Abstract
Introduction. The pattern of failure (POF) after first-line systemic therapy in advanced non-small cell lung cancer (NSCLC) is unknown. We evaluate the POF in this setting to estimate the potential value of consolidative stereotactic body radiation therapy (SBRT). Materials and methods. The records of consecutive NSCLC patients presenting to the University of Colorado, Denver (UCD) between January 2005 and June 2008 were reviewed. Patients with measurable advanced stage NSCLC who received first-line systemic therapy and follow-up at UCD were eligible. In these patients, sites of disease at maximal response were evaluated for theoretical SBRT eligibility, based on institutional criteria. All patients were followed to extracranial progression. The POF was categorized as local (L) for lesions known prior to treatment or distant (D) for new lesions. Results. Among 387 consecutive lung cancer patients (all stages), 64 met the eligibility criteria and 34 were SBRT-eligible. Among all eligible patients, first extra-cranial progression was L-only in 64%, D-only in 9% and L + D in 27%. Among SBRT-eligible patients, POF was L-only in 68%, D-only in 14% and L + D in 18%. In SBRT-eligible patients, time to first progression was 3.0 months in those with L-only failure versus 5.7 month in those with any D failure (HR 0.44; 95% CI 0.22–0.90). Conclusions. The predominant POF in patients with advanced NSCLC after first-line systemic therapy is local-only. The current analysis suggests that SBRT could improve time to progression in a substantial proportion of patients. The estimated increase in time to progression using this approach would be approximately 3 months.
Maintaining durable disease control in patients with advanced or metastatic non-small cell lung cancer (NSCLC) is an elusive goal. Platinum-based doublet chemotherapy is the standard first–line treatment in patients with advanced or metastatic NSCLC. Times to progression with this approach, however, are only 3–4 months Citation[1]. Modest improvements in time to progression with chemotherapy have been achieved in select patients with the addition of Bevacizumab, an anti-vascular endothelial growth factor monoclonal antibody, or of Erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor, particularly in specific subgroups of patients likely to have significant EGFR pathway-addiction Citation[2], Citation[3]. However, despite these advances, disease progression is inevitable and remains the major challenge in the management of patients with advanced or metastatic NSCLC.
Traditionally, radical radiotherapy has been reserved for patients with stage I-III disease, and the most common indication for radiation therapy in the management of patients with metastatic NSCLC has been in the palliative treatment of pain or other symptoms directly resulting from tumor extension in the primary or metastatic site. However, advances in radiation therapy treatment technology have led the development of stereotactic body radiation therapy (SBRT), whereby patients with oligometastatic disease in a variety of sites may safely receive a potent, tightly focused, non-invasive treatment within a conveniently abbreviated regimen of five or fewer treatments. SBRT can efficiently achieve a very high rate of durable control of the treated lesion in a variety of tumor locations Citation[4–7]. Thus, selected patients with a limited burden of metastatic disease may be considered for SBRT with the goal of completely eradicating all measurable disease. This usage is conceptually concordant with surgical metastatectomy for hepatic metastases in colorectal cancer, for example Citation[8].
To our knowledge the POF after modern first-line systemic therapy in advanced and metastatic NSCLC treated without local therapy has not previously been reported. Prospective studies evaluating chemotherapy Citation[1], molecular targeted therapies Citation[9–11] or combinations of each Citation[2], Citation[3], Citation[12–14], have had overall and/or progression-free survival as the primary endpoint, but their analyses have not included detailed information about the POF. While it seems logical that initial progression would most likely occur at a site of known disease, the proportion of patients with first failure in sites of initial disease (local failure) and the median time to local versus distant progression remains unreported. An understanding of the POF is necessary in order to estimate the potential benefit of SBRT applied to sites of residual measurable tumor at the point of maximal response to first-line systemic therapy. This application of SBRT might be referred to “consolidative,” with the goal of extending the progression-free interval and potentially overall survival as well.
In the current study, we performed a detailed analysis of the POF to quantify the likelihood of local-only progression and perform a time-to-event analysis to evaluate for a difference in time to local versus distant progression. These data might then serve to inform the design of future prospective studies of consolidative SBRT at the point of maximal response during or immediately after first-line systemic therapy for advanced NSCLC.
Material and methods
Patients
New patients seen at the University of Colorado, Denver (UCD) with NSCLC in the period from January 2005 to June 2008 were analyzed. Eligibility criteria included presence of advanced measurable disease with treatment and follow up during first-line therapy at UCD. Patients with advanced disease included those with stage IIIB NSCLC with malignant pleural effusion and those with intra-thoracic recurrence after initial local therapy. Patients could not have received prior systemic therapy alone for advanced or metastatic disease; however, patients could have received radiotherapy alone or with concurrent chemotherapy for treatment of localized disease prior to the development of metastases. Furthermore, patients could have received palliative radiotherapy for metastatic disease for emergency situations, as long as there was remaining measurable untreated disease at the initiation of first-line systemic therapy. Patients with brain metastases were included if treated with surgery, radiotherapy or both prior to the initiation of first-line systemic therapy.
Systemic therapy
First-line systemic therapy included cytotoxic chemotherapy and/or molecular targeted therapies administered as part of standard of care regimens or within clinical trials. Systemic therapy regimens are shown in . Systemic therapy in eligible patients was administered at UCD in all cases. Chemotherapy was administered until disease progression, toxicity requiring discontinuation or until the completion of four to six cycles. Molecular targeted therapies, in the form of monoclonal antibodies or tyrosine kinase inhibitors, were administered until disease progression or toxicity requiring discontinuation.
Table I. Systemic therapy regimens (both standard of care regimens and clinical trials)
Patterns of failure
Pre-treatment and follow-up radiological imaging studies with radiology reports, including bi-dimensional measurements of target lesions, were reviewed. Computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) scans were used to analyze progression. Eligible patients were required to have a minimum of two scans available for review. Imaging studies were obtained every 4–8 weeks during chemotherapy and every 4–12 weeks for patients on maintenance targeted therapy. Extra-cranial progression was defined using RECIST (Response Evaluation Criteria in Solid Tumors) criteria, i.e. an increase in the sum of the longest diameters from bi-dimensional measurements by greater than 20% or the appearance of new extracranial lesions Citation[15].
Progression was categorized as local (L) or distant (D). L progression was defined as progression of lesions present prior to the start of first-line systemic therapy, whereas D progression was defined as the emergence of new lesions. Site of first progression was recorded. Actuarial time to progression for patients with first L progression and first D or L + D progression was determined. Intracranial progression was noted but was not counted as D failure.
SBRT eligibility
After the maximum response to first-line therapy prior to progression, disease was categorized as eligible or not eligible for SBRT, based on institutional SBRT trial criteria. Although theoretical SBRT eligibility was determined, none of the patients received “consolidative” or prophylactic radiation therapy prior to first extra-cranial progression. The point of maximal response was defined as the second of two consecutive scans at least one month apart showing stabilization of disease or the last scan prior to disease progression. For patients in the latter group, the first scan showing disease progression was used to assess SBRT eligibility because this is the time point at which consolidation therapy would be considered. SBRT-eligible sites included lung, liver, axial skeleton and soft-tissue sites amenable to treatment to a minimum dose of 30 Gy in 5 fractions. Patients were required to have≤ extra-cranial lesions. Disease in the lung and liver was limited to ≤ 3 sites each. Lung lesions were required to have maximum cumulative diameter (of all lesions) < 7cm. Liver lesions were required to be ≤ 6 cm in maximum dimension.
Patients with a malignant pleural effusion prior to the start of systemic therapy were considered eligible for SBRT if there was complete radiographic resolution of the effusion with systemic therapy. Patients with locally recurrent disease after prior thoracic radiotherapy were considered eligible for SBRT if these criteria were met: (1) the patient had adequate lung function, (2) a minimum period of 6 months had elapsed since prior radiotherapy and (3) a discrete lesion was discernable that could be distinguished from radiation treatment effect by PET-CT and met the remainder of the SBRT-eligibility criteria described above. There was no limitation on prior radiotherapy dose for patients with locally recurrent disease to be eligible for SBRT.
Lesions not considered measurable lesions in the analysis of SBRT eligibility included lung parenchymal lesions ≤ 7 mm, unless these lesions were FDG-avid or new on comparison to prior scans, and non-FDG-avid lesions in a patient with other FDG-avid lesions. Lesions of the spine were eligible if there was no evidence of frank epidural extension. Patients with intra-peritoneal, retroperitoneal and pelvic disease were not considered eligible for SBRT due to unknown tolerance of the intestinal wall to high-dose hypofractionated radiation therapy.
Statistics
The site of first extra-cranial progression was determined for all patients using the product-limit method of Kaplan and Meier Citation[16]. The median time to progression was determined for patients with first failure in the L versus those with first progression in D sites or combined L + D sites and was compared using log rank test Citation[17]. Similarly, the time to first L failure versus any first D failure was determined for the subgroup of patients eligible for SBRT and compared using log rank test. Hazard ratios (HR) for each comparison were reported with 95% confidence intervals.
Results
Patients
The medical records for 387 consecutive patients with NSCLC seen within the University of Colorado, Denver Lung Cancer Program between January 2005, and June 2008, were reviewed. Sixty-four patients with advanced or metastatic disease met the overall inclusion criteria and were analyzed. Fifty-seven (89%) of patients were staged with PET-CT prior to first-line systemic therapy. Patient characteristics are shown in . Median age was 63 years. Adenocarcinoma was the most common histology (64%). Metastatic disease was present in 89%, including brain metastases in 31%, prior to the initiation of systemic therapy. Thirty-four of these patients (53%) were also considered eligible, in theory, for treatment with SBRT. The sites of disease in SBRT-eligible patients are detailed in . The median number of measurable lesions was less for the SBRT-eligible cohort compared with the non-SBRT-eligible cohort (3 lesions vs. 6 lesions). Similarly, the median size of the largest lesion as measured by the product of bi-dimensional measurements was less in SBRT-eligible patients (10.7 cm2 vs. 12.1 cm2).
Table II. Patient characteristics
Table III. Sites of disease in SBRT-eligible patients
Patterns of failure
In the entire group, the POF at time of first extra-cranial progression was L only in 64%, D only in 9%, and both L + D in 27%. Likewise, for the SBRT-eligible group, the pattern of failure at first extra-cranial failure was L only in 68%, D only in 14%, and L + D in 18%. In patients with D or both L + D failure, new lung metastases (n = 13) and liver (n = 7) were the most common site of progression.
The inclusion of patients with treated brain metastases in the analysis did not significantly change the observed POF. Brain metastases represented the first site of progression in six patients (9%) in the entire group and five patients (15%) in the SBRT-eligible cohort. Of the six patients with first progression in the brain, three had brain metastases treated prior to starting first-line systemic therapy. Including brain metastasis in the analysis, the pattern of failure at time of first progression was L only in 63%, D only in 11%, and both L + D in 25%. Likewise, for the SBRT-eligible group, the pattern of failure was L only in 68%, D only in 21%, and both L + D in 11%.
Time to progression
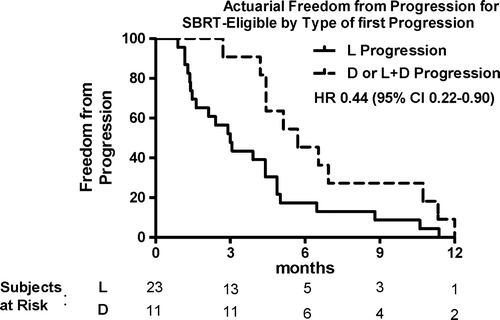
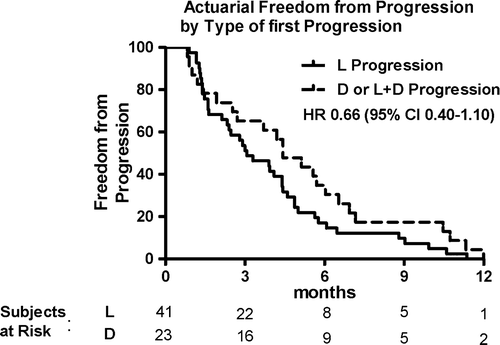
The median time to extra-cranial progression for the entire group was 3.9 months. Among patients with L only failure, the median TTP was 3.1 months, compared with 4.4 months for patients with D only or L + D failure (, HR 0.66; 95% CI 0.40–1.10). Freedom from progression at 6 months for patients with L only failure was 17.1% compared with 34.8% for patients with D or both L + D failure.
Figure 1. Freedom from progression according to type of first progression for all patients. Curves are compared using log rank method.
In the SBRT-eligible group, median TTP was 4.4 months. In SBRT-eligible patients with L only failure, the median TTP was 3.0 months, whereas for those with D only or L + D failure, the median TTP was 5.7 months (; HR 0.44; 95% CI 0.22–0.90). Freedom from progression at 6 months for patients with L only failure was 17.4% versus 45.5% for patients with any aspect of D failure.
Discussion
In the current study, for patients with advanced or metastatic NSCLC treated with first-line systemic therapy, local progression is the dominant pattern of failure. Among SBRT-eligible patients, the first site of extra-cranial progression was local only in 68%, and time to progression in patients with L-only failure was 2.7 months shorter than time to progression in patients with any D failure. While these findings are not necessarily unexpected, to the authors’ knowledge, this represents the first reported detailed analysis of the patterns of failure after first-line therapy for NSCLC. These data suggest that the addition of effective local therapy to known sites of disease using SBRT with consolidative intent after first-line systemic therapy could potentially alter the patterns of failure and prolong the progression-free interval in these patients.
Stereotactic body radiation therapy (SBRT) is a highly conformal, focused method for the delivery of ablative radiation therapy. SBRT has the advantages of being convenient, with treatment delivered in fewer than five fractions, and has demonstrated favorable local control rates within studies evaluating its use in the treatment of metastatic lesions of the lung, liver and spine. In a phase I/II trial of SBRT for patients with 1–3 pulmonary metastases lead by the University of Colorado, 63 lesions were treated in 38 patients using SBRT at a dose of 36–60 Gy in 3 fractions. Twenty-nine patients were treated at the phase II dose of 60 Gy in 3 fractions. Actuarial local control of all SBRT-treated lesions was 96% at 2 years Citation[5]. These same investigators performed a phase I/II trial of SBRT in patients 1–3 liver metastases, using the same dose-escalation scheme and an identical phase II dose. In the 63 SBRT-treated lesions, 2-year actuarial local control was 92% Citation[6]. In addition, Chang and colleagues performed a phase I/II trial of SBRT for the treatment of spinal metastases using prescription doses of 30 Gy in 5 fractions or 27Gy in 3 fractions. For the 74 lesions treated in this study, freedom from progression at 12 months was 84% Citation[7].
Approximately one-half of assessable patients with advanced or metastatic NSCLC in the current study were also considered eligible for treatment with SBRT at the time of maximal response to first line systemic therapy. Eligibility for SBRT in our study was defined using criteria from an institutional SBRT trial. The eligibility criteria for lung, liver and spinal lesions are consistent with criteria from prior studies using SBRT Citation[5–7]. Soft tissue sites were also considered eligible for SBRT if amenable to treatment with a minimum dose of 30 Gy in 5 fractions. Soft tissue lesions in SBRT-eligible patients included the lesions of the axilla in five patients and supraclavicular fossa in one patient (). The minimum dose of 30 Gy in 5 fractions was chosen because of the high rates of local control described in studies using this fractionation regimen in the treatment of other primary and metastatic tumors. As previously described, this regimen was associated with a high rate of local control for metastatic lesions of the spine Citation[7]. Moreover, this regimen has been extensively studied in patients with high risk melanoma, with 5-year local-regional control rates approaching 90%. The reported incidence of late toxicity with this regimen is very low, including in patients with tumors of the axilla and supraclavicular fossa Citation[18], Citation[19].
Mediastinal sites were also considered eligible for SBRT in the current study if these lesions met the other eligibility criteria. Investigators at MD Anderson Cancer Center recently reported their experience using four-fraction SBRT in 27 patients with central (defined as lesions within 2 cm of the proximal bronchial tree) or superiorly located NSCLC. The esophageal dose constraints in this study required that the maximum dose to 1 mL of esophagus be < 35 Gy (8.8 Gy/fx) and that the maximum dose to 10 mL be < 30 Gy (7.5 Gy/fx). Using these constraints, there were no cases of esophagitis observed Citation[20]. Moreover, in a phase II trial of SBRT (50 Gy in 10 fractions) for oligometastatic disease, Milano and colleagues treated thoracic lymph nodes, including mediastinal nodes, in 24 patients and observed no cases of grade 3 esophagitis Citation[21]. Based on these data, we considered 30 Gy in 5 fractions to be a safe and feasible SBRT regimen for mediastinal disease, provided that esophageal constraints can be met.
We chose to evaluate patients as SBRT-eligible or not at the point of maximal response to treatment. This time point was chosen in order to maximize the likelihood of SBRT-eligibility and to maximize the benefit from first-line systemic therapy before considering consolidation therapy. The alternative strategy, of considering only those who are eligible before any cytoreductive effect of systemic therapy, could also be considered in the future. Among SBRT-eligible patients, the median time to local only progression was 2.7 months shorter than the median time to distant only or both L + D progression. These observations suggest that the potential elongation of the progression-free interval with effective consolidation therapy in SBRT-eligible patients would be of the order of 3 months.
Brain metastases were not included in the primary analysis of patterns of failure and in the time to progression analysis. However, a secondary analysis revealed little change in the pattern of first failure when CNS progression was included in the model. We chose not to include CNS progression in our primary analyses because local therapy in the presence of metastatic disease is already an established standard in the treatment for brain metastases. At least three randomized clinical trials have shown improvements in overall Citation[22] and progression-free survival Citation[23] with intensified local therapy in patients with a limited number of brain metastases, though this effect is not uniformly reproducible Citation[24].
In conclusion, roughly half of patients with advanced or metastatic NSCLC are eligible for SBRT after first line systemic therapy and local failure represents the only site of first progression in approximately two-thirds. Among SBRT-eligible patients, time to local progression was 2.7 months shorter than time to distant progression. These findings both provide the foundation for prospective evaluations of SBRT as consolidative therapy to known sites of disease as an addition to the first-line treatment of advanced NSCLC and may be used to estimate sample size requirements for future clinical trials testing whether SBRT would have a significant effect on failure patterns or progression-free survival in patients with advanced NSCLC.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346: 92–8
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355: 2542–50
- Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, et al. TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2005; 23: 5892–9
- McCammon, R, Schefter, TE, Gaspar, LE, Zaemisch, R, Gravdahl, D, Kavanagh BD. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2008, (in press).
- Rusthoven, KE, Kavanagh, BD, Burri, SH, Chen, C, Cardenes, H, Chidel, MA, , et al. Mature results of a multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2008, (in press)
- Rusthoven, KE, Kavanagh, BD, Cardenes, H, Stieber, VW, Burri, SH, Feigenberg, SJ, , et al. Mature results of a multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2008, (in press).
- Chang EL, Shiu AS, Mendel E, Mathews LA, Mahajan A, Allen PK, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine 2007; 7: 151–60
- Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, et al. Liver resection for colorectal metastases. J Clin Oncol 1997; 15: 938–46
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005; 353: 123–32
- Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005; 366: 1527–37
- Lilenbaum R, Axelrod R, Thomas S, Dowlati A, Seigel L, Albert D, et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol 2008; 26: 863–9
- Fukuoka M, Yano S, Giaccone G, Tamura J, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial). J Clin Oncol 2003; 21: 2237–46
- Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller M, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: A phase III trial INTACT 1. J Clin Oncol 2004; 22: 777–84
- Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: A phase III trial INTACT 2. J Clin Oncol 2004; 22: 785–94
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Vereij J, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163–70
- Ang KK, Peters LJ, Weber RS, Morrison WH, Frankenthaler RA, Garden AS, et al. Postoperative radiotherapy for cutaneous melanoma of the head and neck region. Int J Radiat Oncol Biol Phys 1994; 30: 795–8
- Strom EA, Rossi MI. Adjuvant radiation therapy after axillary lymphadenectomy for metastatic melanoma: Toxicity and local control. Ann Surg Oncol 1995; 2: 445–9
- Chang JY, Balter PA, Dong L, Yang Q, Liao Z, Jeter M, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008; 72: 967–71
- Milano MT, Katz AW, Muhs AG, Philip A, Buchholz DJ, Schell MC, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer 2008; 112: 650–8
- Patchell RA, Tibbs PA, Walsh JW, Depsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990; 322: 494–500
- Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet 2004; 363: 1665–72
- Mintz AH, Kestle J, Rathbone MP, Gaspar L, Hugenholtz H, Fisher B, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 1996; 78: 1470–6