To the Editor,
In a recently published Letter-to-the-Editor Citation[1], the authors showed significant characteristics of the EUD and general EUD concepts based on their previous experience and research work Citation[1]. With the present response to this letter we try to expand this discussion including the most significant alternative concepts that have been reported. For many decades, the need for a change in the approach of treatment planning has been realized. Initially the lack of 3-dimensional information in dose delivery led to dose prescriptions to certain points in patient (usually at the center of target). This had as a consequence the introduction of uncertainties in the association of dose with the treatment outcome since different dose distributions having the same prescription dose are usually delivered to the patients. Furthermore, prescribed dose as a concept is an indirect descriptor of the treatment outcome. The clinically applicable prescribed doses were derived from clinical trials utilizing certain treatment techniques and they constitute compromises between the benefits and the side effects of the treatment, which are expressed in terms of tumor control and normal tissues complications. However, this does not mean that they are optimal for different irradiation techniques than those from which they were derived. For this reason there is a need to use a concept that relates the 3-dimensional dose distribution delivered to a certain organ with the control or injury observed to that organ during follow-up. That is because the primary goal of treatment planning is to have a direct association of treatment configuration with the treatment outcome.
The first attempt to reduce the 3-dimensional dose distribution to a single dose that is related to the treatment outcome (expressed in terms of response probability) was made by Brahme Citation[2], Citation[3] with the introduction of the effective dose, Deff concept, which is an alternative. Dose distributions within organs or volumes of interest are never exactly uniform. On the contrary, they can be strongly non-uniform especially for normal tissues. According to Deff, for relatively small dose variations the effect in the target is well related to the mean target dose and for larger dose inhomogeneities, the minimum target dose is more closely related to the effective dose. The drawback of this concept was that although it utilized radiobiological parameters characterizing the dose-response relation of the given organ, its mathematical formulation was not accurate since two different dose distributions could have the same Deff value but different response probabilities. The introduction of the equivalent uniform dose (EUD) from Niemierko Citation[4] partly solved this inconsistency for tumors since it managed to establish a direct relation of the value of EUD with the value of the survival function. This concept assumes that any two dose distributions are equivalent if they eradicate the same fraction of clonogenic cells. Both of these concepts provide a method to account for the biological effects when reporting the absorbed dose. However, they do not apply to all treatment plans since they do not accurately deal with complex targets or organs at risk. Furthermore, they do not provide a common prescription basis for different dose plans. The introduction of the general EUD concept did not solve the previous problems since it also carried the drawback of Deff according to which two different dose distributions could have the same gEUD value but different response probabilities Citation[5]. According to Zhou and colleagues the gEUD and Poisson-based statistical TCP model appear mathematically inconsistent unless there is a finite critical dose or a positive threshold in the tumor response to radiation, that the tumor cell survival fraction obeys and the tumor dose distribution is limited to a certain dose region Citation[6]. Otherwise there is no dose independent constant power index a that will guarantee a tumor uniformly irradiated by gEUD produces the same TCP in Poisson statistics framework as that from the original heterogeneous dose distribution.
All the above mentioned problems were overcome with the introduction of the biologically effective uniform dose () by Mavroidis and colleagues Citation[7], Citation[8], which is a combination of the basic characteristics of the Deff and EUD concepts.
is the uniform dose that causes exactly the same tumor control or normal tissue complication probability as the real dose distribution on a complex target or normal tissue patient. In complex patient cases multiple targets or multiple organs at risk of different radiosensitivities are involved. The general expression of
is defined for a given tumor or tissue from its dose-response relation without dependence on the radiobiological model. The mathematical expressions of the different concepts are given below.
for the binomial model
where denotes the 3-dimensional dose distribution. EUD can also be expressed as:
where
can also be expressed as:
In the present analysis, two step-wise dose distributions are utilized in order to examine how the different concepts are affected from the level of dose inhomogeneity and shape of dose distribution. In both cases, a series of dose distributions are produced, which have the same mean dose (80 Gy) but different relative standard deviations, which range from 0 to 100% by changing the dose to the different fractions of the target. The radiobiological parameters that were used are: and
or
and
and a = − 10 for the gEUD concept. As it is shown in the upper part of , all the examined concepts lie between the mean and minimum dose of the dose distribution, which means that the cold spots have a larger biological weight in parallel-like tissues such as tumors. It can be observed that the
and EUD coincide at the beginning but as the dose inhomogeneity increases they deviate since the Poisson model does not give very accurate results in the region of low doses. Deff behaves similarly but the curves of
and Deff differ from each other in their absolute values. Finally, the gEUD follows closely the curve of Dmin deviating significantly from the curve of
, which is the one that is derived directly from the response probability of the tissue. All these different dose distributions are characterized by the same mean value implying that
is not an appropriate unit to describe a dose distribution. After observing the dependence of the different concepts on the inhomogeneity level of a dose distribution we can investigate their dependence on its shape. In the lower diagram of three pairs of dose distributions were selected from the two series of step-wise dose distributions. The selected dose distributions are characterized by small, medium and large dose inhomogeneities in the target. For each one of them, the response probabilities of the target and the corresponding values of the
, EUD, Deff and gEUD were calculated. The dose distribution having two dose steps is denoted by 1, whereas the dose distribution having four dose steps by 2. We chose pairs of dose distributions that produce the same target response probabilities. It is apparent that at small dose inhomogeneities, dose distributions producing the same response probabilities are associated with biological doses, which have the same value (only gEUD differs slightly) (). At medium dose inhomogeneities, the values of
and EUD coincide, whereas the Deff and gEUD concepts differ from the previous ones in value. Furthermore, they have different values for the two types of dose distributions (which however produce the same response probabilities). The same characteristics are observed at large dose inhomogeneities but even more pronounced. Observable differences between
and EUD can be seen only at very large dose variations, which stem from the differences of the Binomial and Poisson models.
Figure 1. Upper panel: In the left graphs, the two step-wise dose distributions that are tested are shown. In both cases the mean dose is kept constant at the value of 80 Gy while the relative standard deviation varies from 0 to 100% by changing the dose to the different fractions of the target. is related to the minimum dose for parallel structured organs (such as tumors) at large dose variations.
behaves similarly but the curves of
and
differ from each other in their absolute values. All these different dose distributions are characterized by the same mean value implying that
is not an appropriate unit to describe a dose distribution. The curve of EUD follows closely that of
apart from the region of low doses. Finally, the gEUD follows closely the curve of Dmin deviating significantly from the curve of
, which is the one that is derived directly from the response probability of the tissue. Lower panel: By using the two types of step-wise dose distributions shown in the upper panel and selecting distributions of small, medium and large dose inhomogeneities in the target, the response probabilities of the target and the corresponding values of the
, EUD, Deff and gEUD were calculated. It is apparent that the Deff and gEUD concepts are not as accurate as the
and EUD in medium and large dose inhomogeneities.
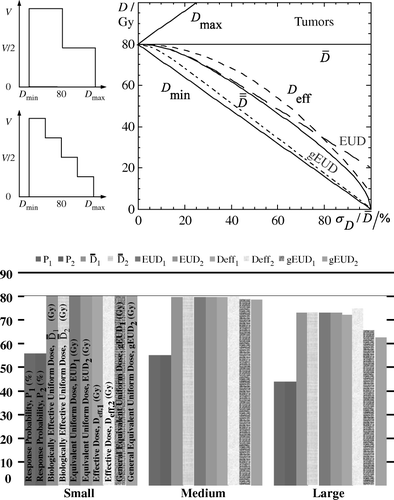
Table I. Comparison of , EUD, Deff and gEUD for different dose distributions and dose ihnomogeneities.
The primary goal of the proposed radiobiological doses is to reduce a given 3D dose distribution to a single radiobiological dose, which should be related to treatment outcome. Since dose distributions are usually inhomogeneous, fractionation correction to a certain dose per fraction should be involved in the process of dose reduction. It has to be mentioned that the original derivation of EUD is not from TCP but from the survival function (SF) leading to the same results for both of the cases when the standard LQ model is applied. However, when the mathematical formula of TCP becomes more complex (e.g. by including more radiobiological mechamisms such as tumor repopulation, reoxygenation, redistribution etc) then the derivation of EUD from the survival function becomes inaccurate and the proper way is to derive it directly from the TCP as it happens in the case of . Generally, different clinics and radiotherapy centers use different radiobiological models to determine the dose-response relations of tumors and normal tissues. In order to use the EUD concept, they have to abandon the model they have experience with and switch to the survival expression used for the EUD. This restriction is not imposed by the
concept and this is one of this model major advantages indicating a wide clinical usability. As it is shown in the example given by Wang and colleagues regarding the hypofractionated EBRT of a prostate cancer, EUD may give reasonable and practical results to the clinicians, however these results are strongly dependent on the accuracy by which the radiobiological parameters are known. Especially, in the case of the general EUD concept, the use of a single only radiobiological parameter is expected to be associated with significantly large confidence intervals (error bars). This is because the number of biological mechanisms involved in the expression of a clinical endpoint is large and the mathematical formulation of each one of them is complex. So, the use of a simple power-law formula is too simplistic to account for all these processes. These weaknesses of the gEUD concept are also discussed by Wang and colleagues.
The delay of using the concepts of TCP, NTCP in the clinic is the lack of trust regarding their accuracy. So, the main task over the past years was to associate certain doses or dose-volume thresholds with a certain clinical endpoint. The benefit of this approach was that these doses or dose-volume thresholds could be accurately measured and be trusted by the clinicians. The approach of using radiobiological parameters (which are determined by clinical trials and are not measured directly) to calculate the biological equivalent of these doses, does not offer to the clinicians the same certainty. So, since the transition from the physical doses to radiobiologically equivalent doses involves the use of radiobiological parameters, it is more reasonable to calculate a measure that is directly associated with the treatment outcome rather than to use a descriptor, which is indirectly related to the treatment outcome. It is becoming clear that a transition from the dosimetric treatment plan evaluation to a radiobiological treatment plan evaluation, where instead of doses to different tissues we discuss in terms of expected responses (response probabilities), becomes a necessity.
Important clinical aspects of D
The significance of calculating a radiobiological dose is to be finally used in conjunction with the corresponding estimated response probability. In dose-response diagrams the mean dose to the PTV is usually used on the dose axis. It is apparent that using this setup it is difficult to compare the corresponding response curves since they move to different places. This is a consequence of the fact that different dose distributions are characterized by different mean target doses for the same response probability. This problem is even more pronounces for targets that have regions of different radiosensitivity (e.g. hypoxic regions). As it is shown in the left diagram of , where a radiobiological treatment plan comparison is performed regarding the clinical effectiveness of three different radiation modalities, the use of on the dose axis has solved this problem since it forces the response probabilities of the whole PTV (PB) to coincide for all the treatment plans under comparison. Since the target response curves of the three dose distributions lie at the same position, the corresponding response curves of the organs at risk can be easily compared individually or combined in the total complication response curve, PI. In this diagram, the confidence intervals of the dose-response curves are also illustrated. It is apparent that the use of the
concept on the dose axis provides the appropriate dose prescription basis for making such comparisons practical and clinical useful. The normalization using
gives emphasis to the therapeutic window, which characterizes each treatment plan. Such as the dose volume histogram chart is a good illustration of the volumetric dose distribution delivered to the patient, so is the biological evaluation plot (
diagram) of a dose plan a good illustration of the expected clinical outcome.
Figure 2. Left diagram: In this diagram, a radiobiological treatment plan comparison is performed regarding the clinical effectiveness of three different radiation modalities illustrating also the confidence intervals of the dose-response curves. It is apparent that the use of the concept on the dose axis provides the appropriate dose prescription basis for making such comparisons practical and clinical useful. Right diagram: The dose-response curve derived using a radiobiological model for a certain tissue is shown. On the same diagram, the dose-response points of each patient have been drawn. Those points were calculated using the individual dose distribution delivered to each patient and the model parameters. P denotes probability and the
is the unit of the dose axis. The patients with response are indicated by crosses while the patients without response by open circles. The histograms represent the observed response rates at the corresponding dose intervals.
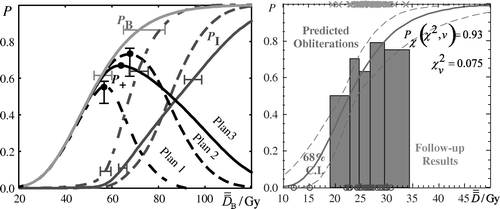
Another important clinical use of is illustrated in the right diagram of . Using a certain set of radiobiological parameters of a given radiobiological model, the dose-response curve of a tissue is calculated for a range of uniform doses. Subsequently, the response probability is calculated for every patient using again those parameters and the individual dose distribution delivered. By applying the concept of biologically effective uniform dose on these probabilities, the corresponding
values are found. Plotting these dose-response points on the existing diagram they will by definition fall exactly on the theoretical dose-response curve. To examine whether the theoretical curve reproduce the observed response rates it is enough to compare these values for the region around the prescribed dose used by the center where the patients were treated (using a statistical method such as the chi-square test). If the two values are close enough then the parameters can be used for predicting the treatment outcome for the applied technique. This is a simple way to examine if a set of parameters is compatible with the clinical practice that a center uses.
References
- Wang JZ, Mayr NA, Yuh TC. Behind EUD. Acta Oncol 2008; 47: 971–86
- Aaltonen P, Brahme A, Lax I, Levernes S, Näslund I, Reitan JB, Turesson I. Specification of dose delivery in radiation therapy. Recommendations by the Nordic Association of Clinical Physics (NACP). Acta Oncol 1997; 36(Suppl 10)1–32
- Brahme, A. Which parameters of the dose distribution are best related to the radiation response of tumours and normal tissues?, In: Proceedings of the Interregional Seminars for Europe, the Middle East and Africa Organized by the Leuven: IAEA; 1994. p 37–58.
- Niemierko A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med Phys 1997; 24: 103–10
- Niemierko A. A generalized concept of equivalent uniform dose (abstr.). Med Phys 1999; 26: 1100
- Zhou SM, Das S, Wang Z, Marks LB. Relationship between the generalized equivalent uniform dose formulation and the Poisson statistics-based tumor control probability model. Med Phys 2004; 31: 2606–9
- Mavroidis P, Lind BK, Van Dijk J, Koedooder K, De Neve W, De Wagter C, et al. Comparison of conformal radiation therapy techniques within the dynamic radiotherapy project ‘Dynarad’. Phys Med Biol 2000; 45: 2459–81
- Mavroidis P, Lind BK, Brahme A. Biologically effective uniform dose () for specification, report and comparison of dose response relations and treatment plans. Phys Med Biol 2001; 46: 2607–30
- Källman P, Lind BK, Brahme A. An algorithm for maximizing the probability of complication free tumor control in radiation therapy. Phys Med Biol 1992; 37: 871–90
- Mavroidis P, Lind BK, Theodorou K, Laurell G, Fernberg JO, Lefkopoulos D, et al. Statistical methods for clinical verification of dose-response parameters related to esophageal stricture and AVM obliteration from radiotherapy. Phys Med Biol 2004; 49: 3797–816
