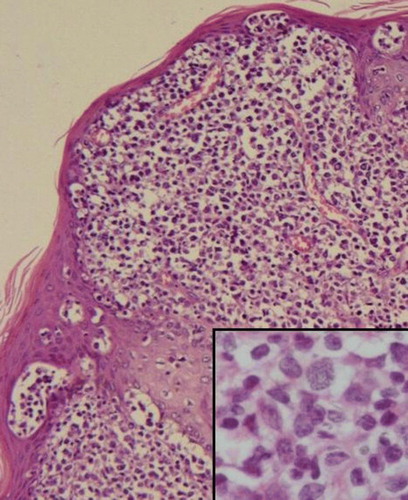
Figure 4. Anaplastic largecell lymphoma pattern. Large lymphoid cells with abundant and eosinophilic cytoplasm and anaplastic nuclei (HE, ×200). Insert: CD30+ cells (×200).
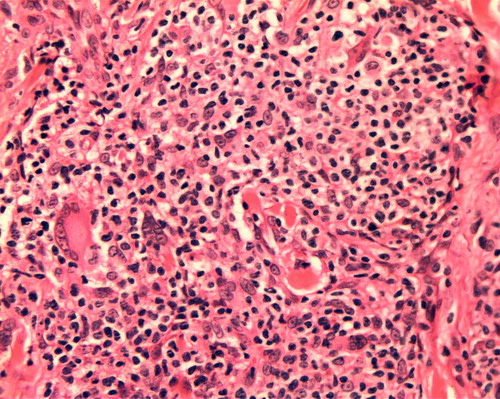
A diffuse infiltration of CD68+ cells with non-necrotizing granulomas was observed in two smoldering cases () with prolonged survival. In one of these cases some giant cells showed atypical nuclei. Specimens stained negative for fungi and acid-fast bacilli. (Grocott and Ziehl-Nielsen stains).
All cases were positive for CD3, CD5 and CD45RO (clones OPD4 and/or UCHL-1), and negative for CD7, CD20 and CD79a. CD25 was positive in 30/33 cases. CD30 was expressed in only two cases. CD8 positivity was observed in 15/52 cases and in 10 of these cases it was associated with CD4 positivity. CD8 was positive in ten cases with MF morphology and in five cases with PTCL-U morphology. Three CD8+ cases with a PTCL-U pattern presented a proliferative index above 18% and only one of these had large cells. No granzyme-B or perforin positivity was observed in the 11 CD8+ patients investigated. TIA positivity varied from 2 to 12%. The ALCL cases were CD30+, ALK-1-negative and presented a high proliferative index (>50%). The proliferative index was >18% in 15/24 cases (62.5%) of PTCL-U and in no case of MF.
Considering the two primary cutaneous forms of ATL, the smoldering type presented 59% of MF morphology and all cases had a proliferative index ≤18% in contrast with the PCT type that presented a PTCL-U morphology and an IP > 18% in 80% of the cases.
Evaluation of survival
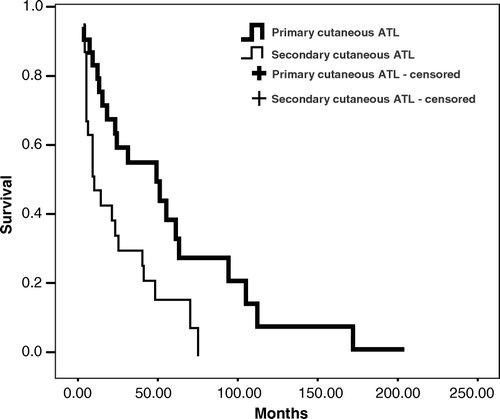
The overall median survival time (MST) was 22 months (95%CI: 1.6–42.4 months). The MST for the different clinical types was: smoldering, 58 months; chronic, 38 months; PCT, 20 months; lymphomatous, 7 months, and acute, 2 months. A statistically significant difference was found between the smoldering type and the acute (p < 0.0001), lymphomatous (p = 0.0021) and PCT (p = 0.019) types, however no statistically significant difference was observed between the smoldering and chronic (p = 0.12) types. The MST in the cases of primary cutaneous ATL (smoldering and PCT types) was 48 months versus 7 months for secondary ATL (). This difference was statistically significant (p < 0.01). The MSTs for the pathologic diagnoses were as follows: PTCL-U, 12 months and MF, 46 months. There was no statistically significant differences in survival between MF and PTCL-U morphologies (p = 0.110), although the cases with MF-like morphologic features had a longer survival. The CD8-positive and negative cases had MSTs of 20 months and 21 months, respectively. This difference was not statistically significant (p = 0.679).
Discussion
The disease manifested itself as erythroderma, plaques, papules, and less frequently as nodules and tumors. There was some correlation between the dermatological lesions and clinical types; tumors were only seen in the more aggressive clinical types (acute, lymphomatous and PCT), nodules were not seen in the smoldering type and erythroderma was not found in the PCT type. The lesions were always multiple and frequently disseminated.
There was a history of severe childhood eczema involving the scalp, possibly representing IDH, in 37.5% of the cases. In addition, one adolescent patient had concurrent IDH, HAM/TSP and ATL Citation[17]. It is generally accepted that IDH, which has been previously described in the Brazilian state of Bahia, predisposes to ATL Citation[6]. In the present study, ATL was also frequently found in association with HAM/TSP, a rare finding in the literature Citation[21]. This association occurred mainly in patients with the smoldering and chronic types.
The histological characteristics of ATL commonly resemble those of a PTCL-U consisting of pleomorphic cells of various sizes and with irregular nuclei described in previous classifications as a pleomorphic T-cell lymphoma Citation[22]. However cases with distinct morphology have been reported Citation[23–25] and in the present study although typical pleomorphic morphology was found in the majority of cases, around one-third of cases had histological characteristics of MF with a neoplastic infiltrate composed of uniformly small and irregular cells and epidermotropism. Many of these cases of ATL presented clinically as erythroderma, while histology revealed epidermotropism and Pautrier abscesses mimicking MF, a cutaneous T-cell lymphoma unassociated with HTLV-I. In cases such as these, clinical and histopathological findings may lead to a diagnosis of MF unless HTLV-I serology is performed. In addition, as previously described Citation[24] two cases in which histology was compatible with ALCL were also observed. In view of the better prognosis of the latter two types of cutaneous T-cell lymphomas, the distinction between these lymphomas and ATL is of clinical relevance.
In this study it was demonstrated that the ATL cases with MF morphology were more frequent in the smoldering and chronic forms and presented a proliferative index <18%, aspects that are indicative of a less aggressive outcome (12). On the other hand, the two ATL cases with ALCL morphology also behaved clinically as less aggressive ATL and had a prolonged follow-up, one of them being still alive. Thus, it is important to classify the ATL cases histologically in order to better evaluate the prognosis.
Diffuse infiltration of histiocytes and non-necrotizing and non-infectious granulomas associated with prolonged survival have been previously reported in ATL Citation[26]. The presence of granulomas has been considered an indication of a host-protective response against ATL progression Citation[26]. In the two present cases with granulomas and diffuse infiltration of histiocytes, survival was prolonged Citation[16], Citation[18].
The majority of the cases presented the classical phenotype of ATL, except for 15 cases, in which CD8 expression was positive, a pattern that has rarely been observed in ATL Citation[13], Citation[27], Citation[28]. These CD8+ cases did not express perforin or granzyme B, indicating that the CD8+ cells had no cytotoxic function, thereby confirming previous findings Citation[28]. TIA-1 was detected in few lymphocytes, but TIA-1 is a cytolytic granule-associated protein that may define a subpopulation of CD8-positive T lymphocytes; however, it is unrelated to activation Citation[29].
Lethality was high, as reflected by the MST of 22 months; however, this was better than the overall MST of 12 months found for the ATL patients in general Citation[12]. MST was shorter in the acute, lymphoma and PCT types, and this finding is in agreement with previous data for ATL patients Citation[12].
In all lymphomas involving the skin, it is important to evaluate whether involvement is primary or secondary, since clinical behavior and prognosis are quite different Citation[19]. A statistically significant difference was found between the MST of patients with primary cutaneous ATL and those with secondary ATL. Two types of primary cutaneous ATL were characterized in this study: the primary cutaneous smoldering type and the PCT type. However ATL was not included as occurring primarily in the skin in the last WHO and in the WHO/EORTC classifications of cutaneous lymphomas Citation[3], Citation[19]. Ratifying a previous observation Citation[12], primary cutaneous smoldering ATL had a much better prognosis (MST of 58 months) as compared to the PCT type (MST of 20 months). In addition, patients with primary cutaneous smoldering ATL mostly died of infections or of causes not directly related to ATL. The MST observed in this study for the primary cutaneous smoldering ATL was higher than those reported by Setoyama et al. (1999) Citation[30]. Provably this difference may be due to different criteria used by these authors.
According to Kamihira et al (1992) Citation[13] the patients with CD8+ cells have a poorer prognosis as compared to patients with the typical CD4 + CD8- phenotype. These authors evaluated patients with acute and chronic ATL. However, in the present study the MST of the CD8+ cases was similar to that of the CD8-negative cases. This may due to the fact that two- thirds of the CD8+ cases in the present study had morphological findings indicative of MF, small cells and a proliferative index ≤18%, aspects that are considered indicative of a better prognosis Citation[12]. Moreover the other one-third had PTCL-U morphology but the proliferative index was >18% in only three cases and only one of these had large cells, aspects suggestive of a poorer prognosis Citation[12]. Considering these findings, CD8 positivity does not seem to be an informative marker of prognosis.
In conclusion, it is important to differentiate between primary and secondary cutaneous ATL and classify the cases histologically in order to better evaluate the prognosis. Considering that there are two types of primary cutaneous ATL, the primary cutaneous smoldering type and the PCT type, which have different characteristics, these types must be also identified. The smoldering type presented a longer survival (58 months) and histological aspects suggestive of better prognosis in contrast to the PCT type that had a shorter survival (20 months) and histological characteristics suggestive of worse outcome.
Acknowledgements
The authors are grateful to the dermatologists and oncologists who provided the follow-up data from their patients. This work was supported by Conselho Nacional de Pesquisa (CNPq) and Fundação de Apoio à Pesquisa no Estado da Bahia (FAPESB). There are no conflicts of interest to be declared.
References
- Barbosa HS, Bittencourt AL, Barreto de Araújo I, Pereira Filho CS, Furlan R, Pedrosa C, et al. Adult T-cell leukemia/lymphoma in northeastern Brazil: A clinical, histopathologic, and molecular study. J Acquir Immune Defic Syndr 1999; 21: 65–71
- Carneiro-Proietti ABF, Catalan-Soares B. Proietti FA and GIPH (Interdisciplinary HTLV-I/II Research Group). Human T Cell Lymphotropic Viruses (HTLV-I/II) in South America: Should it be a public health concern?. J Biomed Sci 2002; 9: 587–95
- Ohshima K, Jaffe ES, Kikuchi M. Adult T-cell leukaemia/lymphoma. WHO Classification of tumors of haematopoietic and lymphoid tissues, SH Swerdlow, E Campo, N Harris, ES Jaffe, SA Pileri, H Stein, et al. IARC, Lyon 2008; 281–4
- Dourado I, Alcantara LC, Barreto ML, Teixeira MG, Galvão-Castro B. HTLV-I in the general population of Salvador, Brazil: A city with African ethnic and sociodemographic characteristics. J Acquir Immune Defic Syndr 2003; 34: 527–31
- de Thé G, Bonford R. An HTLV-I vaccine: Why, how, for whom?. J Acquir Immune Defic Syndr Hum Retrovirol 1993; 9: 381–6
- Oliveira M, de F, Brites C, Ferraz N, Magalhaes P, Almeida F, Bittencourt AL. Infective dermatitis associated with the human T cell lymphotropic virus type I in Salvador, Bahia, Brazil. Clin Infect Dis 2005; 40e: 90–6
- Pombo-de-Oliveira MS, Dobbin JA, Loureiro P, Borducchi D, Maia RC, Fernandes MA, et al. Genetic mutation and early onset of T-cell leukemia in pediatric patients infected at birth with HTLV-I. Leukemia Res 2002; 26: 155–61
- Bittencourt AL, Primo J, Oliveira MFP. Manifestations of the human T-cell lymphotropic virus type I infection in childhood and adolescence. J Ped 2006; 82: 411–20
- Dosaka N, Tanaka T, Miyachi Y, Imamura S, Kakizuka A. Examination of HTLV-I integration in the skin lesions of various types of adult T-cell leukemia (ATL): Independence of cutaneous-type ATL confirmed by Southern blot analysis. J Invest Dermatol 1991; 96: 196–200
- Shimoyama, M, members of the Lymphoma Study Group. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Hematol 1991;79:428–37.
- Tsukasaki, K, Hermine, O, Bazarbachi, A, Ratner, L, Ramos, JC, Harrington, W, Jr, , et al. Definition, prognostic factors, treatment and response criteria of Adult T-cell Leukemia-Lymphoma: A proposal from an international consensus meeting. 2007. J Clin Oncol ( in press).
- Bittencourt AL, Vieira MG, Brites CR, Farré L, Barbosa HS. Adult T-cell leukemia/lymphoma (ATL) in Bahia, Brazil: Analysis of prognostic factors in a group of 70 patients. Am J Clin Pathol 2007; 128: 875–82
- Kamihira S, Sohda H, Atogami S, Toriya K, Yamada Y, Tsukazaki K, et al. Phenotypic diversity and prognosis of adult T-cell leukemia. Leukemia Res 1992; 16: 435–41
- Lee, MW, Korean Dermatopathology Research Group. Characteristics of cutaneous lymphomas in Korea. Clin Exp Dermatol 2003;28:639–46.
- Etoh K, Tamiya S, Yamaguchi K, Okayama A, Tsubouchi H, Ideta T, et al. Persistent clonal proliferation of human T-lymphotropic virus type I-infected cells in vivo. Cancer Res 1997; 57: 4862–7
- Bittencourt AL, Barbosa HS, Requião C, Silva AC, Vandamme AM, Van Weyenbergh J, et al. An exceptional pediatric case of ATL with a mixed CD4+ and CD8+ phenotype and a particularly indolent course. J Clin Oncol 2007; 25: 2480–2
- Farré L, de Oliveira M, de F, Primo J, Vandamme AM, Van Weyenbergh J, Bittencourt AL. Early sequential development of infective dermatitis, human T cell lymphotropic virus type 1-associated myelopathy, and adult T cell leukemia/lymphoma. Clin Infect Dis 2008; 46: 440–2
- Bittencourt AL, Barbosa HS, Pimenta A, Farre L. A case of adult T-cell leukemia/lymphoma(ATL) with a survival of more than 13 years. Acta Oncol 2008; 47: 981–3
- Burg, G, Kempf, W, Cozzio, A, Feit, J, Willemze, R, S Jaffe, E, , et al. WHO/EORTC classification of cutaneous lymphomas 2005: Histological and molecular aspects. J Cutan Pathol 2005;32:647–74.
- Boenish, T. Immunochemical staining methods. Carpinteria: Dako Corporation, 1989.
- Tamiya S, Matsuoka M, Takemoto S, Shimizu K, Yamaguchi K, Mita S, et al. Adult T cell leukemia following HTLV-I-associated myelopathy/tropical spastic paraparesis: Case reports and implication to the natural course of ATL. Leukemia 1995; 9: 1768–70
- Jaffe ES, Ralfkiaer. Mature T-cell and NK-cell neoplasms:introduction. WHO classification of tumors. Pathology and genetics. Tumors of haematopoietic and lymphoid tissues, ES Jaffe, N Harris, H Stein, JW Vardiman. IARC, Lyon 2001; 189–94
- Detmar M, Pauli G, Anagnostopoulos I, Wunderlich U, Herbst H, Garbe C, et al. A case of classical mycosis fungoides associated with human T cell lymphotropic virus type I. Br J Dermatol 1991; 124: 198–202
- Takimoto Y, Tanaka H, Tanabe O, Kuramoto A, Sasaki N, Nanba K. A patient with anaplastic large-cell lymphoma (Ki-1 lymphoma) showing clonal integration of HTLV-1 proviral DNA. Leukemia 1994; 8: 507–9
- D'Incan M, Antoniotti O, Gasmi M, Fonck Y, Chassagne J, Desgranges C, et al. HTLV-I associated lymphoma presenting as mycosis fungoides in an HTLV-I non-endemic area: A viromolecular study. Br J Dermatol 1995; 132: 983–8
- Setoyama M, Katahira Y, Kanzaki T, Kerdel FA, Byrnes JJ. Adult T-cell leukemia/ lymphoma associated with noninfectious epithelioid granuloma in the skin: A clinico- pathologic study. Am J Dermatopathol 1997; 19: 591–5
- Ciminale V, Hatziyanni M, Felber BK, Bear J, Hatzakis A, Pavlakis GN. Unusual CD4 + CD8+ phenotype in a Greek patient diagnosed with adult T-cell leukemia positive for human T-cell leukemia virus type I (HTLV-I). Leukemia Res 2000; 24: 353–8
- Ohshima K, Haraoka S, Suzumiya J, Sugihara M, Kanda M, Shimazaki K, et al. Absence of cytotoxic molecules in CD8- and/or CD56-positive adult T-cell leukemia/lymphoma. Virchows Arch 1999; 435: 101–4
- Anderson P, Nagler-Anderson C, O'Brien C, Levine H, Watkins S, Slayter HS, et al. A monoclonal antibody reactive with a 15-kDa cytoplasmic granules-associated protein defines a subpopulation of CD8+ T lymphocytes. J Immunol 1990; 144: 574–82
- Setoyama M, Katahira Y, Kanzaki T. Clinicopathologic analysis of 124 cases of adult T-cell leukemia/lymphoma with cutaneous manifestations: The smouldering type with skin manifestationshas a poorer prognosis than previously thought. J Dermatol 1999; 26: 785–90