Abstract
Background. The recommendation of adjuvant chemotherapy for colon cancer with lymph node metastases, based on two studies from USA, was reluctantly accepted by Norwegian medical doctors. It was therefore decided to assess the role of adjuvant therapy with 5fluorouracil (5-FU) combined with levamisole (Lev) in a confirmatory randomised study. Material and methods. Four hundred and twenty five patients with operable colon and rectum cancer, Stage II and III (Dukes’ stage B and C), were from January 1993 to October 1996, included in a randomised multicentre trial in Norway. The age limits were 18–75 years. Therapy started with a loading course of bolus i.v. 5-FU (450 mg/m2) daily for 5 days and p.o. doses of Lev (50 mg x 3) for 3 days. From day 28 a weekly i.v. 5-FU dose (450 mg/m2) were administered for 48 weeks. From day 28 also p.o. doses of Lev (50 mg x 3) for 3 days were given every 14 days. In total 214 patients were randomised to 5FU/Lev and 211 were included in the control group with surgery alone. Some did not comply with the inclusion and exclusion criteria, thus leaving 206 evaluable patients in each group. Results. There was no significant survival difference between the two groups at 5 years: Disease-free survival (DFS) was 73% after chemotherapy, 68% (p=0.24) in the control group, and corresponding cancer specific survival (CSS) 75% and 71%, respectively (p=0.69). There was no difference between the two groups when analysed for colon and rectum separately. However, the subgroup of colon cancer with stage III exhibited a statistically significant difference both for DFS, 58% vs. 37% (p=0.012) and CSS, 65% vs. 47% (p=0.032) in favour of adjuvant chemotherapy. The benefit was further statistically significant for women but not for men. Toxicity was generally mild and acceptable with no drug related fatalities. Conclusions. Colon cancer patients with lymph node metastases benefit from adjuvant chemotherapy with 5-FU/Lev with acceptable toxicity. In a subgroup analysis females did better than males. Rectal cancer does not benefit from this regimen.
The “National Institute of Health Consensus Conference” concluded in 1990 that adjuvant chemotherapy should be offered to patients with colon and rectal cancer with lymph node metastases, largely based on three positive studies Citation[1–5]. However, previous results compiled in a meta-analysis concluded that the survival benefit of 5-fluorouracil (5-FU) alone was only marginal Citation[6]. Despite that the combination of 5-FU and Levamisol (Lev) had raised substantial interest, the role of Lev as single agent had earlier been widely used in different cancers without convincing benefit Citation[4], Citation[7–12]. The study by Laurie et al. indicated a benefit from Lev alone, while other studies indicated no benefit of this drug in stage III colon cancer Citation[2], Citation[13]. These results were widely discussed and according to a European recommendation, stage III colon cancer patients unable to enter clinical trials should be offered 5-FU and Lev regimen unless there were medical or psychological contra-indications Citation[3]. After a joint discussion between oncologists and surgeons we decided to perform a Norwegian confirmatory study which was organised as a parallel study with several other Danish and Swedish studies Citation[14]. A joint analysis for overall survival has been previously presented for 2 225 patients included in these parallel Scandinavian studies Citation[15]. We here report data not included in the joint report on cancer specific survival (CSS), disease-free survival (DFS) as well as toxicity from the Norwegian study which recruited 425 patients.
Material and methods
The aim of the study was to confirm the important data presented by Moertel et al. Citation[4]. We therefore decided to include all patients who had a radical resection for an adenocarcinoma of the colon or rectum, with no evidence of distant metastases during surgery or by a standard work-up consisting of chest x-ray, ultrasound of the abdomen or CT of the abdomen, as well as standard blood count including CEA. Age limits were set to 18–75 years, the exclusion criteria are given in .
Table I. Exclusion criteria.
Surgery
The surgery should follow each department's fixed routines. The type of surgery was classified as right-sided hemicolectomy, left-sided hemicolectomy, extended hemicolectomy, sigmoid resection, low anterior resection, abdominoperineal resection with sigmoidostomy or further specified surgical techniques. From 1993 the total mesorectal excision technique was introduced in a separate project in Norway, and this technique was increasingly adopted for rectal cancers as reported elsewhere Citation[16], Citation[17]. Patients with a direct extension of the tumour into neighbouring organs like the bowels, uterus, vagina, and bladder could be included if the affected areas were radically resected (“en block”) as verified by histopathological examination of the surgical specimen. The surgeons should always in a surgery form indicate whether the operation was considered radical or if there was any suspected or verified residual tumour. The operation specimens were sent to the local pathology department where special attention was paid to the selection of lymph nodes for analysis.
Chemotherapy
The treatment should start within 3 to 4 weeks, maximally 42 days after surgery. We followed strictly the Moertel regimen with administration of 5-FU as a short intravenous (i.v.) infusion within 5 minutes at a dose of 450 mg/m2 for 5 consecutive days the first course. Lev was administered orally at a dose of 50 mg 3 times daily the first three days during the loading course. Thereafter 5-FU was given weekly from day 28 at a dose of 450 mg/m2 by a 5 minutes infusion repeated for 48 weeks. The dose was approximated to the nearest 50 mg. Lev was given as 50 mg 3 times daily for 3 days every second week. The administration of therapy was performed at the outpatient section, either from the relevant surgical or oncology department, based upon local tradition. The cost of chemotherapy, diagnostic procedures and follow-up was covered by the Norwegian Labour and Welfare Organisation.
Recording and follow-up
There were fixed forms for recording data at the time of randomisation and surgery, in addition the pathologists had to fill out a trial specific pathology form. For chemotherapy there were separate forms with specific grading of toxicity after each course using WHO grading criteria. After randomisation there were follow-up forms scheduled for 6, 12, 18, 24, 36, 48 and 60 months. The routine examination included rectoscopy if technically feasible, chest x-ray, ultrasound of the abdomen as well as recording of blood count and CEA. All patients had a colonoscopy before surgery or within three months after surgery, thereafter every 3rd year.
Randomisation
Patients were stratified according to hospital, localisation of tumour in colon or rectum and tumour stages, Stage II and III (Dukes’ B or C). The responsible physician had to confirm the check list by phone before the randomisation which followed a computer procedure with weighted block randomisation (unknown for the participants). The program was constructed at the Regional Office for Cancer Research, Health Region West (Bergen), but the practical randomisation was performed at six regional offices where also the forms where collected. The final data handling was centralised to Bergen.
Ethics
The patients were given written information and had to sign a written informed consent form before randomisation. The trial was approved by the official Regional Ethics Committee of Health Region West, and thus followed the official health regulations in Norway.
Statistics
Based on a presumed 10% absolute improvement from 50 to 60% cancer specific survival (CSS) at 5 years (30 to 40% in stage III and 65 to 75% in stage II, a sample size of 1 076 patients was to be recruited (p<0.05, power 0.90; 814 if the required power was 0.80). At the start of the trial we had a joint agreement with the other Scandinavian trials that we should have an interim analysis of the joint studies for overall survival. The time from randomisation to diagnosis of local or distant recurrence was recorded as disease-free survival (DFS), all other events censored. For calculation of CSS death of colorectal cancer or treatment complications were recorded, all other death causes were handled as censored. Survival was recorded by Kaplan-Meier method and the log-rank test was used for assessment of differences, regarding p<0.05 as statistically significant by two-tailed tests.
Results
In total, 425 patients were recruited into the trial from January 1993 to October 1996. Two hundred and eleven patients were allocated to the control group with surgery alone and 214 were randomised to chemotherapy. The patients’ characteristics showed no difference in known prognostic factors between the two groups (data not shown). The total number of lymph nodes examined in the surgery alone group was 8.4 median pr. patient and 9.0 pr. patient in the chemotherapy group.
At the time of final analysis the forms revealed that in the surgery group 3 patients had to be reclassified as stage I (Dukes’ stage A), one patient had colonic metastases from an uterine papillary adenocarcinoma and one patient had pulmonary metastases at staging, leaving 206 fully evaluable patients in the surgery alone group. In the chemotherapy group six patients were in stage I, whereas one patient also had a mammary carcinoma and another had previously been treated for a rectal cancer, leaving 206 evaluable patients in the chemotherapy group. There was no statistically significant difference in known prognostic factors between the two evaluable groups (). The median number of chemotherapy courses actually administered was 43 of scheduled 49, including the loading course. Despite not all patients received the scheduled chemotherapy, all evaluable patients are included in the final analyses based on the intention-to-treat principle. Scheduled start of chemotherapy was within 6 weeks, but the therapy was actually instituted within this time limit in 85% of the patients. Therapy was started within 8 weeks in 92% of the patients.
Table II. Patient characteristics for evaluable patients.
Tumour control and survival
For DFS there was no difference for the two randomisation groups: The 3-year DFS was 73% (95%CI 67–79%) after chemotherapy and 67% (95%CI 61–74%) after surgery alone. The corresponding 5-year DFS was 69% (95%CI 63–75%) and 63% (95%CI 56–70%), respectively, exhibiting a non significant difference of only 5.5% (p=0.24), A. This reflects the difference in distant metastases, as there was no difference at all for local tumour control (data not shown). There was no difference between the two sexes as regards the overall data.
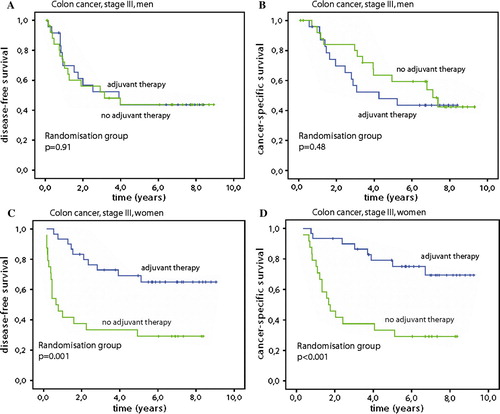
Figure 1. Disease free survival (A) and cancer specific survival (B) for all randomised patients (206 patients in each group). Disease free survival (C) and cancer specific survival (D) for colon cancer stage III patients. Fluorouracil and levamisol was administered for one year as adjuvant chemotherapy after surgery versus surgery alone.
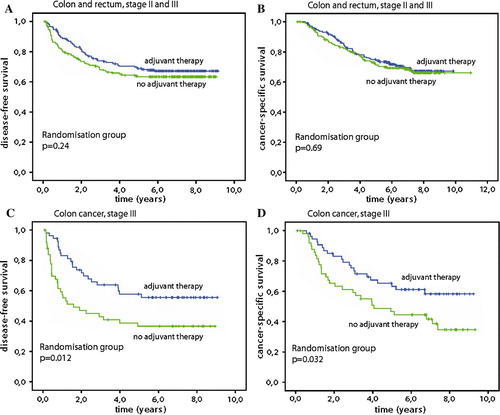
When we analysed for CSS, the main goal of the study, there was no difference between the two groups as the chemotherapy group had 75.0% (95%CI 69–81%) and surgery alone 71.5% (95%CI 65–78%) 5-year CSS, respectively, a difference of only 3.5% (p=0.69), B. Also for overall survival (OS) there was no difference: chemotherapy 71% (95%CI 65–78%) versus 66% (95%CI 60–73%) after surgery alone, with 5% difference (p=0.40). When the factors age, stage, localisation (colon and rectum), sex, randomisation group and total grade of side effects were included in Cox multivariate analyses, only stage (p<0.001) and randomisation group (p=0.047) remained significant for DFS, while only stage (p<0.001) was a significant predictive factor for CSS.
Subgroup analyses
When we analysed patients with primary tumours localised in colon and rectum separately, there was no statistically significant difference between the two locations with regard to the endpoints of the study. However, patients with colon cancer stage III exhibited a highly statistically significant difference between the two groups: With chemotherapy the patients obtained 58% (95%CI 44–71%) DFS at 5 years in contrast to 37% (95%CI 23–50%) after surgery alone (p=0.012), C. This difference was also reflected in a CSS of 65% (95%CI 53–78%) against 47% (95%CI 33–61%) at 5 years (p=0.032), D. When further analysing this subgroup for sex, the female patients had a DFS after chemotherapy of 69% (95%CI 52–86%) against 33% (95%CI 15–52%) (p=0.001) after surgery alone, . This resulted in a CSS benefit of 75% (95%CI 59–91%) after chemotherapy in contrast to 33% (95%CI 15–52%) after surgery alone (p<0.001). There was no significant difference for male patients in stage III who had a DFS of 44% in both groups and CSS survival was also similar at 5 years, 48% (95%CI 28–68%) after chemotherapy and 59% (95%CI 40–79%) in the surgery alone group.
Toxicity
Three patients died of surgical complications, one in the surgery group died of bleeding from varicous veins after operation for a secondary adenocarcinoma near the anastomosis, while two in the chemotherapy group died of bleedings as complications to liver resections for advanced metastases and a misdiagnosed haemangioma, respectively. None of these were related to administration of chemotherapy. In total 190 (92%) of the 206 patients randomised to chemotherapy actually started the treatment. We obtained 186 complete forms on side effects which revealed that the chemotherapy regimen was generally well tolerated and particularly the haematological toxicity was low (): 50% had no side effects, 29% reported WHO grade 1 toxicity, 7% grade 2 side effects, while 1% and 0.7% exhibited WHO grade 3 and 4 toxicity, respectively. The toxicity profile was similar in men and women. There was no relation between the degree of toxicity and freedom from progression and survival.
Table III. Recorded chemotherapy toxicity.
Discussion
Despite not showing a statistically significant benefit of adjuvant chemotherapy with 5-FU and Lev for all patients with rectal and colon cancer, we confirmed that this regimen is effective after surgery for adenocarcinoma of the colon with lymph node metastases (stage III) both for DFS and CSS, as previously reported for overall survival Citation[2], Citation[4], Citation[18–21]. This is in accordance with current recommendations. We could not disclose any benefit for patients with primary tumours of the rectum or patients without lymph node metastases (stage II), but one should note that the study which was prematurely terminated, was underpowered to demonstrate small benefits. The role of adjuvant chemotherapy in stage II is still an open question as the benefit remains limited with 5-FU based regimens Citation[18], Citation[19], Citation[22–26]. Lev was rapidly replaced by calcium folinate in combination with 5-FU and Lev is not currently used for colorectal cancer Citation[27–33]. These studies also revealed that 6 months with 5-FU and calcium folinate was as effective as 12 months with 5-FU and Lev.
For rectal cancer patients we could not demonstrate any benefit of adjuvant 5FU and Lev. This is in accordance with the pooled Scandinavian analysis Citation[15]. The type of surgery, standard or total mesorectal excision was not recorded. However, based on experience with postoperative chemotherapy and radiation before introduction of the total mesorectal excision technique and a joint analysis of three Japanese trials of oral fluoropyrimidines added to the same type of surgery, adjuvant 5-FU based chemotherapy is widely recommended for four to six months both in USA and Europe Citation[34–39]. Limitation of adjuvant chemotherapy to the patients not previously exposed to preoperative radiation is also proposed Citation[40]. In a German randomised trial preoperative chemoradiotherapy reduced the local recurrence rate but had no effect on distant recurrence rate compared with postoperative chemoradiotherapy Citation[41]. Recently a benefit of adjuvant chemotherapy also in rectal cancer was reported in two groups each including 474 patients in the Quasar study, with a relative risk of recurrence of 0.58 (p=0.01) and relative risk of dying of 0.77 (p=0.05) Citation[42]. In an EORTC trial, preoperative or postoperative chemotherapy added to radiation only reduced local recurrences with no effect on distant metastases Citation[43]. A subgroup analysis indicated that only good-prognosis patients benefited from adjuvant chemotherapy as also shown as a non-significant trend (p=0.09) in our joint Scandinavian report for Stage II rectal cancer Citation[15], Citation[44]. Thus, despite widely practiced, prolonged adjuvant chemotherapy except in conjunction with radiation, remains based on limited evidence. More studies on adjuvant chemotherapy should be carried out in rectal cancer as still about one third of these patients die from distant metastases.
In the present study the patients should start chemotherapy as soon as possible, and not later than 6 weeks. Actually 85% of the patients had their first treatment within this period, and 92% within 8 weeks. This may be the reason for our observed benefit of chemotherapy for DFS and CSS in colon cancer stage III. Some studies have indicated improved survival in patients starting chemotherapy within 8 weeks compared to those who started after 8–12 weeks Citation[45–47], while others reported no significant difference Citation[48]. Also for breast cancer delaying adjuvant chemotherapy start seems detrimental if started later than 3 months after surgery Citation[49]. As these data is based on retrospective analyses with limited power, a firm conclusion cannot yet be drawn. However it seems wise to start as soon as the patients are fit for chemotherapy, and no later than 8 weeks after colon surgery Citation[45].
Generally colorectal cancer localisation differ by sex, females tend more often to develop right sided cancers in higher ages, while men tend to develop left sided tumours Citation[50], Citation[51]. In our subgroup analysis of colon cancer patients stage III, the observed benefit was statistically significant for women only. This observation may be due to low numbers or chance, but has been previously reported in a nonrandomised comparison of patients treated with or without adjuvant chemotherapy from Australia Citation[52]. Mortality ratios show more favourable trends for females with colon cancer in Europe and Norway Citation[53–55]. A similar observation has been reported also for rectal cancer patients with postoperative short-term chemotherapy and chemoradiotherapy Citation[56]. However, it must be underlined that this is not a general finding based on fluoropyrimidines as adjuvant chemotherapy Citation[18], Citation[38].
Despite low toxicity of the adjuvant regimen, there was reduced compliance -- only 90% of randomised patients actually started chemotherapy. The motivation of the patients to proceed with chemotherapy was also low as many withdrew after experiencing low grade toxicity, typically grade 1-2 diarrhoea or general fatigue. This may reflect the written information where it was clearly stated that the alternative was no therapy at all. As most chemotherapy was administered at the local surgical outpatient clinics in this trial, it may also reflect lack of proper support and motivation of the patients to proceed with therapy despite some side effects. This is standard for all established chemotherapy indications where the therapy is regarded as necessary.
The addition of oxaliplatin to the combination of 5-FU and calcium folinate has more recently further improved the DFS and CSS in randomised trials, and the FOLFOX regimen is currently the international standard therapy after curative resection of adenocarcinomas of the colon stage III Citation[57], Citation[58]. Oral fluoropyrimidines is equally effective as 5-FU and calcium folinate as adjuvant therapy in patients not tolerating oxaliplatin based regimens Citation[59], Citation[60]. Despite its efficacy in advanced colorectal cancer, irinotecan failed to demonstrate improved tumour control as adjuvant treatment Citation[61]. This underlines that adjuvant chemotherapy can not be based on presumptions, but must rely on data from prospective trials.
Conclusion
Despite premature closure, the randomised study which accrued 412 evaluable patients with colon and rectal adenocarcinoma stage II and III (Dukes’ B and C), confirms that adjuvant chemotherapy with 5-FU and Lev is an effective treatment for colon cancer stage III but is ineffective for rectal cancer. Females with colon cancer stage III benefited from chemotherapy in a further subgroup analysis, while there was no statistically significant effect in men.
Acknowledgements
The Norwegian Gastointestinal Cancer Group received grants from The Norwegian Cancer Society.
References
- NIH Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. J Am Med Assoc 1990;264:1444–50.
- Laurie JA, Moertel CG, Fleming TR, Wieand HS, Leigh JE, Rubin J, et al. Surgical adjuvant therapy of large-bowel carcinoma: An evaluation of levamisole and the combination of levamisole and fluorouracil. J Clin Oncol 1989; 7: 1447–56
- Metzger U. Adjuvant therapy for colon and rectal cancer. NIH Consensus Development Conference. Eur J Cancer 1990; 26: 753–5
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990; 322: 352–8
- Wolmark N, Fisher B, Rockette H, Redmond C, Wickerham DL, Fisher ER, et al. Postoperative adjuvant chemotherapy or BCG for colon cancer: Results from NSABP Protocol C-01. J Natl Cancer Inst 1988; 80: 30–6
- Buyse M, Zeleniuch-Jacquotte A, Chalmers TC. Adjuvant therapy of colorectal cancer. Why we still don't know. J Am Med Assoc 1988; 259: 3571–8
- Quirt IC, Shelley WE, Pater JL, Bodurtha AJ, McCulloch PB, McPherson TA, et al. Improved survival in patients with poor-prognosis malignant melanoma treated with adjuvant levamisole: A phase III study by the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1991; 9: 729–35
- Schnall SF, Macdonald JS. Adjuvant therapy in colorectal carcinoma. Semin Oncol 1991; 18: 560–70
- Spitler LE. A randomized trial of levamisole versus placebo as adjuvant therapy in malignant melanoma. J Clin Oncol 1991; 9: 736–40
- Stevenson HC, Green I, Hamilton JM, Calabro BA, Parkinson DR. Levamisole: Known effects on the immune system, clinical results, and future applications to the treatment of cancer. J Clin Oncol 1991; 9: 2052–66
- Verhaegen H, De Cree J, De Cock W, Verhaegen-Declerq ML, Verbruggen F. Levamisole therapy in patients with colorectal cancer. Immunotherapy of Human Cancer. Rosenberg SA. New York: Elsevier; 1982. p 225–9.
- Windle R, Bell PRF, Shaw D. Five year results of a randomized trial of adjuvant 5fluorouracil and levamisole in colorectal cancer. Brit J Surg 1987; 74: 569–72
- Arnaud JP, Buyse M, Adloff M, Nordlinger B, Pector JC, Duez N. Interim analysis of a double-blind phase-III clinical trial of adjuvant levamisole versus control in resectable Dukes-C colon cancer: A study of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Rec Results Cancer Res 1988; 110: 101–3
- Pahlman L, Glimelius B. [Treatment of colorectal cancer with antineoplastic agents. Too early to introduce routine adjuvant therapy]. Lakartidningen 1991; 88: 208–9
- Glimelius B, Dahl O, Cedermark B, Jakobsen A, Bentzen SM, Starkhammar H, et al. Adjuvant chemotherapy in colorectal cancer: A joint analysis of randomised trials by the Nordic Gastrointestinal Tumour Adjuvant Therapy Group. Acta Oncol 2005; 44: 904–12
- Wibe A, Carlsen E, Dahl O, Tveit KM, Weedon-Fekjaer H, Hestvik UE, et al. Nationwide quality assurance of rectal cancer treatment. Colorectal Dis 2006; 8: 224–9
- Wibe A, Møller B, Norstein J, Carlsen E, Wiig JN, Heald RJ, et al. A national strategic change in treatment policy for rectal cancer – implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum 2002; 45: 857–66
- Gill S, Loprinzi CL, Sargent DJ, Thome SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much?. J Clin Oncol 2004; 22: 1797–806
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes' B2 colon cancer. J Clin Oncol 1995; 13: 2936–43
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: A final report. Ann Intern Med 1995; 122: 321–6
- Wolmark N, Rockette H, Mamounas E, Jones J, Wieand S, Wickerham DL, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes' B and C carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol 1999; 17: 3553–9
- Arkenau HT, Bermann A, Rettig K, Strohmeyer G, Porschen R. 5-Fluorouracil plus leucovorin is an effective adjuvant chemotherapy in curatively resected stage III colon cancer: Long-term follow-up results of the adjCCA-01 trial. Ann Oncol 2003; 14: 395–9
- Au E, Ang PT, Seow-Choen F, Soo KC, Low CH, Chng HC, et al. Adjuvant chemotherapy for patients with resected Dukes' C and high-risk B2 colon cancer with fluorouracil and levamisole. Ann Acad Med Singapore 1998; 27: 733–7
- Benson AB, 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 2004; 22: 3408–19
- Marsoni S. Efficacy of adjuvant fluorouracil and leucovorin in stage B2 and C colon cancer. International Multicenter Pooled Analysis of Colon Cancer Trials Investigators. Semin Oncol 2001; 28: 14–9
- Zaniboni A, Labianca R. Adjuvant therapy for stage II colon cancer: An elephant in the living room?. Ann Oncol 2004; 15: 1310–8
- Anonymous. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet 1995;345:939–44.
- Anonymous. Comparison of flourouracil with additional levamisole, higher-dose folinic acid, or both, as adjuvant chemotherapy for colorectal cancer: A randomised trial. QUASAR Collaborative Group. Lancet 2000;355:1588–96.
- Francini G, Petrioli R, Lorenzini L, Mancini S, Armenio S, Tanzini G, et al. Folinic acid and 5-fluorouracil as adjuvant chemotherapy in colon cancer. Gastroenterology 1994; 106: 899–906
- Haller DG. An overview of adjuvant therapy for colorectal cancer. Eur J Cancer 1995; 31A: 1255–63
- Mamounas E, Wieand S, Wolmark N, Bear HD, Atkins JN, Song K, et al. Comparative efficacy of adjuvant chemotherapy in patients with Dukes' B versus Dukes' C colon cancer: Results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C-01, C-02, C-03, and C-04). J Clin Oncol 1999; 17: 1349–55
- O'Connell MJ, Mailliard JA, Kahn MJ, Macdonald JS, Haller DG, Mayer RJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 1997; 15: 246–50
- Schippinger W, Jagoditsch M, Sorre C, Gnant M, Steger G, Hausmaninger H, et al. A prospective randomised trial to study the role of levamisole and interferon alfa in an adjuvant therapy with 5-FU for stage III colon cancer. Br J Cancer 2005; 92: 1655–62
- Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. New Engl J Med 1985;312:1465–72.
- Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. New Engl J Med 1991; 324: 709–15
- Minsky BD. Adjuvant management of rectal cancer: The more we learn, the less we know. J Clin Oncol 2007; 25: 4339–40
- O'Neil BH, Tepper JE. Current options for the management of rectal cancer. Curr Treat Options Oncol 2007; 8: 331–8
- Sakamoto J, Ohashi Y, Hamada C, Buyse M, Burzykowski T, Piedbois P. Efficacy of oral adjuvant therapy after resection of colorectal cancer: 5-year results from three randomized trials. J Clin Oncol 2004; 22: 484–92
- Wolpin BM, Meyerhardt JA, Mamon HJ, Mayer RJ. Adjuvant treatment of colorectal cancer. CA Cancer J Clin 2007; 57: 168–85
- Glynne-Jones R, Mathur P, Elton C, Train ML. The multidisciplinary management of gastrointestinal cancer. Multimodal treatment of rectal cancer. Best Pract Res Clin Gastroenterol 2007; 21: 1049–70
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–40
- , Quasar Collaborative GroupGray R, Barnwell J, McConkey C, Hills RK, Williams NS, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007;370:2020–9.
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006; 355: 1114–23
- Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: Does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol 2007; 25: 4379–86
- Berglund A, Cedermark B, Glimelius B. Is it deleterious to delay the start of adjuvant chemotherapy in colon cancer stage III?. Ann Oncol 2008; 19: 400–2
- Chau I, Norman AR, Cunningham D, Tait D, Ross PJ, Iveson T, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol 2005; 16: 549–57
- Hershman D, Hall MJ, Wang X, Jacobson JS, McBride R, Grann VR, et al. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer 2006; 107: 2581–8
- Andre T, Quinaux E, Louvet C, Colin P, Gamelin E, Bouche O, et al. Phase III study comparing a semimonthly with a monthly regimen of fluorouracil and leucovorin as adjuvant treatment for stage II and III colon cancer patients: Final results of GERCOR C96.1. J Clin Oncol 2007; 25: 3732–8
- Lohrisch C, Paltiel C, Gelmon K, Speers C, Taylor S, Barnett J, et al. Impact on survival of time from definite surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol 2006; 24: 4888–94
- Elsaleh H. The microsatellite instability phenotype in human colorectal carcinoma: Relationship to sex, age and tumor site. Gastroenterology 2001; 121: 230–4
- Matanoski G, Tao XG, Almon L, Adade AA, Davies-Cole JO. Demographics and tumor characteristics of colorectal cancers in the United States, 1998-2001. Cancer 2006; 107: 1112–20
- Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B. Assosiation of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet 2000; 355: 1745–50
- Angell-Andersen E, Tretli S, Coleman MP, Langmark F, Grotmol T. Colorectal cancer survival trends in Norway 1958-1997. Eur J Cancer 2004; 40: 734–42
- Burdy G, Panis Y, Alves A, Nemeth J, Lavergne-Slove A, Valleur P. Identifying patients with T3-T4 node-negative colon cancer at high risk of recurrence. Dis Colon Rectum 2001; 44: 1682–8
- Fernandez E, Bosetti C, La Vecchia C, Levi F, Fioretti F, Negri E. Sex differences in colorectal cancer mortality in Europe, 1955-1996. Eur J Cancer Prev 2000; 9: 99–104
- Tepper JE, O'Connell M, Niedzwiecki D, Hollis DR, Benson AB, 3rd, Cummings B, et al. Adjuvant therapy in rectal cancer: Analysis of stage, sex, and local control–final report of intergroup 0114. J Clin Oncol 2002; 20: 1744–50
- Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350: 2343–51
- Kuebler JP, Wieand HS, O'Connell MJ, Smith RE, Colangelo LH, Yothers G, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol 2007; 25: 2198–204
- Lembersky BC, Wieand HS, Petrelli NJ, O'Connell MJ, Colangelo LH, Smith RE, et al. Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol 2006; 24: 2059–64
- Twelves C, Wong A, Nowacki MP, Abt M, Burris H, 3rd, Carrato A, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005; 352: 2696–704
- Saltz LB, Niedzwiecki D, Hollis D, Goldberg RM, Hantel A, Thomas JP, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J Clin Oncol 2007; 25: 3456–61