Abstract
Introduction. Nearly 40% of patients with advanced NSCLC are in performance status (PS) 2. These patients have a shorter life expectancy than PS 0/1 patients and they are underrepresented in clinical trials. Data on how platinum-based combination chemotherapy affects Health Related Quality of Life (HRQOL) of patients with PS 2 are scarce and the treatment of this important group of patients is controversial. Methods. A national multicenter phase III study on platinum based chemotherapy to 432 advanced NSCLC patients included 123 patients with PS 2. To explore the treatment impact on HRQOL, the development of HRQOL during the first nine weeks were compared between PS 2 and PS 0/1 patients. We used the EORTC QLQ-C30 and QLQ-LC13 questionnaires. Standardized area under the curve for all HRQOL items, and HRQOL responses classified as better, stable or worse, were compared between the groups. Results. Whereas the demographic data at baseline were well balanced between the groups, the PS 2 patients had significantly worse function and more severe symptoms than the PS 0/1 patients. In response to combination chemotherapy, the PS 2 patients had a more profound improvement of global QOL, cognitive function, fatigue, dyspnea, sleeping problems and appetite loss in comparison to the PS 0/1 group. Conclusions. PS 2 NSCLC patients seem to achieve valuable HRQOL benefits from platinum-based combination therapy. Prospective clinical studies with predefined HRQOL outcomes in PS 2 patients are needed to confirm these findings.
Non-small cell lung cancer (NSCLC) is a common malignancy and a leading cause of cancer-death worldwide. The majority of NSCLC patients present with advanced disease Citation[1] and palliation and health related quality of life (HRQOL) are thus important aspects of their treatment.
It is estimated that 30 – 40% of the advanced NSCLC patients present with performance status (PS) 2 Citation[2], Citation[3]. These patients have shorter life expectancy, and their poor PS is suspected to make them more vulnerable to treatment-related side effects Citation[4]. PS is also the strongest predictor of survival in patients with advanced NSCLC Citation[5]. Despite these important facts, PS 2 patients have been greatly underrepresented in clinical trials.
The importance of HRQOL as an outcome of chemotherapy trials for patients with cancer is widely acknowledged. A review of 32 randomized trials examining HRQOL in patients with advanced NSCLC undergoing chemotherapy, has confirmed the superiority of chemotherapy over best supportive care regarding HRQOL and symptom improvement Citation[6]. An Outcomes Working Group Citation[7] within the American Society of Clinical Oncology has concluded that, even in the absence of prolonged survival, treatment guidelines can be based on improvements of HRQOL alone.
Investigations on symptomatic improvements and HRQOL benefits as trial endpoints are strongly recommended by a European Experts Panel Citation[8]. Furthermore, the NICE guidelines on lung cancer Citation[9] call for further research into the effects of chemotherapy on HRQOL in patients with advanced NSCLC and PS 2.
Bottomley et al. Citation[10] reviewed HRQOL methods in 29 randomized controlled NSCLC trials. In general, they found limited details in the reporting of HRQOL results. HRQOL was mainly used as a secondary endpoint, and limited space was used for the presentation of these data. As a result, the authors suggested separate HRQOL publications in order to make adequate explanations and presentations of the findings.
Platinum-based 2-drug combination chemotherapy is the established first line treatment of advanced NSCLC Citation[11], but remains controversial in the treatment of patients with PS 2. Several studies have concluded that combination chemotherapy should not be recommended for PS 2 patients Citation[4], Citation[12–14]. It has been pointed out that combination chemotherapy to PS 2 patients may lead to unacceptable toxicity and that this would further compromise their already reduced HRQOL. On the other hand, combination chemotherapy was associated with improved survival when compared to single-agent therapy in advanced NSCLC PS 2 patients Citation[15].
Limited data are available on how combination chemotherapy affects the HRQOL of PS 2 NSCLC patients. In our recent publication of treatment outcome in PS 2 advanced NSCLC patients administered platinum-based combination chemotherapy Citation[16], a subgroup analysis of some selected HRQOL items according to performance status favored patients with PS 2. In this study we further explore the impact of combination chemotherapy on HRQOL in the PS 2 patients.
Material and methods
Patients
In our national multicenter phase III study in advanced NSCLC patients, three cycles of vinorelbine/carboplatin were compared to three cycles of gemcitabine/carboplatin with no significant differences in survival and HRQOL between the two treatment arms Citation[17]. The study was designed to detect differences in survival and predefined HRQOL aspects between the two treatment arms. Chemonaive patients at all ages with histologically or cytologically confirmed NSCLC stage IIIB or IV, adequate bone marrow-, renal- and hepatic functions were included. PS 0–2 were allowed, using the performance status scale classified by the Eastern Cooperative Oncology Group Citation[18]. At inclusion, patients were stratified according to PS 0/1 vs. PS 2. In the patient population, 123 PS 2 patients were identified and their complete HRQOL data analyzed and compared to the PS 0/1 group. Survival and toxicity in the PS subgroups are published earlier Citation[16]. Median and 1-year survival of PS 2 patients was 4.5 mths and 10% vs. 8.9 mths and 37% among PS 0/1 patients.
Chemotherapy
In both arms, three courses of chemotherapy were given at 3-week cycles. Carboplatin Chatelut AUC = 4 (equals Calvert AUC = 5), was administered day 1, and vinorelbine 25 mg/m2 or gemcitabine 1 000 mg/m2 day 1 and 8 in each course. Patients ≥ 75 years received 75% of standard doses. Chemotherapy was terminated in case of disease progression, unacceptable toxicity, intercurrent disease or patients’ wish.
Assessment of HRQOL
We collected patient-assessed HRQOL data using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C30 Citation[19] and the lung cancer specific module QLQ-LC13 Citation[20]. The QLQ-C30 is a “core questionnaire” which incorporates a range of physical, emotional and social health issues relevant to a broad spectrum of cancer patients. Global QOL, physical-, role-, emotional-, cognitive- and social function are multi-item scales, as are fatigue, nausea/vomiting and pain. Dyspnea, insomnia, appetite loss, constipation and diarrhea are single-item measures.
The lung cancer module is validated for use in lung cancer patients. Pain, coughing, sore mouth, dysphagia, peripheral neuropathy, alopecia and hemoptysis are measured by single items while dyspnea is a three-item scale addressing dyspnea at rest, by walking and by climbing stairs.
The HRQOL questionnaires completed at baseline, before second and third chemotherapy cycle and three weeks after completion of chemotherapy are considered of primary interest. Time windows of ±10 days from onset of second and third chemotherapy courses and ±14 days for controls at week 9 were assigned.
Statistical considerations
All HRQOL items were explored, and these were scored for each patient according to the EORTC QLQ-C30 scoring manual Citation[21]. HRQOL-item scores range from 0 to 100. A high score in functioning scales represents good function, whereas a high score in symptom scales represents more symptoms.
The mean baseline scores for each HRQOL item were calculated and differences between the PS 0/1 and PS 2 patients were tested using the Mann-Whitney U-test.
Area under the Curve (AUC) of HRQOL scores plotted against time is a summary measure of HRQOL Citation[22]. This provides each patient's longitudinal HRQOL experience as a single quantity and was calculated for each item Citation[23]. To adjust for baseline differences, the AUC calculation for each patient was based on changes from baseline. Missing data were imputed. If data from one assessment point were missing, the mean value of the two adjacent ones was used. For patients who withdrew or dropped out before week 9, the last value carried forward was used to impute the missing subsequent values. This may introduce a bias if the main reason for drop-out was deterioration. To examine this possibility, comparisons were performed with data based on the worse possible score for the missing data. Standardized AUC (SAUC) was estimated as AUC divided by time. SAUC allows for differences in patient survival and corresponds to calculating the average HRQOL. SAUC from baseline to week 9 was compared between PS 0/1 and PS 2 patients using ANOVA.
Patients’ responses were also classified as improved, stable or worse for all HRQOL items at week 9 according to the NCIC CTG standard QOL analysis framework Citation[24]. Symptom or function items were considered worse if the change from baseline was >10 points towards worse without improvement at any time-point after baseline. Significant improvement was defined as ≥10 points towards bettering in patients who did not deteriorate. Patients, who had less than 10-point changes from baseline at every HRQOL assessment or failed to meet the criteria for worsening or improvement, were considered stable. Distributions of the categories were tested by χ2.
Due to multiple comparisons, p-values of <0.01 were considered significant and p < 0.05 indicating a tendency.
Results
Patients
Patient characteristics according to performance status are given in . The PS groups were well balanced regarding baseline demographic, clinical and histological data. Of the 123 PS 2 patients, 61 were treated with vinorelbine/carboplatin and 62 with gemcitabine/carboplatin. Five did not complete the baseline QLQ, 4 did not receive any chemotherapy and 20 completed only the baseline QLQ. Among PS 0/1 patients the corresponding numbers were 10, 2 and 19. This leaves 372 patients for HRQOL analyses, 278 PS 0/1 patients and 94 PS 2 patients.
Table I. Patient characteristics at baseline.
Chemotherapy completion
Significantly less patients in the PS 2 group received three courses of chemotherapy when compared with the PS 0/1 patients (68% vs. 85%; p < 0.01, ).
Table II. Completion of Chemotherapy according to Performance Status.
HRQOL
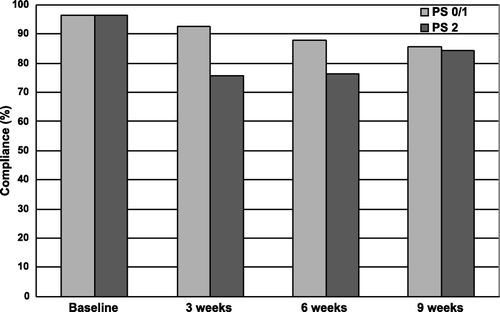
The compliance rate with respect to completion of the HRQOL questionnaires was 97% in both groups at baseline (). The overall compliance during the study period was 91% and 83%, for the PS 0/1 and PS 2 group, respectively. At 3 and 6 weeks, the compliance was significantly lower in the PS 2 group (76% vs. 93% and 76% vs. 88%, p < 0.01). The rates of completed questionnaires within the time window of ±10 days from onset of second and third chemotherapy courses were 95% and 94%, and 89% within ±14 days of follow-up at week 9 without significant differences between the two subgroups.
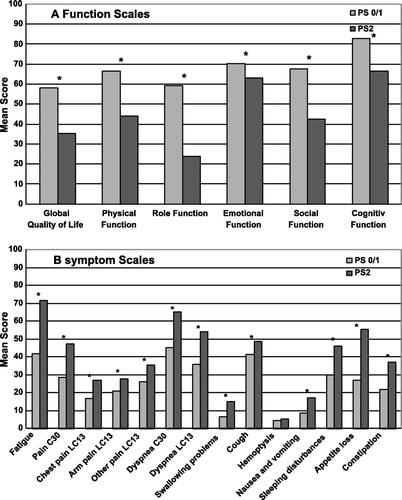
The two PS subpopulations differed significantly at baseline (). The PS 2 patients reported lower function for all the functional scales (p < 0.01). They also had significantly more severe symptoms with more fatigue, pain, dyspnea, swallowing problems, cough, nausea, insomnia, appetite loss and constipation (symptom scales, p < 0.01).
Figure 2. Mean HRQOL scores at baseline. Panel A: Functioning scales. A high function score represents good function. Panel B: Symptom scales. A high symptom score represents more symptoms. *p < 0.01.
The SAUC based on imputation by carrying the last value forward, is presented in . Results from imputation of worst possible scores were consistent with the presented data. Regarding the function scales, a tendency towards improved global QOL was achieved among PS 2 patients. For symptoms, they achieved significantly relief of fatigue, dyspnea and sleeping problems, and they tended towards less pain and appetite loss. In no items did PS 2 patients experience significant deterioration when compared to PS 0/1 patients.
Table III. Standardized Area under Curve According to Performance Status for the HRQOL items.
The proportions of patients classified as improved, stable or worse are presented in . PS 2 patients achieved improvement in global QOL and cognitive function and tended towards improvement of role function. They also experienced relief of dyspnea measured by QLQ-C30, and tended to improvement of fatigue, swallowing problems and appetite.
Table IV. Health related Quality of Life Responses according to Performance Status
Discussion
In the present study of combination chemotherapy, PS2 patients had more improvement of global QOL, cognitive function, fatigue, pain, dyspnea, sleeping problems and appetite loss than PS 0/1 patients.
The differences in HRQOL gains are not surprising. Taking into account the heavier baseline symptom burden of PS 2 patients, they clearly have the greatest potential for palliation and HRQOL improvements.
The HRQOL benefits seen among our PS 2 patients are consistent with previous studies. Billingham and Cullen found superior palliation among PS 2 patients in comparison to PS 0/1 Citation[12] in two randomized trials using mitomycin, ifosfamide and cisplatin in the treatment of unresectable NSCLC Citation[25]. Furthermore, in a recent randomized phase II study on first line erlotinib versus standard chemotherapy of PS 2 advanced NSCLC patients, HRQOL tended to improve rather than worsen in both treatment arms Citation[26]. In fact, the authors concluded that unselected advanced NSCLC PS 2 patients are best treated with combination chemotherapy in first-line.
A major strength of this prospective study is HRQOL analyses based on an unselected lung cancer population, largely reflecting the everyday clinical setting. The high average age and the large proportion of PS 2 patients reflects the high grade of representativity, as nearly 40% of diagnosed advanced NSCLC patients nationally during the accrual period were included in this study.
Missing data in trials involving HRQOL represents, on the other hand, a well known and described challenge Citation[27]. Deteriorating patients are likely to have an increased drop-out rate in completing HRQOL questionnaires Citation[28], and the lower compliance among PS 2 patients at weeks 3 and 6 may be a weakness. Another important issue is the chance of type I error as false positives may result from multiple testing in post hoc analyses Citation[29]. Although a significance level defined at p < 0.01 to a certain degree can compensate for this Citation[30], the results of our HRQOL analyses should be interpreted with caution. Nevertheless, new HRQOL data on PS 2 patients treated with combination chemotherapy are essential and provide valuable information.
Dyspnea, pain and fatigue are described as the most distressing symptoms in advanced NSCLC Citation[31] and consequently these symptoms are of substantial clinical interest. In our population, these symptoms were at baseline significantly worse, but actually palliated to a larger extent in PS 2 patients when compared to PS 0/1. Surprisingly, PS 2 patients did not seem to deteriorate in any of the HRQOL dimensions when compared to the PS 0/1 patients.
Concerns have been raised that chemotherapy to PS2 patients may further deteriorate already compromised HRQOL aspects. Current guidelines have cautioned against the use of combination chemotherapy in these patients Citation[11]. The results of the present study challenge these conservative therapeutic attitudes towards PS 2 patients. We found clinically relevant palliation of traumatic symptoms like fatigue, pain, dyspnea, appetite loss, sleeping problems and improved global QOL, role function and cognitive function in these patients.
In conclusion, combination chemotherapy to motivated PS 2 NSCLC patients should not be controversial from a HRQOL perspective. There are no convincing data indicating that such treatment deteriorates HRQOL in this patient population. On the contrary, clinically meaningful improvements of symptoms and function can be achieved. Further prospective studies with predefined HRQOL outcomes in PS 2 patients are warranted to confirm these findings.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin 2006; 56: 106–30
- Kelly K. Challenges in defining and identifying patients with non-small cell lung cancer and poor performance status. Semin Oncol 2004; 31: 3–7
- Paesmans M, Sculier JP, Libert P, Bureau G, Dabouis G, Thiriaux J, et al. Prognostic factors for survival in advanced non-small-cell lung cancer: Univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1,052 patients. The European Lung Cancer Working Party. J Clin Oncol 1995; 13: 1221–30
- Sweeney CJ, Zhu J, Sandler AB, Schiller J, Belani CP, Langer C, et al. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: A Phase II trial in patients with metastatic nonsmall cell lung carcinoma. Cancer 2001; 92: 2639–47
- Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst 1980; 65: 25–32
- Fallowfield LJ, Harper P. Health-related quality of life in patients undergoing drug therapy for advanced non-small-cell lung cancer. Lung Cancer 2005; 48: 365–77
- Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. American Society of Clinical Oncology. J Clin Oncol 1996;14:671–9.
- Gridelli C, Ardizzoni A, Le CT, Manegold C, Perrone F, Thatcher N, et al. Treatment of advanced non-small-cell lung cancer patients with ECOG performance status 2: Results of an European Experts Panel. Ann Oncol 2004; 15: 419–26
- National Institute for Clinical Excellence. Clinical Guideline 24, Lung cancer: the diagnosis and treatment of lung cancer. 2005, (Report).
- Bottomley A, Efficace F, Thomas R, Vanvoorden V, Ahmedzai SH. Health-related quality of life in non-small-cell lung cancer: Methodologic issues in randomized controlled trials. J Clin Oncol 2003; 21: 2982–92
- Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S, Jr, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: Update 2003. J Clin Oncol 2004; 22: 330–53
- Billingham LJ, Cullen MH. The benefits of chemotherapy in patient subgroups with unresectable non-small-cell lung cancer. Ann Oncol 2001; 12: 1671–5
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346: 92–8
- Soria JC, Brisgand D, Le Chevalier T. Do all patients with advanced non-small-cell lung cancer benefit from cisplatin-based combination therapy?. Ann Oncol 2001; 12: 1667–70
- Lilenbaum RC, Herndon JE, List MA, Desch C, Watson DM, Miller AA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: The cancer and leukemia group B (study 9730). J Clin Oncol 2005; 23: 190–6
- Helbekkmo N, Aasebo U, Sundstrom SH, von Plessen C, Brunsvig PF, Bremnes RM. Treatment outcome in performance status 2 advanced NSCLC patients administered platinum-based combination chemotherapy. Lung Cancer 2008; 62: 253–60
- Helbekkmo N, Sundstrom SH, Aasebo U, Brunsvig PF, von Plessen C, Hjelde HH, et al. Vinorelbine/carboplatin vs gemcitabine/carboplatin in advanced NSCLC shows similar efficacy, but different impact of toxicity. Br J Cancer 2007; 97: 283–9
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–55
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–76
- Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M. The EORTC QLQ-LC13: A modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer 1994; 30A: 635–42
- Fayers, PM, Aaronson, NK, Bjordal, K, Groenvold, M, Curran, D, Bottomley, A. EORTC QLQ-30. Scoring Manual 3rd ed. 2001.
- Fairclough DL. Summary measures and statistics for comparison of quality of life in a clinical trial of cancer therapy. Stat Med 1997; 16: 1197–209
- Fayers, PM, Machin, D. Quality of Life. Assessment, analysis and interpretetation. 1st ed. Chichester: John Wiley and Sons Ltd.; 2000.
- Osoba D, Bezjak A, Brundage M, Zee B, Tu D, Pater J. Analysis and interpretation of health-related quality-of-life data from clinical trials: Basic approach of The National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer 2005; 41: 280–7
- Cullen MH, Billingham LJ, Woodroffe CM, Chetiyawardana AD, Gower NH, Joshi R, et al. Mitomycin, ifosfamide, and cisplatin in unresectable non-small-cell lung cancer: Effects on survival and quality of life. J Clin Oncol 1999; 17: 3188–94
- Lilenbaum R, Axelrod R, Thomas S, Dowlati A, Seigel L, Albert D, et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol 2008; 26: 863–9
- Bernhard J, Cella DF, Coates AS, Fallowfield L, Ganz PA, Moinpour CM, et al. Missing quality of life data in cancer clinical trials: Serious problems and challenges. Stat Med 1998; 17: 517–32
- Herndon JE, Fleishman S, Kosty MP, Green MR. A longitudinal study of quality of life in advanced non-small cell lung cancer: Cancer and Leukemia Group B (CALGB) 8931. Control Clin Trials 1997; 18: 286–300
- Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine-reporting of subgroup analyses in clinical trials. N Engl J Med 2007; 357: 2189–94
- Osoba D, Tannock IF, Ernst DS, Neville AJ. Health-related quality of life in men with metastatic prostate cancer treated with prednisone alone or mitoxantrone and prednisone. J Clin Oncol 1999; 17: 1654–63
- Tishelman C, Degner LF, Rudman A, Bertilsson K, Bond R, Broberger E, et al. Symptoms in patients with lung carcinoma: Distinguishing distress from intensity. Cancer 2005; 104: 2013–21