Abstract
Background. Single points placed on Dose–Volume Histograms (DVHs) for treatment plan acceptance are still widely used compared to the Equivalent Uniform Dose (EUD). The aim of this work is to retrospectively measure and compare the ability of both criteria in correctly predicting two clinical outcomes, RTOG grade 2 acute gastrointestinal (GI) and genitourinary (GU) complications in 137 patients treated for prostate cancer. Material and methods. For both complications, the best predictions have been achieved by fitting the EUD parameter and a tolerance dose (for a varying DVH point) by maximization of the Area Under the Receiver Operating Curve (AUROC). A complementary likelihood fitting of the Lyman's Normal Tissue Complication Probability (NTCP) allowed a graphical comparison between expected and observed frequencies, and to derive the associated parameters. Results and discussion. No significant differences were found in the AUROC values obtained by using dose–volume or EUD criteria, but all the results highlighted the role of high doses. Limiting V65 (for grade 2 GI) or V73 (for grade 2 GU) was as predictive as limiting EUD value, with n equal to 0.09 or 0.06 respectively, but in all cases AUROC values were low (<0.7). Likelihood fitting gave m = 0.195 and TD50 = 72.5 Gy (fixing n = 0.06 for acute GU) and m = 0.19 and TD50 = 66 Gy (fixing n = 0.09 for acute GI). Both AUROC and likelihood values revealed a better fit for acute GI than for acute GU. The use of a fractionation correction, new clinical contours or previous risk factors could improve these values.
In radiotherapy, the ability to predict the possibilities of suffering side effects after irradiation is crucial prior to starting any treatment. In radiotherapy treatment planning, some criteria are needed to avoid plans that could be pernicious for the patients’ quality of life. Up to now, such criteria can be split into two categories: dose–volume points (used as constraints), and probabilistic radiobiological models. The aim of this study is to propose a way to compare the ability of both criteria to achieve correct predictions. It has been applied to genito-urinary and gastro-intestinal acute complications from a clinical data base composed of 137 patients treated for prostate cancers.
Correlated with the clinical outcomes, recommendations about DVH points have been tabulated specifying that a certain fraction of the total volume receiving at least some tolerance dose (TD) corresponded to a certain level of risk of complication. Such recommendations, sorted by Organ At Risks (OARs) as done by Emami et al. in 1991 Citation[1], are still widely used in practice. However, there are two major drawbacks when using DVH points in such a way. Since they are defined for a few points of the DVH curves, the main one is that they do not take into account the whole dose distribution. The second one is that there is no information tabulated about intermediate levels of risk, especially when several DVH points are considered. Although some studies have been carried out; assuming a linear relationship such as in Linear Discriminant Analysis Citation[2], using Principal Component Analysis Citation[3] or neural networks Citation[4]; conclusions highly depend on the assumptions made and are quite uneasy to use in practice. For simplicity reasons, this study is focused on defining a single DVH point, for a single tolerance dose to be found.
Conversely, by shrinking (reducing) the entire DVH into a single one-dimensional metric, radiobiological models based on dose–volume reduction schemes allow the clinicians to compute precise levels of complication probability. The most commonly used dose–volume reduction scheme is given by the gEUD formulation Citation[5]. As all dose–volume reduction schemes, it assumes a one-to-one relationship between its value (here in Grays) and the level of complication probability. Hence, by estimating the AUROC value on a clinical patient data base it is possible to quantify the ability of this metric to correctly classify patients into those who have suffered complications and those who have not. ROC curve analysis has already been used in radiotherapy for evaluating the link between dose–volume or radiobiological models with clinical outcomes, as in Citation[6]. In this study, AUROC value has been considered as a very useful non-parametric estimator to fit and compare both the gEUD and dose–volume point predictions.
gEUD formalism has been initially introduced in addition to the Lyman-Kutcher-Burman (LKB) Normal Tissue Complication Probability (NTCP) model. All NTCP model being probabilistic models; likelihood maximization, which estimates the highest probability that a set of parameters fit a set of data, is the most indicated statistical method to obtain a fit of the best NTCP parameters. It is possible then to estimate the predicted frequencies of affected patients and to compare them with the observed frequencies of patients suffering complications. Similarly, observed frequencies have been plotted versus volumes receiving the tolerance dose, in order to graphically represent the observed relationship. These plots can help the clinicians to define intermediate levels of risk, and appreciate at a glance the uncertainties of the criteria.
Material and methods
Patient data base
The clinical database (clinical and dosimetric information) of 137 patients treated of prostate carcinoma used in this study was furnished by the General Hospital of Valencia (ERESA), Spain. Relevant clinical information was composed of registered grades of gastro-intestinal and genito-urinary acute complications. Dosimetric information was provided by means of cumulated Dose-Volume Histograms (DVHs) for bladder and rectum. The contours of bladder for the evaluation of the bladder DVHs included the whole external contour of the bladder (filled with a contrast product for planning). Rectum was contoured from the anal sphincter until the upper limit of union between rectum and sigmoid. Prescription doses were delivered in 38 or 40 sessions for 76 or 80 Gy at the isocenter, respectively, according to a standard fractionation scheme of 2 Gy per fraction. Intensity Modulated Radiation Therapy (IMRT) has been used in patients treated until 80 Gy, while patients receiving 76 Gy have been treated by 3D Conformational Radiotherapy (3DCRT). Planning Target Volumes (PTVs) included prostate volume and seminal vesicles and, eventually, the pelvic lymph nodes. In the case of using IMRT, some patients received IMRT for all fields including lymph nodes, while others received IMRT only for those delimitating the prostate and seminal vesicles while lymph nodes were treated with conventional 3DCRT fields (see ).
Table I. Numbers of patients by technique (or group). PTV1 is composed of prostate and seminal vesicles, PTV2 refers as the lymphatic nodes.
Reported grades for acute effects considered in this study were defined according to RTOG scale for gastro-intestinal (GI) and genito-urinary (GU) acute injury Citation[7]. Positive endpoint based on acute GI (corresponding to the rectal DVHs) was selected to be equal or more than 2. For acute GU, grade 2 was also chosen. Since complications studied in the scope of our research deal with acute but not late effects, no follow-up time considerations after treatment was necessary. summarizes some characteristics of patient population.An in-house software was developed for this work using version 6.5 of Matlab (the mathworks company).
Table II. Characteristics of patient population (percent of ill patients in respect to total population, mean dose of ill and safe patients, and standard deviation in the mean doses).
Selection of the tolerance doses
The selection of the best tolerance doses is based on the concept of AUROC, which is evaluated from estimates of the True Positive Rates (TPR) and the False Negative Rates (FNR). The closer to one is the AUROC, the better (in terms of sensitivity and specificity) is the performed diagnostic. Furthermore, AUROC has the fundamental property to be equal to the Mann-Whitney (Wilcoxon) statistic Citation[8], Citation[9], which gives the probability that a positive patient has to have a higher rank than a negative one. If AUROC equals 0.5, patient diagnostic is as good as a random decision. If AUROC equals 1, diagnosis concerning any patient of the clinical data base never fails.
In this work, best dose–volume constraint has been selected as it corresponded to the largest AUROC value. In order to compute this value for a dose–volume point defined at a certain tolerance dose Dtol, corresponding cumulated volumes V at dose Dtol are collected from each patient of the data base. Then, patients with V > Vmax are classified as positive (with complications), and patients with V < Vmax are classified as negative patients (free from complications), allowing the calculation of the TPR and FNR rates. By varying Vmax and evaluating the TPR and FNR, ROC curve and so AUROC value are derived. By varying tolerance dose Dtol, a set of AUROC values is available and the tolerance dose corresponding to the major AUROC is retained.
A graphical appreciation of the relationship between the increasing risk of complication per increasing volume at the selected tolerance dose has also been plotted. Frequencies of observed patients with complications for the ith bin of volume, , has been estimated:
where
and
are the number of patients suffering complications and the total number of patients whose part v of their volume receiving at least the retained tolerance dose, is comprised between
and
(vi increase of at a given time). Since
depends on i, a gaussian uncertainty
has been assumed to be equal to:
This formula has been obtained after assuming that, for a single patient, maximal error on estimating the complication frequency should be of 100% (). As for patients this error must be divided by
(assuming that data are normally distributed), it yields that for
maximal error bar can be estimated through Equation 2.
Selection of the gEUD dose–volume parameter and NTCP likelihood fitting
The generalized equivalent uniform dose (gEUD) is an isoeffect model which quantifies the clinical outcome of both tumor and healthy tissues. It is given by:
where k is the structure for which the equivalent dose is evaluated, vj is the fraction of the volume irradiated with dose d(j), and a is the dose–response parameter of tissue for the considered endpoint (for a tumor parameter a is supposed to be negative, and for an Organ At Risk a is supposed to be positive). For an OAR, gEUD is strictly equivalent to the Lyman-Wolbarst DVH effective dose reduction scheme Citation[10] for Lyman-Kutcher-Burman Normal Tissue Complication Probability (NTCP) Citation[11] with a=1/n (in this study, using n rather than a parameter was preferred, in order to have the same notation for both effective and generalized equivalent dose):
where “erf” stands for the error function responsible for the typical sigmoidal dose–response relationship of the NTCP:
and t is computed as follows:
Here Deff is given by:with a=1/n.
With this definition, gEUD is the uniform dose for OARs that would yield the same NTCP calculated by means of the Lyman model as the true delivered heterogeneous dose distribution. Notice that when gEUD equals TD50 the value of NTCP equals 50% of chance of complication. Its value is always comprised between the mean and the maximum dose of the dose distribution.
Parameter n (=1/a) of Equations 3and7for calculating gEUD was selected using the same principle as for the selection of tolerance doses, that is to say by looking for the highest AUROC value. By varying n parameter from 0 to 1 (with a step of 0.01), and computing the ROC curves corresponding to the two distributions of EUD values (for ill and safe patients), the value of the n parameter that maximizes AUROC value was retained. Then, best gEUD AUROC value was compared with best tolerance dose based AUROC value.
In order to compare the predicted NTCP values for particular values of gEUD, knowledge of TD50 and m parameters is compulsory. These parameters have been obtained by likelihood maximization, computed as follows:
where n is the number of patients in the data base, xi equals 1 if patient i suffers the complication and 0 if he is free of it. Likelihood L is the probability that parameters m and TD50 truly fit the clinical data base, so that its maximum gives their best fit. In this study likelihood landscapes are shown in order to appreciate the goodness of the fit (its width giving an indication about the uncertainties on m and TD50), and ensuring not to get trapped into a local maximum.
As for the selection of the tolerance doses, the observed frequencies by bins of gEUD have been plotted, this time in addition to the predicted NTCP values. Frequencies and uncertainties have been calculated as previously mentioned, by defining a bin of gEUD () instead of a bin of volume (, see Equations 1and 2).
Results
Analysis of the acute GI complications
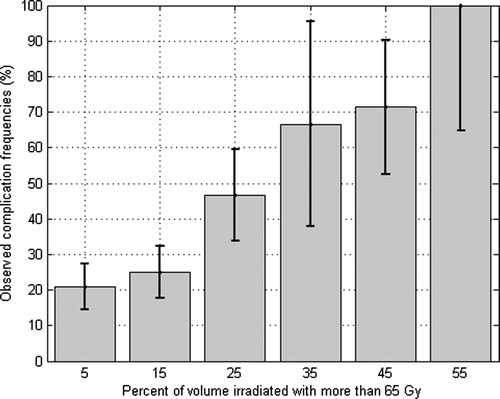
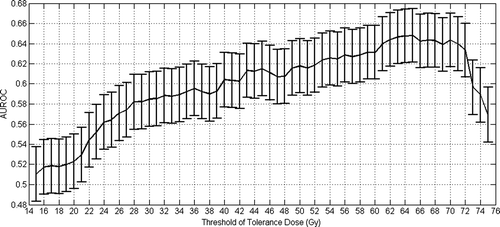
The selected tolerance dose is 65 Gy, so that cumulated volume receiving at least 65 Gy discriminate patients with an AUROC value of 0.65 (standard error value of 0.05). It corresponds to the best AUROC value obtained, with an uncertainty which is illustrated on , where standard errors have been plotted according to Equation A.3 of Appendix 1.
Figure 1. Selection of best tolerance dose for an acute GI grade larger than 2 based on maximum AUROC value.
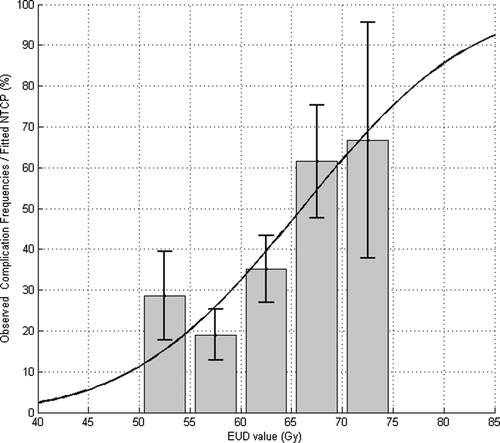
Observed frequencies of complications versus V65 (with a bin width of 10% of total volume of the rectum) is plotted on . Fifty percent of complications are observed at 25 to 50% of the rectal volume irradiated.
Analysis of the AUROC corresponding to EUD constraints indicates that the maximum is reached when n parameters equals to 0.09 (for an AUROC equals to 0.64, and standard error value of 0.05), as shown in , and so EUD value is approximately equal to the highest doses. Nonetheless, the standard errors are so, that this value of n discriminates slightly better among patients than when EUD is used with an n equal to one. It could be explained by the fact that EUD values of patients when n is equal to 0.09 are strongly correlated with EUD values of patients when n is equal to 1. Indeed, we computed a Pearson correlation coefficient of 0.904 between both sets of EUD values. By fixing n equal to 0.09 and varying m (ranging from 0.005 to 1 by a step of 0.005) and TD50 (ranging from 0.5 to 100 by a step of 0.5 Gy), likelihood reached its maximum (3.7 x 10 -35) for m=0.19 and TD50=66 Gy (see ). These parameters allow to plot NTCP values and observed frequencies of patients suffering of grade 2 acute GI versus the gEUD on a same graph (see ). It is interesting to note that the TD50 value fitted thanks to a likelihood analysis is very close to the best tolerance dose obtained by maximizing the AUROC, which can be due to some statistical artifacts and not necessarily involving a theoretical reason.
Figure 3. Selection of EUD n parameter an acute GI grade larger than 2 based on maximum AUROC value.
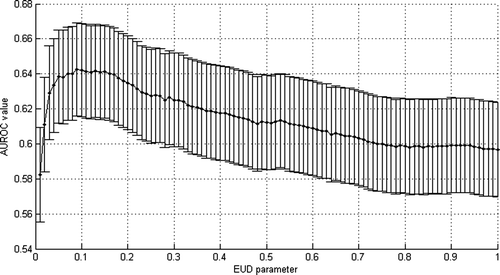
Figure 4. Likelihood fitting of acute GI by Lyman's NTCP model and fixing n parameters equal to 0.09.
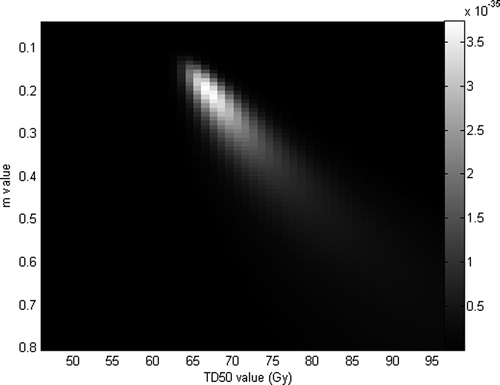
Figure 5. Observed frequencies of patients suffering acute GI (grade larger than 2) by bin of EUD versus NTCP expected values.
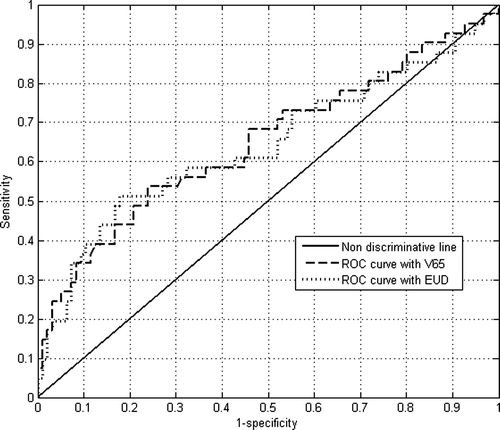
The comparison of both AUROC values (based on cumulated volumes at 65 Gy and EUD calculated with n equal to 0.09) has been performed by means of a two-tail z-test (Equation A.2). Because z equals 0.52 and hence p equals to 0.60, the difference between AUROC values is not statistically significant (since p > 0.05). ROC curves are quite similar, even if they both lead to relatively low AUROC values, as observed in .
Analysis of the acute GU complications
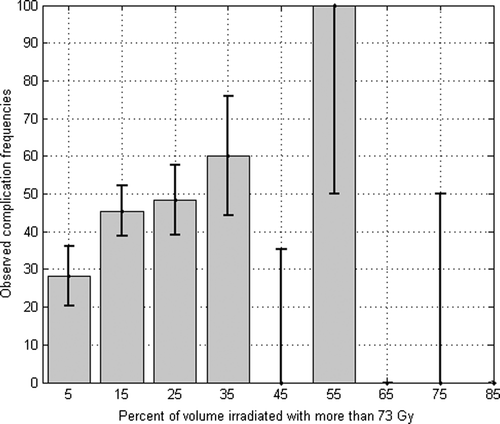
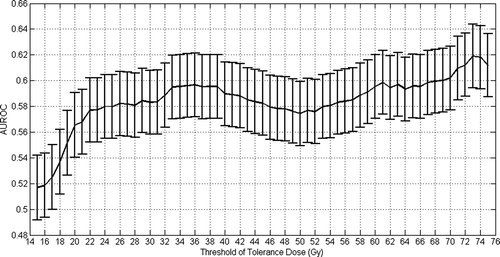
The same analysis has been performed in the case of an acute GU toxicity higher than 2 associated with the dose received by the bladder. In this case, best tolerance dose has been obtained for 73 Gy (AUROC of 0.62 and a standard deviation of 0.05). Nonetheless, as shown in , a relatively high local maximum for the AUROC value has been obtained at 36 Gy (AUROC 0.59 and a standard deviation of 0.05). Observed frequencies of complications versus V73 (with a bin width of 10% of total volume of the rectum) is plotted on . Fifty percent of complications can be observed for a range of 15 to 75% of the volume irradiated of bladder.
Figure 7. Selection of best tolerance dose for an acute GU grade larger than 2 based on maximum AUROC value.
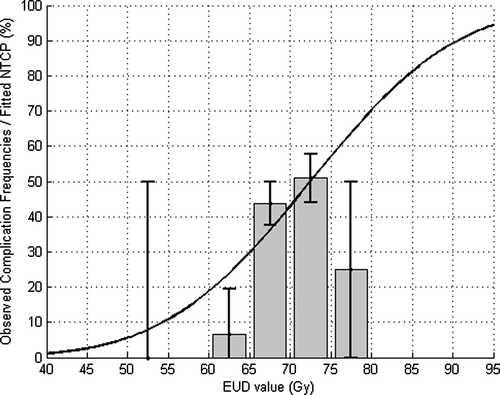
The analysis of the AUROC values calculated by the mean of EUD values for different values of the n parameter indicates that there is no privileged parameter, all of them leading to a similar AUROC (see ). Nonetheless, n equal to 0.06 was retained (AUROC value 0.60 with standard deviation 0.05), to compare with the AUROC value obtained by varying constraint on V73. By fixing n equal to 0.06 and varying m (ranging from 0.005 to 1 by a step of 0.005) and TD50 (ranging from 0.5 to 100 by a step of 0.5 Gy), likelihood reached its maximum (4 x 10 -40) for m=0.195 and TD50=72.5 Gy (see ). These parameters allow to plot NTCP values and observed frequencies of patients suffering of grade 2 acute GI versus the gEUD on a same graph (see ). Here again, the TD50 value fitted thanks to a likelihood analysis is very close to the best tolerance dose obtained by maximizing the AUROC.
Figure 9. Selection of EUD n parameter an acute GU grade larger than 2 based on maximum AUROC value.
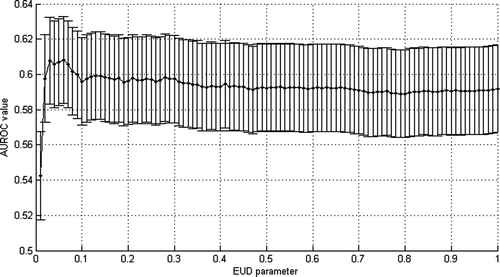
Figure 10. Likelihood fitting of acute GU by Lyman's NTCP model and fixing n parameters equal to 0.06.
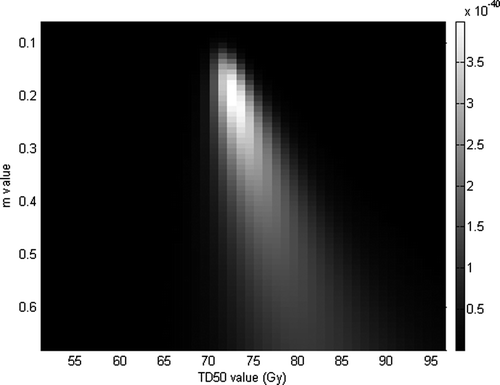
Figure 11. Observed frequencies of patients suffering acute GU (grade larger than 2) by bin of EUD versus NTCP expected values.
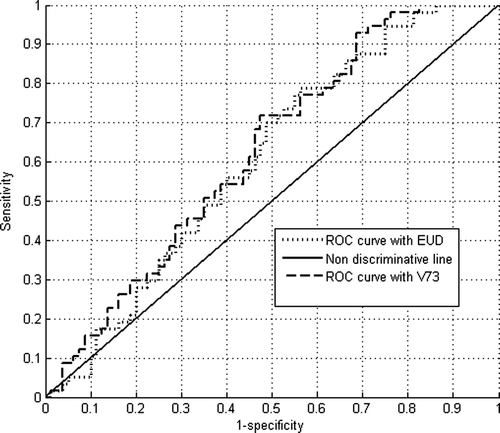
As for the rectum, both descriptions lead to quite the same (and low) AUROC value (z equals 0.67, and then p = 0.50 as computed by Equation A.2). The difference between AUROC values is not statistically significant, since p > 0.05, which is merely due to random variation in the sample and, in addition, ROC curves are quite similar ().
For both GU and GI acute complications; numerical results of AUROC values and fitted parameters are summarized in , while obtained LKB NTCP parameters are summarized in .
Table III. Summary of AUROC and parameter values by criterion and endpoint.
Table IV. Fitted LKB NTCP m and TD50 parameters by keeping n equal to 0.09 (Acute GI) and n equal to 0.06 (acute GU), their values obtained for the AUROC comparisons.
Conclusions and discussion
Regarding the analysis of the acute GI complications carried out, it is interesting to note that AUROC reaches a global maximum monotonically, and then falls down, as it can be seen in . It is a reasonable result since it indicates that gradually increasing doses better discriminate patients (as occurrence of the complication is a dose–response relationship, the curve is likely to have this shape), but after reaching the maximum, very high tolerance doses do not increase patient discrimination (the AUROC falling down).
Finally, it was impossible to declare that EUD criterion was better than a single dose–volume point on volume receiving at least 65 Gy to discriminate safe from ill patients.
In a recent study Citation[12] using a slightly modified Citation[13] RTOG acute GI and GU toxicity, the role of intermediate and high doses in predicting GI toxicity (as partial cumulated volume V65) without a volume effect has been pointed out. In another study dealing with the dosimetric predictors for acute side effects after prostate irradiation, Karlsdóttir et al. Citation[14] found that grade 2+ was mostly correlated with V70. Similarly, Nuyttens et al. Citation[15] found a statistical significant increase in grade 2+ gastrointestinal acute complications when the absolute rectal volume receiving more than 70 Gy undergoes 16.5 cm3. The results shown above corroborate these previous results, as V66 has been found here for best tolerance dose (instead of V65); and an n equal to 0.09 emphasizing the role of the highest doses (slight volume effect).
Regarding the analysis of the acute GU complications, as for the rectum, the presence of a relatively high local maximum for the AUROC is unlikely to occur. In , there is no strong correlation between V73 and V36 since there is a Pearson correlation coefficient of 0.61 between them. A relatively high local maximum in the AUROC values between V36 and V73 only could be explained by statistical noise.
It is interesting to notice the poorer quality of the fit respect to the analysis of the GI complications, underlined by lower values of maximum likelihood value and AUROC value for GU complications respect with GI complications. In previous studies, Cheng et al. Citation[16], Nuyttens et al. Citation[15] and Odrázka et al. Citation[17] also encountered some difficulties in finding out dosimetric factors for acute GU complications, as these ones concluded with no predictive dosimetric factors when using bladder DVHs. This could be explained by the fact that, in this study, standard DVHs have been used instead of Dose–Surface Histograms for the bladder's wall, as shown in Citation[12]. Indeed, Peeters et al. found a dose–surface effect for acute GU complications (for absolute surfaces receiving doses higher than 40, 45 and 65 Gy, neo adjuvant hormonal therapy being an important prognostic factor). Since bladder DVHs contain dosimetric information of volumes that are not expected to respond to doses, it could explain that fitting GU acute side effects with such conventional DVHs led us to poorer results. Zelefsky et al. Citation[18] already noted a paradoxal absence of benefit for acute GU toxicities using IMRT rather than 3DCRT, in spite of a better sparing of the bladder. In a report of acute toxicities followed by 87 patients receiving IMRT for prostate cancer; Sanguineti et al. Citation[19] invoked the probable reason that the dose received by the urethra, which passes through the prostate gland, would be a key factor in predicting acute GU toxicities. Anyway, using bladder DVHs Karlsdóttir et al. Citation[14] also showed that V14 to V27 seemed to correlate with acute GU complications. Even though we found a local maximum at V36; the presence of great uncertainties (see ) show the importance of V30 to V40 could corroborate the results of Karlsdóttir et al. and, to a certain extent, those of Peeters et al. However, it should be fruitful to correlate acute GU toxicities with other dosimetric information of other volumes.
As observed in and , higher values of AUROC and likelihood point out that GI grade 2 acute complication is best modeled and fitted than acute grade 2 GU complication; which can be graphically observed by comparing and . As aforementioned, the use of dose–wall histograms Citation[20] or other volumes could help to improve the quality of the fit, especially for acute GU complications. It has also been shown in the literature that, as mentioned by Sanguinetti et al. Citation[19], a retrospective study should include detailed information about some prognostic factors such as the presence of previous gastrointestinal symptom or previous surgery; and information about the age of the patients.
As a global conclusion, two different criteria have been compared to predict the occurrence of RTOG grade 2 gastrointestinal and genitourinary acute side effects. Partial cumulated volumes at 65 Gy (for acute GI effects) and at 73 Gy (for acute GU effects) were selected as the better discriminate variables, corresponding to AUROC values of 0.65 and 0.62, respectively. Again, n parameters of the equivalent (effective) uniform doses were 0.09 (acute GI) and 0.06 (acute GU), corresponding to maximum AUROC values 0.64 and 0.59, respectively. Likelihood fitting of m and TD50 parameters of Lyman's NTCP model gave m = 0.19 and TD50 = 66 Gy for GI complications; and m = 0.195 and TD50 = 72.5 Gy for GU complications. Notice that best tolerance doses (65 and 73 Gys) are quite close to fitted TD50 values (66 and 72.5 Gys). It could also corroborate the similarities between the criteria used, even if this point is harder to interpret.
An analysis of the statistical significance of both models (for both endpoints) revealed that it should be possible to use either model to predict the defined endpoints. In treatment plan optimization, limiting V65 or V73 should lead to the same biological impact on the RTOG grade 2 acute GI or GU complications than limiting EUD values calculated with a n equals to 0.09 or 0.06 respectively.
Nonetheless, further studies should be carried on to include in the data base other volumes than rectum for GI complication prediction Citation[21], to use dose–wall histograms or normalized histograms taking into account the fractionation effect Citation[22]. Presumably, with these modifications the EUD model could be more predictive than a single dose–volume point. Hopefully, the introduced methodology in this work could be extended to other clinical data bases, as it still remains an important topic in radiotherapy.
Acknowledgements
This work has been possible thanks to the “Health Research Grant” (Fondo de Investigaciones Sanitarias – FIS). Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991; 21: 109–22
- Schinkel C, Stavrev P, Stavreva N, Fallone BG. A theoretical approach to the problem of dose-volume constraint estimation and their impact on the dose-volume histogram selection. Med Phys 2006; 33: 3444–5
- Dawson LA, Biersack M, Lockwood G, Eisbruch A, Lawrence TS, Ten Haken RK. Use of principal component analysis to evaluate the partial organ tolerance of normal tissues to radiation. Int J Radiat Oncol Biol Phys 2005; 62: 829–37
- Munley MT, Lo JY, Sibley GS, Bentel GC, Anscher MS, Marks LB. A neural network to predict symptomatic lung injury. Phys Med Biol 1999; 44: 2241–9
- Niemierko A. A generalized concept of equivalent uniform dose (EUD). Med Phys 1999; 26: 1100
- Das SK, Baydush AH, Zhou S, Miften M, Yu X, Craciunescu O, et al. Predicting radiotherapy-induced cardiac perfusion defects. Med Phys 2005; 32: 19–27
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–6
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143: 29–36
- Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983; 148: 839–43
- Lyman JT, Wolbarst AB. Optimization of radiation therapy, IV: A dose-volume histogram reduction algorithm. Int J Radiat Oncol Biol Phys 1989; 17: 433–6
- Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys 1991; 21: 123–35
- Peeters ST, Hoogeman MS, Heemsbergen WD, Slot A, Tabak H, Koper PC, et al. Volume and hormonal effects for acute side effects of rectum and bladder during conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2005; 63: 1142–52
- Peeters ST, Heemsbergen WD, van Putten WL, Slot A, Tabak H, Mens JW, et al. Acute and late complications after radiotherapy for prostate cancer: Results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys 2005; 61: 1019–34
- Karlsdóttir A, Johannessen DC, Muren LP, Wentzel-Larsen T, Dahl O, et al. Acute morbidity related to treatment volume during 3D-conformal radiation therapy for prostate cancer. Radiother Oncol 2004; 71: 43–53
- Nuyttens JJ, Milito S, Rust PF, Turrisi AT. Dose-volume relationship for acute side effects during high dose conformal radiotherapy for prostate cancer. Radiother Oncol 2002; 64: 209–14
- Cheng JC, Schultheiss TE, Nguyen KH, Wong JY. Acute toxicity in definitive versus postprostatectomy image-guided radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2008; 71: 351–7
- Odrázcka K, Vanásek J, Vaculíková M, Petera J, Zouhar M, Zoul Z, et al. Conformal radiotherapy for prostate cancer—Longer duration of acute genitourinary toxicity in patients with prior history of invasive urological procedure. Acta Oncol 2001; 40: 810–5
- Zelefsky MJ, Fuks Z, Hunt M, Yamada Y, Marion C, Ling CC, et al. High-dose intensity modulated radiation therapy for prostate cancer: Early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys 2002; 53: 1111–6
- Sanguineti GA., Endres EJ, Parker BC, Bicquart C, Little M, Chen G, et al. Acute toxicity of whole-pelvis IMRT in 87 patients with localized prostate cancer. Acta Oncol 2008; 47: 301–10
- Meijer GJ, Van den Brink M, Hoogeman MS, Meinders J, Lebesque JV. Dose-wall histograms and normalized dose-surface histograms for the rectum: A new method to analyze the dose distribution over the rectum in conformal radiotherapy. Int J Radiat Oncol Biol Phys 1999; 45: 1073–80
- Tho LM, Glegg M, Paterson J, Yap C, MacLeod A, McCabe M, et al. Acute small bowel toxicity and preoperative chemoradiotherapy for rectal cancer: Investigating dose-volume relationships and role for inverse planning. Int J Radiat Oncol Biol Phys 2006; 66: 505–13
- Park CS, Kim Y, Lee N, Bucci KM, Quivey JM, Verhey LJ, et al. Method to account for dose fractionation in analysis of IMRT plans: Modified equivalent uniform dose. Int J Radiat Oncol Biol Phys 2005; 62: 925–32
Appendix 1: Comparison of EUD-based constraint and dose-volume based constraint AUROC values
Hanley and Mc Neil Citation[8] explained how to compute p-value to determine whether two different AUROC values are significantly different or not, where two different models are evaluated on two statistically independent populations. In Citation[9] the authors showed how to calculate the estimated significance when two models are evaluated on the basis of a same population, as in this study.
Let us note, A1 as an AUROC value corresponding to the first model and A2 for the second model, SE1 and SE2 their respective standard deviations derived below from the Wilcoxon statistic theory. So p-value can be derived from the z test:and so:
where c is the Pearson correlation coefficient, which was evaluated in this study through leave-one-out cross validation estimation of the Pearson correlation coefficient between A1 and A2.
The standard deviations SE1 and SE2in Equation A.2 can be evaluated as follows, using Wilcoxon statistic:where θ is the AUROC value of the corresponding model evaluated on a sample of an infinite number of cases (in practice A1 or A2), n+ and n- the respective numbers of positive and negative cases, Q1 is the probability that two randomly chosen ill patients will be ranked with greater suspicion than a randomly chosen safe patient, and Q2 the probability that two randomly chosen ill patients will be ranked with greater suspicion than two randomly chosen safe patients.
As mentioned, Q1 and Q2 can be estimated by the following equations (yielding from a negative exponential model fitting the ROC curve), which leads to good a approximation of the value of standard deviation:and
Then, after having computed z-value, it is possible to calculate the significant level of null hypothesis. This hypothesis states that the difference found between both AUROC values is just due to randomness, or, equivalently, that ideally A1=A2. Corresponding p-value must be evaluated with the one-tailed z distribution, if it is supposed that one specific model must lead to an AUROC higher than the other (for some intrinsic reason that can be explained a priori), or with a two-tailed z distribution, if it is not known which one should have an AUROC higher than the other. In this work p-values have been evaluated with a two tailed z distribution.