Abstract
Background: To analyze the relationship between mean radiation dose to the bowels and the anal-sphincter and occurrence of ‘defecation into clothing without forewarning’, a specific and serious fecal incontinence symptom after gynecological radiotherapy. Additional potential risk factors associated with the symptom are explored.
Material and methods: Data were collected for 519 eligible gynecological cancer survivors, treated with pelvic radiotherapy, with a median follow-up of 5.8 years, using a study-specific questionnaire and medical records. Correlations between defecation into clothing without forewarning and mean dose to organs at risk; the anal-sphincter region, the rectum, the sigmoid and the small intestines were investigated, also taking other risk factors into account.
Results: Twelve percent reported having had the symptom at least once in the preceding six months. Mean doses >50 Gy to the anal-sphincter region, the rectum, the sigmoid and the small intestines were related to the occurrence of the symptom. Significantly associated risk factors were deliveries with high birth weight, heart failure and lactose and/or gluten intolerance. After adjusting for these factors, mean doses >50 Gy to the anal-sphincter region, the sigmoid and the small intestines remained related to the occurrence of the symptom.
Conclusion: Mean doses to the bowels and anal-sphincter region are related to the risk of defecation into clothing without forewarning in long-term gynecological cancer survivors treated with pelvic radiotherapy. Further radiobiological modeling may distinguish which organ(s) contribute most to development of the symptom.
Involuntary defecation into clothing is a devastating event remembered for many years. Among gynecological cancer survivors, we have described a symptom in which the survivor suddenly and unexpectedly defecates into the clothes [Citation1], a defecation using the reflexes seen during a regular toilet visit. This symptom, here cited as ‘defecation into clothing without forewarning’, is thus a completely different symptom than having fecal leakage without the reflexes of defecation. The underlying conditions include a combination of decreased sensitivity for rectal filling as well as an increased activity of the bowel as seen in frequent defecation urgency. This endpoint is associated with a decreased sensitivity that entails not being able to sense the need to go to the toilet and defecate and it also includes an irritative component that is responsible for the sudden emptying of a large volume of stools.
Having the symptom ‘defecation into clothing without forewarning’ may result in adverse psychological, sexual and social consequences [Citation1–3]. At present we lack dose-volume data that allow us to examine possible associations of dose-volume with this devastating symptom.
Fecal continence is maintained by the pelvic floor muscles, the anal-sphincter and the rectum [Citation4]. Dysfunction leads to fecal incontinence and the anorectal function has been extensively evaluated after pelvic radiation therapy [Citation5,Citation6]. Weakness of the anal-sphincter, reduction in rectal compliance, change in rectal sensitivity, and altered stools consistency have been reported among gynecological cancer survivors treated with pelvic radiation therapy [Citation7,Citation8]. It is evident that the mechanism leading to fecal incontinence after radiation therapy is complex [Citation9] perhaps involving many organs. However, most attention thus far has been given to the anal-sphincter and the rectum. Other organs at risk (OARs) have not been studied as carefully as they should be in order to understand the mechanism. Furthermore, the concept of ‘fecal incontinence’ as commonly used encompasses a variety of symptoms and thus may be too general. We have found that by atomizing the term ‘incontinence’ into detailed patient-reported symptoms, it may be possible to reveal specific radiation pathophysiologies that otherwise would remain hidden. Information on dose to normal tissue and outcome data will help to predict the risk of normal tissue injury and thereby guide the radiotherapist in the choice of competing treatment plans [Citation10].
In Sweden unique personal identity numbers and official population-based registers offer excellent conditions to follow cancer survivors without selection-induced problems. In 2006 a population-based study was performed among 616 gynecological cancer survivors on late symptoms after pelvic radiation therapy. A matched control population of 344 non-irradiated women was included. Information was provided through a study-specific validated questionnaire comprising questions on demographics, physical and psychological symptoms, sexuality and social functioning. We found a higher prevalence of the symptom defecation into clothing without forewarning at least occasionally during the preceding six months among the survivors (12%) than among the controls (less than 1%) [Citation2]. In the present study we report on the relationship between mean dose to bowel OARs and the occurrence of this specific fecal incontinence symptom in 519 survivors for whom we had electronically stored external beam radiation therapy (EBRT) dose plans.
Material and methods
We have followed the hierarchical step model [Citation11] in the design of the data collection and the interpretation of the results. A detailed description of the study methodology has previously been published [Citation2]. In previous papers from our group on ‘defecation of stools into clothing without forewarning’ we used the phrasing directly reported by the survivors, i.e. ‘emptying of all stools into clothing without forewarning’.
The Regional Ethics Committee at the Karolinska Institute approved the study.
Survivors’ characteristics
Clinical data and three-dimensional (3D) EBRT plans including dose-volume histograms (DVHs) for the anal-sphincter region, the rectum, the sigmoid and the small intestines were collected for the 519 eligible gynecological cancer survivors. The treatments were given with curative intent at Radiumhemmet, Stockholm or at Jubileumskliniken, Gothenburg in 1991–2003. Endometrial cancer and cervical cancer were the most common diagnoses, 62% and 22%, respectively. In addition there were survivors of ovarian, fallopian tube, vaginal and vulvar cancers and uterine sarcomas. In addition to EBRT, approximately 90% also had had surgery, 82% brachytherapy and 27% chemotherapy. The chemotherapy received was single cisplatinum, combination platinum-taxane, combination platinum-antracycline or other. Median time since completing EBRT was 5.8 years (range 2–14 years).
Treatment planning and delivery
EBRT 3D treatment planning was based on computed tomography (CT) scans performed prior to therapy. Scans were made in the supine position on a flat table top, using laser markers and conversion factors to electron density. Slice thickness was usually 5–10 mm. The EBRT dose was prescribed either at the isocenter or as the mean dose to the target covering at least 95% of the planning target volume [Citation12]. The treatment was administered with linear accelerators or a racetrack accelerator with 6–50 MV photons using two opposing fields or a four-field box technique with prescribed daily fractions of 1.6, 1.8 or 2.0 Gy. EBRT treatment position was verified by portal image films and check-and-confirm systems.
Prescribed doses for endometrial cancer was 40–46 Gy and to uterine sarcomas 50 Gy. For cervical cancer similar techniques were used in an initial treatment phase and in a second phase a boost covering a smaller volume with a prescribed total dose of 55–70 Gy, depending if brachytherapy was added or not. Ovarian and fallopian tube cancers had a prescribed dose of 20 Gy to the abdomen and an additional 20 Gy to a volume with lowered cranial margin.
Brachytherapy (BT) was applied using standardized techniques and applicator templates. The BT dose was prescribed according to local practice. Orthogonal x-ray images verified the position of the BT applicators. High-dose rate BT for endometrial cancer was prescribed at 5 Gy per fraction in 2 fractions or 3.75 Gy per fraction in 3 fractions. For cervical cancer low-dose rate BT was prescribed at 10.0–24.0 Gy per fraction in 1–3 fractions depending on tumor size and EBRT dose or as high-dose rate BT at 4.0 Gy per fraction in 3 fractions.
Organs at risk and dose-volume histograms
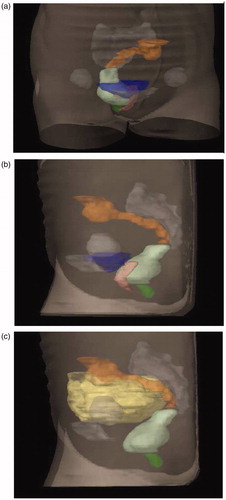
In the present study we contoured four intestinal OARs; anal-sphincter region, rectum, sigmoid and small intestines. The ‘anal-sphincter region’ was represented by the inner muscle layer of the sphincter up to the anal verge. The ‘rectum’ was depicted by its outer contour with filling extending from the anal verge to the recto-sigmoid junction. The ‘sigmoid colon’ was contoured from where the rectum deviates from its mid-position to where it turns cranially in the left part of the abdomen connecting to the colon descendens. The ‘small intestines’ were all visible small bowels in the small pelvic cavity to the caudal part of the sacroiliac joints. Continuous CT slices resulted in 3D volumes () where the absorbed doses were calculated. The contouring was performed by two persons at each clinic under the supervision of senior oncologists (H.L. and A.-C.W.) during 2006 and 2007. Guidance was provided by a Contouring Manual with illustrations (Supplementary material, available online at http://www.informahealthcare.com) and written instructions. The DVHs were exported for the four OARs for each patient using the TMS (Nucletron, Veenendaal, the Netherlands), Cadplan or Eclipse (Varian Medical Systems, Palo Alto, CA) treatment planning systems.
Statistical analyses
Cancer survivors were dichotomized into having had or not having had defecation into clothing without forewarning at least once during the preceding six months. We used risk ratios (RRs) and odds ratios (ORs) with 95% CI to compare symptom prevalence between the two groups. RRs and p-values were estimated through the log-binomial model. For multivariable modeling we used ORs estimated through logistic regression.
Characteristics of the study population and univariate RRs are given in and .
Table 1. Demographic and clinical characteristics for 519 gynecological cancer survivors with and without defecation into clothing without forewarning after pelvic radiation therapy.
Table 2. Diagnosis and treatment characteristics for 519 gynecological cancer survivors with and without defecation into clothing without forewarning after pelvic radiation therapy.
In order to identify potential co-variates for the symptom, we performed an exploratory variable selection () using logistic regression with forward selection. We used complete cases and α = 0.05 as inclusion criteria (). Survivors were sorted into five mean EBRT dose intervals and symptom prevalence was calculated within each dose level for the OARs, using the previously identified factors for adjustments (). Exclusion of survivors who had received BT with iridium >11.25 Gy or radium +/− cesium was made to test the impact of BT. Correlations between mean doses in the four OARs were calculated using the Spearman’s correlation coefficient.
Table 3. Potential risk factors for defecation into clothing without forewarning after pelvic radiation therapy in 519 gynecological cancer survivors.
Table 4. Mean dose to organs at risk and risk of defecation into clothing without forewarning after pelvic radiation therapy.
Differences between mean DVHs of those with and without the symptom were assessed with pointwise t-tests for each dosebin. All tests were performed two-sided and at the 5% significance level; individuals with missing data were excluded in each calculation. Calculations were performed using SAS software (version 9.2, SAS Institute Inc., Cary, NC). The EBRT doses have been corrected to 2 Gy per fraction using the linear-quadratic model with an α/β-ratio of 3 Gy [Citation13].
Results
Demographic and clinical characteristics of the 63 gynecological cancer survivors with the symptom defecation into clothing without forewarning and the 456 survivors without the symptom occurring at least once the past six months are presented in and . Deliveries with high birth weight, cardiovascular disease, lactose intolerance, rheumatism and thrombosis were more frequent among survivors with the symptom.
Cervical cancer and uterine sarcomas were overrepresented compared to endometrial cancer, which was the reference level. Affected survivors had more often been treated with radiation as only treatment and usually to higher doses compared to survivors treated following surgery ().
Mean total EBRT dose was higher among survivors with the symptom than among those without the symptom and 52% of the affected survivors had had a total dose >45 Gy compared to 35% of non-affected survivors. There was no difference regarding field technique or target area. BT was less common among survivors with the symptom. There was no statistically significant increased risk of developing the symptom with time after EBRT, OR =1.03 (95% CI 0.94–1.12) per year.
The multivariable analyses identified three risk factors for the symptom ‘defecation into clothing without forewarning’, in addition to EBRT. Delivery of at least two children with birth weight exceeding 4 kg (RR =2.2, 95% CI 1.2–4.1), heart failure (RR =3.4, 95% CI 2.0–6.0), and lactose intolerance and/or gluten intolerance (RR =2.6, 95% CI 1.4–4.7) were significantly associated with a risk of having the symptom ().
Mean doses to the four OARs were too closely correlated to be included in the same regression analysis, with Spearman’s correlation coefficients ranging from 0.412 to 0.724. The prevalence of ‘defecation into clothing without forewarning’ was higher among survivors with mean doses >50 Gy for at least one of the OARs than among those with lower mean doses (). The corresponding unadjusted RRs and ORs for mean doses >50 Gy were significantly increased. Adjustment for the risk factors resulted in losing the significantly increased OR for mean dose >50 Gy to the rectum. The risk of the outcome was highest among patients with four OARs with mean dose >50 Gy.
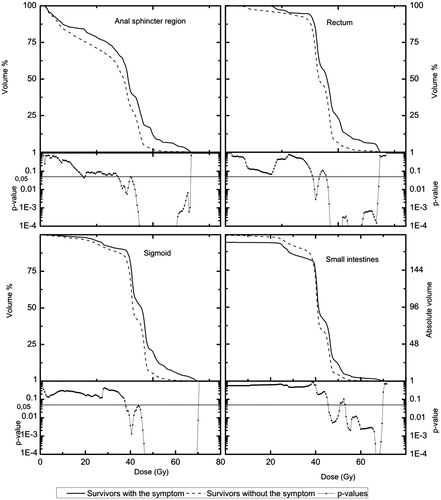
Further analyses were restricted to survivors treated with iridium BT dose ≤11.25 Gy. All OARs with the exception of the rectum showed significantly increased ORs for mean doses >50 Gy [anal-sphincter: 27.5 (5.0–150.1), rectum: 9.4 (0.9–95.9), sigmoid: 8.8 (20–39.3), small intestines: 7.0 (1.5–32.5)]. After adjusting for risk factors the significantly increased ORs for mean doses >50 Gy remained for all OARs except for the rectum [anal-sphincter: 36.3 (6.0–221.7), rectum: 11.1 (0.9–136.6), sigmoid: 9.9 (2.0–48.2), small intestines: 9.3 (1.7–50.7)]. The differences in average dose DVHs with p-values for EBRT for survivors with and without defecation into clothing without forewarning for the four OARs are presented in . The DVHs for the anal-sphincter region were significantly separated (p < 0.05) for doses in the interval of 34.5–66.5 Gy, for the rectum in 39.0–41.5 Gy and 45.0–68.0 Gy, respectively, for the sigmoid in 38.0–70.0 Gy and for the small intestines in the interval of 45.5–50.5 and 53.0–69.5 Gy, respectively.
Discussion
Our results show a dose-effect relationship between mean doses >50 Gy to the anal-sphincter region, the rectum, the sigmoid, and the small intestines and the occurrence of defecation into clothing without forewarning among long-term gynecological cancer survivors treated with pelvic radiation therapy. The dose distributions for patients with and without the symptom were significantly separated for the studied OARs for intermediate and high doses indicating that the doses to these OARs is an important factor for the development of the symptom ‘defecation into clothing without forewarning’. In a recent publication we reported on a study of a subgroup of the survivors treated without BT. The DVHs for these OARs were also significantly separated for intermediate and high doses [Citation14].
To the best of our knowledge, these are the first studies investigating the relationship between dose-distribution data of EBRT to the bowels and the anal-sphincter region and the occurrence of involuntary defecation among gynecological cancer survivors treated with pelvic radiation therapy. In contrast, a vast number of studies have reported on the relationship between anorectal dose parameters and the risk of late fecal incontinence in prostate and other pelvic cancers [Citation15]. In a study by Fiorino et al., dosimetric rectal data from 506 prostate cancer patients were analyzed, where rectal volume receiving ≥40 Gy (V40) and surgery were the strongest predictors of fecal incontinence defined as ‘use of pads’ [Citation16]. Based on 641 prospectively scored (RTOG/EORTC scale) prostate cancer survivors Peeters et al. found fecal incontinence requiring pads to be associated with anal wall parameters [Citation17]. Similar results have previously been reported by our own group for prostate cancer, where a significant correlation between mean dose in the interval of 45–55 Gy to the anal-sphincter region and the risk of fecal incontinence was found [Citation18].
Some researchers favor the hypothesis that symptoms may originate from specific anatomic regions. Smeenk et al. reported on urgency and incontinence, which originated from both the anal wall and rectal wall, while frequency seemed mostly associated with rectal wall dysfunction [Citation19]. In addition they found that dose-effect relations differed between the described symptoms. The importance of discriminating between different symptoms and their origin in order to increase specificity is supported by Heemsbergen et al., who performed an anorectal dose-surface map analysis and found a dose-effect relation for fecal incontinence in the anal region and lower rectum [Citation20]. In the study by Fonteyne et al., the sigmoid colon was suggested as being co-responsible for the development of lower intestinal toxicity beside the anal-sphincter and the rectum. They also found that the volume of the small bowel receiving doses in the range of 50–60 Gy is predictive for the development of late side effects, which is in line with our results [Citation21]. In our recent paper we found steep dose-response relationships for the anal-sphincter, rectum, sigmoid and the small intestines and the development of ‘defecation into clothing without forewarning’. The mean doses to the OARs were however highly correlated with each other, and it is difficult to say if only one or if multiple organs are involved in the development of the symptom [Citation14]. However, Andreyev et al. have recently questioned the anatomically based approach arguing that symptoms originating from the pelvic area have multiple causes [Citation22]. We consider ‘defecation into clothing without forewarning’ neither to be fecal incontinence nor a pure urgency symptom. This implies the engagement of all the investigated OARs and is in line with our result that the risk of the symptom ‘defecation into clothing without forewarning’ is highest among patients with mean dose >50 Gy to all four of the investigated OARs. We strongly support the importance of proper diagnostic procedures and that increased knowledge of radiotherapy-related atomized symptoms may lead to refinement of treatment and development of less radiotherapy induced long-term side effects. We would like to note that the treatment technique used for the survivors included in the study was either opposing fields or four-field box. Nowadays, in order to decrease toxicity, it is recommended to use IMRT and VMAT for the EBRT treatment.
Normal tissue injury induced by ionizing radiation is thought to be a progressive process. However, there are reports showing both an increase and a decrease of rectal symptoms in prostate cancer survivors with time [Citation23]. In the present study we did not find any statistically significant increase in risk for developing defecation into clothing without forewarning during follow-up from 2 to 14 years after pelvic radiation therapy.
One of the strengths of this study is the large population-based survivor cohort. The use of unique personal identity numbers, public registers and the fact that all gynecological cancer patients in Sweden belong to one out of six geographical catchment areas minimizes the risk of selection-induced problems. Interviewer-induced bias was avoided by the use of a numbered postal questionnaire, a practice that mimics the technique of blinding. Access to all medical records has ensured correct information regarding clinical characteristics. The large cohort in combination with the long follow-up time has enabled us to investigate the symptom prevalence over time. Our data were based on women under the age of 80 years, and the results may not be possible to generalize to older populations.
The major limitation of this study comprises the difficulties in estimating the contribution from BT. A variety of techniques were used regarding isotopes, applicators, anatomical arrangements and doses. In addition treatments were performed without the aid of 3D BT planning systems. Based on our clinical experience, we made the assumption that iridium BT in the adjuvant setting to treat endometrial cancer patients postoperatively with a total prescribed dose of 10–11.25 Gy did not substantially affect the dose to the studied OARs. The survivors that were treated with this kind of BT had in general a low prescribed EBRT dose. We therefore excluded survivors receiving iridium >11.75 Gy, or radium with or without cesium, in an attempt to investigate the impact of BT given with ‘high’ doses. The resulting prevalence ratios were even higher indicating that EBRT to the four OARs is related to the occurrence of defecation into clothing without forewarning.
To improve the specificity the contouring of the OARs was made with a zero margin in order not to unintentionally include parts of other organs. Reviewing of the contouring was applied to decrease inter-observer variety. A limitation of our study is the potential effect of organ motions and variations in setup of patients since the contouring is based on pretreatment CT scans, which represents a static picture. We have previously reported that the position of the sigmoid may vary and has the largest deviation anteriorly but also that overlapping dose is most pronounced in the anterior rectal wall [Citation24].
This study shows that mean dose to bowel organs and anal-sphincter region is related to the occurrence of ‘defecation into clothing without forewarning’. Our results suggest that not only the rectum and anal-sphincter should be acknowledged in radiation therapy planning but also the sigmoid and the small intestines. The results should be taken into consideration when comparing competing dose plans. In addition there is a need of more advanced radiobiological modeling to further explore the contribution from each OAR for predicting the risk of ‘defecation into clothing without forewarning’.
COntouring_manoual_4.pdf
Download PDF (76.1 KB)COntouring_manoual_3.pdf
Download PDF (63 KB)COntouring_manoual_2.pdf
Download PDF (69.5 KB)COntouring_manoual_1.pdf
Download PDF (68.6 KB)Acknowledgments
We thank all women who participated in this study. This study was funded by the Swedish Cancer Society CAN 2006/1321 and CAN 2009/1099, The Cancer Research Funds of Radiumhemmet, The King Gustav V Jubilee Clinic Cancer Foundation in Gothenburg and The Swedish State under the ALF agreement in Stockholm and Gothenburg. We would like to thank Anna Carlander for helpful assistance in the delineation of organs at risk and for collection of dose-volume histograms and Dr Lawrence Lundgren for skillful linguistic revising.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bergmark K, Åvall-Lundqvist E, Dickman PW, Henningsohn L, Steineck G. Patient-rating of distressful symptoms after treatment for early cervical cancer. Acta Obstet Gynecol Scand 2002;81:443–50.
- Lind H, Waldenström AC, Dunberger G, Al-Abany M, Alevronta E Johansson KA, et al. Late symptoms in long-term gynaecological cancer survivors after radiation therapy: a population-based cohort study. Br J Cancer 2011;105:737–45.
- Dunberger G, Lind H, Steineck G, Waldenström AC, Nyberg T Al-Abany M, et al. Fecal incontinence affecting quality of life and social functioning among long-term gynecological cancer survivors. Int J Gynecol Cancer 2010;20:449–60.
- Bajwa A, Emmanuel A. The physiology of continence and evacuation. Best Pract Res Clin Gastroenterol 2009;23:477–85.
- Maeda Y, Hoyer M, Lundby L, Norton C. Faecal incontinence following radiotherapy for prostate cancer: a systematic review. Radiother Oncol 2011;98:145–53.
- Putta S, Andreyev HJ. Faecal incontinence: A late side-effect of pelvic radiotherapy. Clin Oncol (R Coll Radiol) 2005;17:469–77.
- Yeoh E, Sun WM, Russo A, Ibanez L, Horowitz M. A retrospective study of the effects of pelvic irradiation for gynecological cancer on anorectal function. Int J Radiat Oncol Biol Phys 1996;35:1003–10.
- Dunberger G, Lind H, Steineck G, Waldenström AC, Onelöv E, Åvall-Lundqvist E. Loose stools lead to fecal incontinence among gynecological cancer survivors. Acta Oncol 2011;50:233–42.
- O'Brien PC. Radiation injury of the rectum. Radiother Oncol 2001;60:1–14.
- Bentzen SM, Constine LS, Deasy JO, Eisbruch A, Jackson A Marks LB, et al. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys 2010;76:S3–S9.
- Steineck G, Hunt H, Adolfsson J. A hierarchical step-model for causation of bias-evaluating cancer treatment with epidemiological methods. Acta Oncol 2006;45:421–9.
- ICRU. Prescribing, recording, and reporting photon beam therapy; 1993.
- Emami B, Lyman J, Brown A, Coia L, Goitein M Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–22.
- Alevronta E, Lind H, Al-Abany M, Waldenström AC, Olsson C Dunberger G, et al. Dose-response relationships for an atomized symptom of fecal incontinence after gynecological radiotherapy. Acta Oncol 2013;52:719–26.
- Fiorino C, Rancati T, Valdagni R. Predictive models of toxicity in external radiotherapy: dosimetric issues. Cancer 2009;115:3135–40.
- Fiorino C, Fellin G, Rancati T, Vavassori V, Bianchi C Borca VC, et al. Clinical and dosimetric predictors of late rectal syndrome after 3D-CRT for localized prostate cancer: preliminary results of a multicenter prospective study. Int J Radiat Oncol Biol Phys 2008;70:1130–7.
- Peeters ST, Lebesque JV, Heemsbergen WD, van Putten WL, Slot A Dielwart MF, et al. Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2006;64:1151–61.
- Al-Abany M, Helgason AR, Cronqvist AK, Lind B, Mavroidis P Wersäll P, et al. Toward a definition of a threshold for harmless doses to the anal-sphincter region and the rectum. Int J Radiat Oncol Biol Phys 2005;61:1035–44.
- Smeenk RJ, Hopman WP, Hoffmann AL, van Lin EN, Kaanders JH. Differences in Radiation Dosimetry and Anorectal Function Testing Imply That Anorectal Symptoms May Arise from Different Anatomic Substrates. Int J Radiat Oncol Biol Phys 2010;13:1–8.
- Heemsbergen WD, Hoogeman MS, Hart GA, Lebesque JV, Koper PC. Gastrointestinal toxicity and its relation to dose distributions in the anorectal region of prostate cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys 2005;61:1011–18.
- Fonteyne V, De Neve W, Villeirs G, De Wagter C, De Meerleer G. Late radiotherapy-induced lower intestinal toxicity (RILIT) of intensity-modulated radiotherapy for prostate cancer: the need for adapting toxicity scales and the appearance of the sigmoid colon as co-responsible organ for lower intestinal toxicity. Radiother Oncol 2007;84:156–63.
- Andreyev HJ, Wotherspoon A, Denham JW, Hauer-Jensen M. “Pelvic radiation disease”: new understanding and new solutions for a new disease in the era of cancer survivorship. Scand J Gastroenterol 2011;46:389–97.
- Odrazka K, Dolezel M, Vanasek J, Vaculikova M, Zouhar M Sefrova J, et al. Time course of late rectal toxicity after radiation therapy for prostate cancer. Prostate Cancer Prostatic Dis 2009;13:138–43.
- Waldenström AC, Alsadius D, Pettersson N, Johansson KA, Holmberg E Steineck G, et al. Variation in position and volume of organs at risk in the small pelvis. Acta Oncol 2010;49:491–9.