Abstract
Introduction. The aim of this retrospective analysis was to analyze the results of conventional radical radiotherapy in the treatment of oropharyngeal cancer and to identify pre-treatment and treatment-related prognostic factors for outcome. Material and methods. The records of 627 patients with oropharyngeal cancer treated with radical radiotherapy with conventional techniques were analyzed. Results. The median age was 56 years. History of tobacco abuse was present in 80.5%. Eighty six percent had stage III or IV disease. Radical radiotherapy alone was the treatment modality for 71.2% and concomitant or neoadjuvant chemotherapy was used in 28.8%. The 3-year local control (LC), loco-regional control (LRC), disease-free survival (DFS) and overall survival (OS) was 49%, 40.6%, 38.9% and 36.1% respectively. The 3-year DFS rates were 80.3% for stage I, 65.8% for stage II, 46.1% for stage III and 25.2% for stage IV disease. Multivariate analysis was performed for prognostic factors. Prior history of tobacco abuse was an independent prognostic factor for both DFS and LRC. Karnofsky Performance Score (KPS) < 80, higher nodal stage, lower total radiotherapy dose (<66 Gy) in those receiving > 60 Gy, and overall treatment time > 50 days were other independent prognostic factors for inferior DFS and LRC. KPS < 80, higher T stage, higher nodal stage, RT dose < 66 Gy and longer overall treatment time (>50 days) were independent prognostic factors for poorer local control. Conclusions. Several patient-, disease- and treatment-related variables independently affect survival outcomes after radical radiotherapy for oropharyngeal cancer. Oropharyngeal cancers in those without a history of tobacco abuse may be biologically different and more amenable to cure with radiotherapy.
Squamous cell carcinoma of the oropharynx is a common malignancy of the head and neck. Worldwide age-adjusted incidence rates for men and women are 3.8 and 0.8 per 100 000 populations respectively, with a substantial variation in different regions and countries Citation[1]. In India the age-adjusted incidence rates in several population based registries are among the highest in the world Citation[2]. Although the strongest etiological association of this cancer is with the abuse of tobacco and alcohol, there is now an established association with human papilloma virus (HPV) infection as well Citation[3–6]. All over the world, most cases of this cancer present in locoregionally advanced disease. This is especially true for developing countries.
The treatment of oropharyngeal cancer most commonly involves the use of definitive radiotherapy (RT), now with an increasing use of concomitant chemotherapy (CT). Results of treatment of oropharyngeal cancer with radical radiotherapy have been previously published Citation[7–13]. However, nearly all of this data originates from western literature. The patterns of presentation, stage distributions, tumor bulk, biology and tolerance to intensive radical treatment may differ in developing countries and different ethnic populations. Oropharyngeal cancers comprise a substantial proportion of head and neck malignancies at our institute, and we decided to retrospectively analyze outcomes in the patient population receiving radical radiation to generate our treatment outcomes with conventional radiotherapy techniques as well as to identify both pretreatment and treatment related prognostic factors that affect outcomes in this disease.
Material and methods
Patients
The medical records of a single radiotherapy unit in the institute between 1990 and 2004 were reviewed retrospectively. The records of 627 consecutive patients with oropharyngeal carcinoma treated with radical radiotherapy were isolated for analysis. The decision of treatment with radical RT in each case was based on a multidisciplinary joint clinic assessment. Prior to treatment all the patients had detailed evaluation that included a) complete history; b) documentation of risk factors especially tobacco or alcohol abuse (tobacco abuse was defined as a chronic abuse of one or more forms of smoked or smokeless tobacco); c) comprehensive clinical examination including a flexible endoscopy; d) histological diagnosis; e) staging workup including blood chemistry, chest x-ray and appropriate imaging of the face and neck. Patients were staged according to the prevalent American Joint Committee for Cancer (AJCC)/International Union Against Cancer (UICC) staging system. For the purpose of this analysis they were reclassified according to the current AJCC 2002 system.
Radiotherapy treatment
All the patients were irradiated with megavoltage beams (cobalt or 6MV linear accelerator) with parallel opposed portals with shrinking field technique. Appropriate immobilization and tissue compensators were used during the treatment. They were treated with conventional daily fractionation of 2 Gy, 5 fractions per week, to a dose of 66–70 Gy to the gross primary and nodal disease as well as adjacent nodal regions at high risk of microscopic metastasis. The remaining electively treated neck received between 50–60 Gy at 2 Gy daily fractions. Selected stage I/II patients were planned for total doses between 60 and 66 Gy in the population treated in the early 1990s. Spinal cord shielding was applied after 46 Gy in 23 fractions. A posterior neck electron portal with appropriate energies was added after spinal shielding when indicated on the basis of pretreatment nodal extent.
Chemotherapy
Chemotherapy was administered with radiation in a proportion of patients either in neoadjuvant or concomitant setting. The practice of chemotherapy varied with the time period, and was individualized according to the age, performance status and stage of the disease. The most commonly used regimen consisted of concurrent weekly cisplatinum, at a dose of 30–35 mg/m2.
Follow-up
During treatment, all patients were reviewed weekly or more frequently depending on the need and evaluated for tolerance and compliance to treatment, weight loss, performance status, skin and mucosal reactions, blood counts and need for symptomatic treatment.
The patients were followed-up at 6 to 8 weeks from completion of therapy to assess response and persistent toxicity. Acute toxicity was reported utilizing the Radiation Therapy Oncology Group (RTOG)/ European Organization for Research and Treatment of Cancer (EORTC) toxicity criteria Citation[14]. Response was documented by the WHO response grading Citation[15].
Subsequent follow-up visits were scheduled at 3 monthly intervals for the first 2 years, then every 6 months till the 5th year and annually thereafter. All efforts were made to update the disease status of patients by the medical records department through reply-paid postcards or telephonic contact. Patients not responding to above measures were considered lost to follow-up and censored for statistical analysis.
Statistical analysis
Local failure was defined as persistence of disease or reappearance of disease at or in close vicinity to the primary site. Loco-regional failure was defined as persistence of disease or reappearance of disease either at the primary site and/or draining regional lymph nodes, or the appearance of a second primary in the upper aero-digestive tract. All patients were included in the survival analysis. The local control (LC), loco-regional control (LRC) and disease-free survival (DFS) were calculated using the method of Kaplan-Meier Citation[16]. All estimates were calculated from the date of initiation of therapy till the defined event if any or until last contact or death. Prognostic factors were analyzed using the log-rank test for univariate analysis and Cox-regression analysis using a stepwise backward conditional model for multivariate analysis. All analyses were done using the statistical package SPSS version 14.0 (SPPS Inc. Chicago, USA).
Results
Patient and disease characteristics
Details of 627 patients with oropharyngeal cancer receiving radical radiotherapy as the primary treatment were analyzed. Patient and disease characteristics are presented in . The median age of the cohort was 56 years (range 24–86 years) and the sex ratio was 7.7:1 in favor of males. History of tobacco abuse was present in 80.5% of the patients. Most (74%) had a KPS of 80 or more. The majority (65.4%) had T3 or T4 tumors. Clinicoradiologic evidence of nodal enlargement was present in 66.8%. Overall nearly 38% had stage III and another 48% stage IV disease. Base of tongue (BOT) and vallecular primaries (57.1%) outnumbered tonsillar and soft palate carcinoma (42.4%). All tumors were squamous cell carcinoma on histopathological examination of biopsy specimens.
Table I. Patient and treatment characteristics.
Treatment characteristics
All patients were planned for radical radiotherapy with or without chemotherapy. Treatment details are also presented in . Radical RT alone was the treatment modality for 71.2% of the patients. The remaining received either concomitant (23.1%) or neoadjuvant (5.7%) chemotherapy. The characteristics of patients receiving chemotherapy vs. RT alone are shown in . The total dose of radiation delivered varied from 46 to 72 Gy (median 70 Gy). Doses of 66 Gy or more were delivered to 74.3%. The overall treatment time (OTT) ranged from 32–102 days (median 50 days). The OTT exceeded 50 days in 45.6% of patients without any planned treatment break. In the group receiving concomitant chemotherapy, the median number of chemotherapy cycles was 4 (range 1 to 8). Eighty-eight of 145 (60.6%) patients received at least five cycles and 73 patients (50.3%) receiving concurrent chemoradiation completed radiotherapy to a dose ≥66 Gy.
Table II. Patient and treatment characteristics on the basis of use of chemotherapy.
Salvage treatment at relapse was individualized and included surgery and/or palliative chemotherapy as appropriate. However this analysis did not attempt to consider the impact of salvage therapy on final outcome restricting itself to first failures alone.
Acute toxicity
The overall incidence of RTOG grade III skin and mucosal reactions were 24.7% and 23.1% respectively. The incidence of grade III acute skin toxicity was 22.4% and 28.3% (p = 0.31) with radiotherapy alone and with chemotherapy respectively. Grade III mucositis was also more common with chemotherapy (28.3% vs. 21.7%, p = 0.23). A significantly higher incidence of grade ≥II haematological toxicity was seen with chemoradiation (10.1% vs. 0.3%, p < 0.001).
Response and survival
The patients were assessed for response after 6–8 weeks of completion of treatment. Complete response was seen in 255 (40.7%) patients. Isolated residual disease at primary site was seen in 10.4%, and at the nodal site in 38% of patients. Residual disease at both primary and nodal regions was present in 113 patients (18.0%).
The median and mean follow-up for the whole group was 11 months and 21 months respectively (range 0–184 months). Corresponding values for patients free of disease were 17 and 27 months. Thirty eight percent of disease-free patients had follow-up greater than 2 years. A total of 124 patients (19.8%) had disease recurrence. Of these 58 (46.8%) recurred at primary site alone followed by isolated recurrences at nodal sites in 34 (27.4%). Recurrence at both primary as well as nodal site was seen in 18 (14.5%) patients. Distant metastasis occurred in 13 patients (10.5%) among whom two patients had simultaneous locoregional failure. One patient had a second primary cancer in the irradiated region.
The 3-year LC, LRC, DFS and OS for the whole cohort of patients were 49%, 40.6%, 38.9% and 36.1% respectively. The 3-year DFS rates were 80.3% for stage I, 65.8% for stage II, 46.1% for stage III and 25.2% for stage IV disease.
Univariate and multivariate analysis for prognostic factors
The impact of different prognostic factors on LC, LRC, and DFS was analyzed. Some variables were grouped into appropriate categories for comparison.
The results of univariate analysis are shown in . KPS < 80, higher T stage (T3/T4), higher N stage (N2/N3), advanced TNM stage group, lower total RT dose (<66 Gy) in those receiving ≥60 Gy, and longer OTT (>50 days) in those receiving ≥66 Gy had significantly poorer DFS, LRC and LC. Prior history of tobacco abuse predicted for a significantly poorer DFS and LRC ().
Figure 1. Disease free survival and locoregional control in those with and without prior history of chronic tobacco abuse. The numbers tabulated below represent individuals at-risk at different time points.
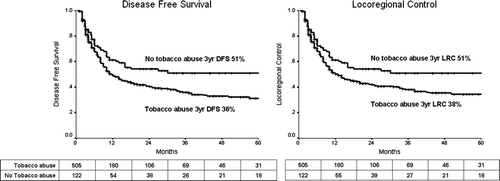
Table III. Univariate analysis of prognostic factors.
Age (>65 years or ≤65 years), sex, primary site (BOT and vallecula vs. tonsil and soft palate) did not make a significant difference to any of the outcome parameters. The use of chemotherapy (neoadjuvant or concurrent) also did not affect outcomes. There was a trend towards improved DFS with chemotherapy in stage IV tumors (3 year DFS 33.4% vs. 19.7%, p = 0.12), but without statistical significance.
Multivariate analysis was performed using the above parameters. The results are depicted in . KPS < 80, higher T stage (T3/T4 vs. T1/T2), higher nodal stage (N2/N3 vs. N0/N1), lower total RT dose (<66 Gy) in those receiving >60 Gy, and longer OTT (>50 days) were independent prognostic factors for poorer local control. Prior history of tobacco abuse, KPS < 80, higher nodal stage, lower total RT dose, and OTT were independently prognostic for inferior DFS and LRC.
Table IV. Multivariate analysis of prognostic factors.
Discussion
This retrospective audit of 627 patients with oropharyngeal squamous cell carcinoma represents one of the largest series reporting results of conventional radical radiotherapeutic treatment of this common cancer and an analysis of prognostic factors.
As reflected in the disease characteristics, the vast majority (87%) of cases present with locoregionally advanced disease. The standard curative primary treatment for oropharyngeal cancers in our hospital has been radical radiotherapy with or without chemotherapy, with surgery usually reserved for salvage if feasible. The results of radical radiotherapy presented here correspond with previously published retrospective reports and prospective studies of radical radiotherapy in oropharyngeal cancer Citation[8], Citation[10–13], Citation[17], depicted in .
Table V. Selected large series reporting results and/or prognostic factors after radical radiotherapy for oropharyngeal cancer.
Prognostic factors for DFS, LRC and LC have been analyzed using both univariate and multivariate analysis. Several pre-treatment and treatment related factors have been identified to be of prognostic relevance. Among patient related factors, the performance status was found to be an independent predictor of all outcome parameters. The prognostic importance of performance status has been reported previously as an independent predictive factor for disease free and overall survival Citation[18], Citation[19]. Age was not a predictor of outcome in our series. The gender was also not predictive, though it has been reported as a factor of significance in some series Citation[11], Citation[12].
The prognostic importance of prior tobacco abuse (smoking or smokeless forms of tobacco) in our patient population is of great interest. All patients are comprehensively counseled regarding smoking cessation and compliance is high. Therefore, the inferior outcomes cannot be attributed to smoking during or after radiotherapy. To our knowledge, no large series in head and neck has previously reported an independent predictive value of prior tobacco abuse. Significantly better outcomes in non-abusers of tobacco may well represent a different biology of cancer Citation[20]. It is possible that squamous oropharyngeal cancers in those without history of tobacco abuse are related to human papilloma virus (HPV) infection and therefore of a more indolent nature. HPV related cancers have been described most often in the oropharynx Citation[3], Citation[21] and have been previously reported to have better outcomes after treatment compared to those without HPV related changes Citation[3], Citation[6], Citation[22]. Although we have not formally tested our patient population for HPV, it would be a strong risk factor for cancer in those without tobacco exposure. Specific testing for HPV in cancers of this subsite may elucidate their role further and explain the marked difference in outcomes between patients based on their history of tobacco abuse.
T-stage and N-stage has been previously shown to be of prognostic relevance in several reports Citation[7], Citation[19], Citation[23–25]. We found the N-stage to be the strongest predictor of all three parameters of control among those studied. T-stage was an independent predictor of LC but not for DFS and LRC. This represents the relatively greater importance of the nodal stage over the local stage in the ultimate disease control.
The prognostic importance of the total RT dose needs emphasis. Within the patient population receiving RT doses higher than 60 Gy, the delivery of doses ≥66 Gy was found to be an independent predictor of improved LC, LRC and DFS. Those receiving lower radiation doses (usually due to poor tolerance or noncompliance) were excluded to remove an obvious bias resulting from under-treatment. Other authors have reported on the importance of total RT dose, with a benefit for doses between 66–70 Gy Citation[24–26]. Whether doses of 70 Gy or more have added to the benefit is not completely clear. In our group of patients the 3 year LRC was not significantly better in those receiving ≥70 Gy compared to those who received 66–69 Gy (46.2% vs. 37.8%, p = 0.25), though the patient numbers may not have been adequate to detect a small improvement.
The OTT was also found to be an independent prognostic factor for outcome measures. The importance of OTT has been previously reported Citation[7], Citation[25–29]. A substantial proportion of patients in our series have had a prolongation of OTT beyond 50 days. In nearly all cases the cause of this prolongation has been due to unplanned breaks caused by non-compliance or poor tolerance. This has very likely had an impact on the response rates. Our results represent one the largest bodies of evidence validating the importance OTT in radical radiotherapy in head and neck cancer, and confirms that a prolongation of OTT beyond 50 days may prove to be significantly detrimental to locoregional outcomes. Prevention of unplanned treatment breaks and methods to compensate for them need serious consideration in day-to-day practice Citation[30]. Treatment schedules that reduce the OTT further (accelerated fractionation schedules) have shown improved results in pharyngeal and laryngeal cancers with a suggestion of a small benefit in survival Citation[31]. We are also a part of an ongoing prospective multinational trial of accelerated fractionation Citation[32]. A previous study from India has recently reported a relatively greater benefit of accelerated fractionation with concomitant boost in the subgroup with oropharyngeal primaries Citation[33].
The practice of concomitant or neoadjuvant chemotherapy has varied over the time period studied, with an increasing use of concomitant chemotherapy as a radiosensitizer in routine care after 2000 with the emergence of evidence of a small but significant survival benefit Citation[34]. This explains the relatively small number of patients receiving chemotherapy. Our results do not demonstrate a difference in outcomes between groups receiving or not receiving chemotherapy. An analysis in the retrospective setting is not the optimal way to comment on the role of chemotherapy in our patient population. A higher proportion of patients receiving chemotherapy belonged to stage IV compared to those receiving RT alone (66% vs. 41%, p < 0.001, see ). We also found a trend (though statistically nonsignificant) towards improved outcomes with chemotherapy in this stage. This modest benefit must also be weighed against the incidence of higher toxicity and impact of resultant treatment breaks (62% vs. 55% with OTT > 50 days) on outcomes.
This analysis is limited by the relatively short follow-up. Every effort was made to contact patients by reply-paid postcards or telephone, but with limited success owing to socioeconomic reasons, the lack of literacy and the constraints of repeatedly traveling long distances to the hospital. However, in those free of disease, the duration of follow-up was acceptable, considering that the majority of failures in head and neck cancer occur within the first two years.
Conclusion
Disease related parameters are important determinants of locoregional control, but patient-related factors like the performance status may also have an independent effect. Even in busy and resource-limited settings, careful attention to adequate dose delivery and OTT may improve overall disease control. The biology of cancers in those without a history of tobacco abuse may be different and needs further investigation. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ferlay, J, Bray, F, Pisani, P, Parkin, DM. GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide. IARC Cancer Base No. 5, version 2.0. Lyon: IARC Press; 2004.
- National Cancer Registry Programme. Two year report of population based cancer registries 1997-1998. Bangalore: Indian Council of Medical Research; 2002.
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000; 92: 709–20
- Ringstrom E, Peters E, Hasegawa M, Posner M, Liu M, Kelsey KT. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res 2002; 8: 3187–92
- Pintos J, Black MJ, Sadeghi N, Ghadirian P, Zeitouni AG, Viscidi RP, et al. Human papillomavirus infection and oral cancer: A case-control study in Montreal, Canada. Oral Oncol 2008; 44: 242–50
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008; 100: 261–9
- Bataini JP, Asselain B, Jaulerry C, Brunin F, Bernier J, Pontvert D, et al. A multivariate primary tumour control analysis in 465 patients treated by radical radiotherapy for cancer of the tonsillar region: Clinical and treatment parameters as prognostic factors. Radiother Oncol 1989; 14: 265–77
- Jaulerry C, Rodriguez J, Brunin F, Mosseri V, Pontvert D, Brugere J, et al. Results of radiation therapy in carcinoma of the base of the tongue. The Curie Institute experience with about 166 cases. Cancer 1991; 67: 1532–8
- Regueiro CA, Aragon G, Millan I, Valcarcel FJ, de la Torre A, Magallon R. Prognostic factors for local control, regional control and survival in oropharyngeal squamous cell carcinoma. Eur J Cancer 1994; 30A: 2060–7
- Perez CA, Patel MM, Chao KS, Simpson JR, Sessions D, Spector GJ, et al. Carcinoma of the tonsillar fossa: Prognostic factors and long-term therapy outcome. Int J Radiat Oncol Biol Phys 1998; 42: 1077–84
- Johansen LV, Grau C, Overgaard J. Squamous cell carcinoma of the oropharynx–an analysis of treatment results in 289 consecutive patients. Acta Oncol 2000; 39: 985–94
- Mendenhall WM, Morris CG, Amdur RJ, Hinerman RW, Malyapa RS, Werning JW, et al. Definitive radiotherapy for tonsillar squamous cell carcinoma. Am J Clin Oncol 2006; 29: 290–7
- Mendenhall WM, Morris CG, Amdur RJ, Hinerman RW, Werning JW, Villaret DB. Definitive radiotherapy for squamous cell carcinoma of the base of tongue. Am J Clin Oncol 2006; 29: 32–9
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–6
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–14
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81
- Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 2004; 22: 69–76
- Cooper JS, Farnan NC, Asbell SO, Rotman M, Marcial V, Fu KK, et al. Recursive partitioning analysis of 2105 patients treated in Radiation Therapy Oncology Group studies of head and neck cancer. Cancer 1996; 77: 1905–11
- Jeremic B, Milicic B. Pretreatment prognostic factors of local recurrence-free survival in locally advanced squamous cell carcinoma of the head and neck treated with radiation therapy with or without concurrent chemotherapy. Am J Clin Oncol 2008; 31: 213–8
- Ragin CC, Taioli E, Weissfeld JL, White JS, Rossie KM, Modugno F, et al. 11q13 amplification status and human papillomavirus in relation to p16 expression defines two distinct etiologies of head and neck tumours. Br J Cancer 2006; 95: 1432–8
- Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol Biomarkers Prev 2005; 14: 467–75
- Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int J Cancer. 2007; 121: 1813–20
- Lee WR, Mendenhall WM, Parsons JT, Million RR, Cassisi NJ, Stringer SP. Carcinoma of the tonsillar region: A multivariate analysis of 243 patients treated with radical radiotherapy. Head Neck 1993; 15: 283–8
- Mak-Kregar S, Baris G, Lebesque JV, Balm AJ, Hart AA, Hilgers FJ. Radiotherapy of tonsillar and base of the tongue carcinoma. Prediction of local control. Eur J Cancer B Oral Oncol 1993; 29B: 119–25
- Hannisdal K, Boysen M, Evensen JF. Different prognostic indices in 310 patients with tonsillar carcinomas. Head Neck 2003; 25: 123–31
- Johansen LV, Overgaard J, Overgaard M, Birkler N, Fisker A. Squamous cell carcinoma of the oropharynx: An analysis of 213 consecutive patients scheduled for primary radiotherapy. Laryngoscope 1990; 100: 985–90
- van Putten WL, van der Sangen MJ, Hoekstra CJ, Levendag PC. Dose, fractionation and overall treatment time in radiation therapy–the effects on local control for cancer of the larynx. Radiother Oncol 1994; 30: 97–108
- Withers HR, Peters LJ, Taylor JM, Owen JB, Morrison WH, Schultheiss TE, et al. Local control of carcinoma of the tonsil by radiation therapy: An analysis of patterns of fractionation in nine institutions. Int J Radiat Oncol Biol Phys 1995; 33: 549–62
- Hoffstetter S, Marchal C, Peiffert D, Luporsi E, Lapeyre M, Pernot M, et al. Treatment duration as a prognostic factor for local control and survival in epidermoid carcinomas of the tonsillar region treated by combined external beam irradiation and brachytherapy. Radiother Oncol 1997; 45: 141–8
- Agarwal, JP, Budrukkar, A, Laskar, SG. Unplanned interruptions: Unresolved issues: in regard to Bese et al. (Int J Radiat Oncol Biol Phys 2007;68:654–61). Int J Radiat Oncol Biol Phys 2007;69:1335–6, Author reply 1336
- Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet 2006; 368: 843–54
- Overgaard J, Mohanti B, Bhasker S, Begum N, Ali R, Agarwal J, et al. Accelerated versus conventional fractionated radiotherapy in squamous cell carcinoma of the head and neck (SCCHN). A randomized international multicenter trial with 908 patients conducted by the IAEA-ACC study group. Int J Radiat Oncol Biol Phys 2006; 66: S13
- Ghoshal S, Goda JS, Mallick I, Kehwar TS, Sharma SC. Concomitant boost radiotherapy compared with conventional radiotherapy in squamous cell carcinoma of the head and neck--a phase III trial from a single institution in India. Clin Oncol (R Coll Radiol) 2008; 20: 212–20
- Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-analysis of chemotherapy on head and neck cancer. Lancet 2000; 355: 949–55
