Abstract
Background: Electrochemotherapy, the combination of electroporation and chemotherapy, is mainly used in the palliative setting for treatment of cutaneous and subcutaneous metastases; however, new applications are continuously being explored. Patients with head and neck cancer are primarily treated with surgery and/or radio-chemotherapy. In the setting of local recurrence with no further curative treatment options available, electrochemotherapy could be of value. We therefore performed a systematic search of the present literature.
Materials and methods: Eligible studies presented data from patients with head and neck cancer treated across the mucosal surface with electrochemotherapy. The search resulted in 11 studies with a total of 72 patients.
Results: Overall complete response was reported as good, especially in primary small tumors. Side effects were minor in primary tumors whereas large, recurrent tumors displayed more frequent side effects and some serious adverse events. Design and structure of the studies differed considerably, making general comparisons difficult.
Conclusion: Few studies concerning electrochemotherapy on mucosal head and neck tumors are available and they are not easily comparable. Overall response to treatment is good; nonetheless, further systematic studies are warranted.
Introduction
Head and neck cancer is the seventh most frequent cancer form in the world with over 500 000 new cases of oral and pharyngeal cancers globally [Citation1]. Standard treatment regimens consist of surgical resection and/or radio-chemotherapy but both modalities can severely affect quality of life by altering appearance, speech, swallowing and respiration. Furthermore, recurrences are difficult to treat and new treatment modalities are warranted, particularly for patients for whom further surgery or radiotherapy is not possible.
Electrochemotherapy is a treatment modality that may be applicable to recurrent head and neck cancer patients; the purpose of this review is to examine the use of electrochemotherapy for mucosal head and neck cancer.
Electrochemotherapy
The combination of electroporation and chemotherapy is termed electrochemotherapy. By the use of electric fields, i.e. electroporation, the cell membrane is depolarized, making it permeable for molecules, which normally do not easily pass across the cell membrane [Citation2–5]. Electroporation is used in vitro and in vivo in a number of settings, but clinically electrochemotherapy is mainly used in treatment of cutaneous and subcutaneous metastases [Citation6].
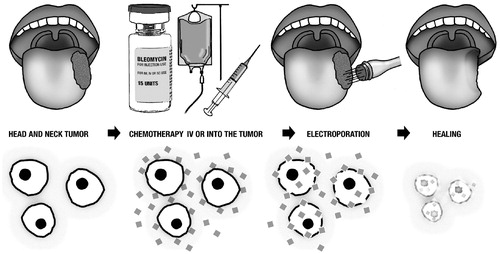
The technique is simple (): first, administration of the chemotherapeutic drug, next penetration of electrodes into the tumor, and ultimately delivery of short electric pulses, which destabilize the membrane through creation of an electric field. As this electric field surpasses a threshold, pore-like structures are temporarily created in the membrane [Citation3,Citation4]. Consequently, the chemotherapeutic drug may get access to the cell cytosol.
Bleomycin, a large hydrophilic molecule with the size of approximately 1500 daltons, is the preferred chemotherapeutic agent for electrochemotherapy [Citation7]. Before electroporation, bleomycin is introduced to the extracellular space by intra-tumoral or intravenous injection. Once inside the cell, bleomycin causes single- and double-strand DNA breaks leading to quick cell death by pseudoapoptosis [Citation8]. The combination of electroporation and bleomycin enhances the cytotoxic effect of bleomycin by 300–700-fold [Citation9,Citation10], resulting in an effective local cancer treatment against various classes of histopathology [Citation11,Citation12].
The first in vivo clinical trial using electrochemotherapy was performed 1991 in France [Citation13,Citation14]. Since then, electrochemotherapy has undergone numerous trials on advanced malignant melanoma, and non-melanoma skin cancer [Citation11,Citation12,Citation15–18], recurrent breast cancer [Citation19] and Kaposi’s sarcoma [Citation20]. Today, electrochemotherapy is mainly used for cutaneous treatment of primary or recurrent tumors and metastases [Citation21]; however, research is ongoing for the use of electrochemotherapy in the treatment of colorectal cancer [Citation22] and liver metastases [Citation23]. Furthermore, novel drug agents are being explored such as calcium [Citation24] and mitomycin C [Citation25]. The advantage of incorporating molecules into the cell by electroporation has led to new research about DNA vaccine delivery [Citation26] and gene therapy [Citation27].
In 2006, a comprehensive guideline was published, European Standard Operating Procedures of Electrochemotherapy (ESOPE), [Citation28] depicting how to select patients and choose the appropriate type of anesthesia, route of bleomycin administration, follow-up period etc. The guideline was based on previous studies on electrochemotherapy. In addition, National Institute for Health and Care Excellence (NICE, UK, www.nice.org.uk) has issued guidelines for electrochemotherapy on skin tumors. However, there are currently no existing guidelines for electrochemotherapy on mucosal tumors in the head and neck region.
Aim of review
The purpose of the review is to clarify the current knowledge of the use of electrochemotherapy for mucosal head and neck tumors; providing general information regarding number of patients treated, tumor stage and response to treatment. As electrochemotherapy is mainly used in the palliative setting, we searched for comparisons made to other palliative treatments. Ideally, the review will identify the knowledge gaps that need to be explored to establish a guideline for future treatment.
The academic definition of head and neck cancer differs from country to country; many include skin cancer within the head and neck region. This review will focus only on mucosal tumors in the head and neck region. We define these tumors as located in, or derived from, the mucosal surface in the oral cavity, pharynx, larynx, and sinuses, or from the salivary glands.
Methods
This review was constructed according to the PRISMA group statement [Citation29]. There was no available protocol for this review; neither institutional nor ethics board approval was necessary as all data were retrieved from previously published data.
A systematic literature search was conducted on the 3 February 2016 from the following databases: MEDLINE, EMBASE and COCHRANE (Cochrane database of systematic review and Cochrane central register of clinical trials). Initially, we performed a MeSH search in MEDLINE; however, too many articles were not MeSH indexed and this search strategy was deselected. Instead, an advanced search was performed, taking all electrochemotherapy-synonyms and tumors located in the head and neck into account. The following search terms were used: electrochemotherapy, electroporation, electropermeabilization, electropermeabilization, and head neck cancer.
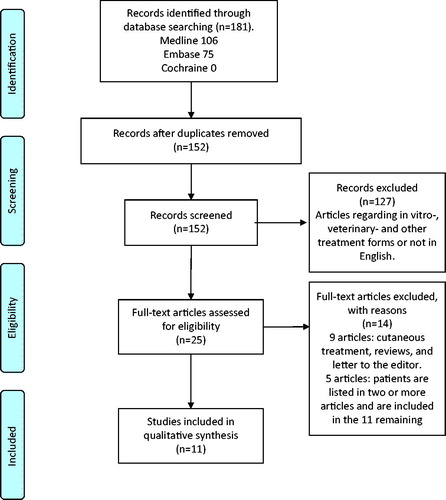
A total of 152 articles were found. All abstracts were examined and screened, excluding 114 articles regarding in vitro or veterinary treatments, well defined non-mucosal head and neck cancer or non-English papers. Full text assesment of the remaining 25 articles resulted in 11 eligible articles. The process of screening abstracts and full text assesment was independently performed by three authors ().
As the field of electrochemotherapy on mucosal head and neck tumors is small, all articles were included without consideration of publication year or length of follow-up.
Inclusion criteria: Eligible studies had to describe trans-mucosal electrochemotherapy of tumors located in the oral cavity, pharynx, larynx, sinuses or salivary glands. Clinical trials, original studies and case reports from articles in peer-reviewed journals were included.
Exclusion criteria: Papers concerning electrochemotherapy of skin cancers and lymph nodes were not included. Cutaneous electrochemotherapy of salivary glands and the thyroid was not included. In vitro treatments, veterinary studies, general reviews, editorials and letters to the editor were excluded. Abstracts in all languages were screened, but non-English articles were excluded from full text assesment.
Data extraction: Full text assesment retrieved the following data: study design, patient number, tumor localization, tumor size, histology, status of disease (primary/recurrent), curative/palliative intention, type of anesthesia, route of bleomycin administration, choice of electrodes, side effects, length of follow-up and overall response.
Bias: Several articles describe electrochemotherapy of head and neck tumors without differentiating between cutaneous and mucosal tumors. This represents a possible bias; studies using mucosal electochemotherapy may be overlooked, if it is not clearly described in the published article.
Results
Eleven clinical trials and case reports published from 2001 to 2015 were included (; Supplementary Table 1, available online at http://www.informahealthcare.com). In summary, 72 patients received electrochemotherapy of mucosal head and neck tumors, but of the 11 included studies only three performed electrochemotherapy solely on mucosal tumors [Citation32,Citation35,Citation36]. The remaining eight studies were a combination of cutaneous and mucosal treatment, of which only treatments of mucosal tumors are included in the present analysis.
Table 1. Overview of included studies.
All 11 studies provided information regarding age; patients treated were distributed from 19 to 100 years of age, with a median age from 56.6 years [Citation35] to 76.7 years [Citation37]. Both genders were treated, but none of the studies provided information about comorbidity of the patients. Nevertheless, several studies described detailed exclusion criteria. One study provided information about performance status ≤2 [Citation33] and one study had focus on providing electrochemotherapy to the elderly, or to patients in poor general condition [Citation34].
Thirty-six patients had primary tumors and 34 patients had recurrent/advanced stage disease. Only one study which had two patients did not categorize the stage of disease [Citation34]. The intention of electrochemotherapy was curative in two studies [Citation32,Citation35], palliative in one study [Citation38], and either curative or palliative in three studies [Citation30,Citation34,Citation36]. Five studies did not describe their intention of treatment.
The treated tumors were primarily located in the oral cavity or oropharynx (65 patients). In addition, electrochemotherapy was performed on tumors in the nasal cavity, sinuses, nasal pharynx and larynx. Apart from two cases of adenocarcinoma and one with clear cell carcinoma, the histology was squamous cell carcinoma (69 patients).
Tumor stage was primarily defined by the use of the TNM classification system. The TNM classification described the tumor size at the time of diagnosis. Nevertheless, four studies either did not describe tumor size [Citation37,Citation39] or described the maximum tumor size, which they were treating [Citation31,Citation40]. In addition, one study classified the tumors into three different groups depending on tumor diameter [Citation33]. TNM classification in the remaining 49 patients were: T1-T2: 39 patients, T3: one patient, T4: seven patients and Tx: two patients.
The procedure was predominantly performed during general anesthesia. In six studies an intra-tumoral injection of bleomycin was chosen [Citation30–32,Citation35,Citation36,Citation40], whereas three studies preferred an intravenous route of bleomycin administration [Citation34,Citation37,Citation38]. Skarlatos et al. [Citation39] and Campana et al. [Citation33] used both administration routes depending on tumor size; a large tumor could more easily be reached and obtain a more homogenous distribution of bleomycin by intravenous administration.
Bleomycin is measured in either units (U) or international units (IU or IE); 1 U is equal to 1000 IU and contains 0.56–0.66 mg of bleomycin [Citation6]. All intravenous injections were administered at a dose of 15 000 IU/m2 [Citation33,Citation34,Citation37–39]. When administered intra-tumorally, bleomycin was delivered in a dose of 1 U/cm3 tumor [Citation30–32,Citation40] or as 1000 IE/cm3 tumor [Citation35,Citation36]. Two studies administered different doses depending on tumor size, ranging from 250 to 1000 IU/cm3 with a lower concentration for the larger tumors [Citation33,Citation39]. Allegretti and Panje [Citation30] and Bloom and Goldfarb [Citation31] calculated tumor volume using this formula: (A × B × C × 3.14)/6. Both studies by the Landström group [Citation35,Citation36] used: 0.5(a + 1cm)(b +1cm)2, thereby adding 1 cm to the tumor volume. Tijink et al. [Citation40] calculated tumor volume adding 0.5 cm as a margin.
The electric pulses were delivered with either a MedPulser [Citation42] or Cliniporator device [Citation28]. All the studies provided information regarding choice of electrode: six needle array-, hexagonal-, linear-, plate- or finger electrode. The electrodes are differently designed and thereby cover different areas and depths [Citation6]. Pulse duration was generally 100 μs in all studies but field strengths (V/cm), as well as number of pulses per application delivered, differed.
Response to treatment was described in all the studies. Nonetheless, a pooling of the responses from all 11 studies was problematic: response was defined and measured either by clinical examination, histological examination, or from imaging. Furthermore, two studies treated the tumor with surgical tissue removal or radio-chemotherapy after electrochemotherapy, thereby obscuring the response [Citation32,Citation35]. Due to the different response measurements, we decided not to pool the overall response data but to display it for each study (Supplementary Table 1).
A complete response (CR) was unanimously described as being no tumor left. A partial response (PR) was either loss of at least 25% [Citation38] or 50% [Citation31,Citation34,Citation39] tumor volume. The studies treating small, primary T1-T2 tumors demonstrated high CR of 100% in Landström et al. [Citation35] and 83% in Burian et al. [Citation32]. In comparison, a study by Allegretti and Panje focused on recurrent tumors [Citation30], showed CR of 33% and PR of 50%.
The response was evaluated between four weeks and two months after electrochemotherapy. There was no consistency regarding how the length of follow-up was reported; described as either the planned follow-up period [Citation31], the actual periods [Citation37], or the median follow-up period [Citation34,Citation35]. The follow-up period ranged from 45 days to 5 years.
Side effects and safety
Three studies described the healing process of a mucosal tumor that differs from a cutaneous tumor. Initially, a swelling phase occurs lasting 3–10 days. On Day 2–3 after electrochemotherapy a white demarcation of the treated tissue becomes visible. In a second phase, the mucosal tumor enters a necrotic phase with yellow-gray debris, lasting 3–5 weeks. Finally, the mucosa heals within 6–9 weeks depending on the success of the treatment [Citation32,Citation38,Citation45].
A common side effect was post-operative pain. If not treated, the pain slowly increased after treatment and reached a peak at Week 3–4 [Citation32,Citation45]. Gradually, during the healing phase, the pain would decrease and depending on the success of the treatment, disappear. The studies did not report a systematic graduation or evaluation of pain, e.g. by the VAS score; however, Landström et al. [Citation35] used the European Organization of Research and Treatment of Cancer (EORTC) H&N35 questionnaire at baseline and one year after treatment in which part of the questions are regarding pain.
shows the reported side effects from each study. Serious adverse events occurred in five studies all treating recurrent and advanced stage disease. One patient died three days after electrochemotherapy of a myocardial infarction [Citation37]. Two patients had severe hemorrhage from the treated area, in one case fatal – 7.5 weeks and 6 weeks after electrochemotherapy, respectively [Citation31,Citation35]. Four studies reported infections (cellulitis, localized mucositis, osteomyelitis) [Citation30,Citation31,Citation33,Citation38] and one patient died from septicemia [Citation38].
Table 2. Description of side effects in each included article.
None of the studies reported lung fibrosis, which is, although dose-dependent, a known irreversible side effect to bleomycin. The cumulative doses of bleomycin used in these studies were much lower than standardly used in the treatment of, e.g. testicular carcinoma.
Discussion
Overall, the number of papers reporting electrochemotherapy on mucosal tumors in the head and neck region is very small. Our search of the literature resulted in 72 patients with mucosal head and neck cancer treated with electrochemotherapy during a 14-year period. Consequently, this limited amount of literature will affect our conclusions.
The field of electrochemotherapy on head and neck tumors may very well be larger than we are able to present in this review. ‘Gray literature’ as in studies never published or literature from courses, posters, patents etc., is difficult to assess thoroughly. To ensure that this present review is based on valid data, we decided to include only papers published in peer-reviewed journals.
In the literature describing head and neck cancer it is not always clear whether the cancer location is cutaneous or mucosal. As this study focuses on mucosal tumors, we have omitted studies where the distinction between cutaneous and mucosal tumors was not clear.
A common tumor group in the head and neck region derives from the salivary glands and several papers describe electrochemotherapy on tumors in the parotid gland. These patients are not included because they are treated trans-cutaneously. In total, we have discovered 13 cases of cutaneous treatment on tumors in the parotid gland. Likewise, cases of electrochemotherapy on peristomal tumors are not included because the treatment is performed cutaneously [Citation46]. Another excluded paper describes palliative electrochemotherapy on 16 circumferential esophageal tumors: electrochemotherapy was performed in order to improve dysphagia and was performed with a custom-designed electrode demonstrating overall good results and few side effects [Citation47]. Even though treatment was done on mucosal tumors, the study was excluded because the tumors were localized below the head and neck region.
Response to electrochemotherapy on mucosal head and neck tumors is overall high. The positive result has to be correlated to the tumor size; 39 of the 72 patients had a T1 or T2 tumor. It is generally accepted that the smaller the tumor, the better result with electrochemotherapy. Study 1 divided their tumors into two groups: ‘T1-T2’ and ‘advanced cancers T4-Tx’ [Citation30]. The response was CR 4/5 and CR 0/7, respectively. A CR can only be reached if the entire tumor is penetrable for the electrodes. The results could thereby indicate that the majority of patients were selected with accessible tumors not being too deeply seated.
Comparison between the individual studies is complicated due to different study designs: both in regard to tumor size, evaluation form, and time of evaluation. The response to electrochemotherapy was recorded from a clinical examination, imaging or biopsy. When using imaging, response was either evaluated according to RECIST criteria [Citation48] or WHO criteria. The reason for these different methods of evaluation might be based on the diminishing use of the WHO criteria for tumor response as a result of the introduction of the RECIST criteria in the year 2000. The shift in tumor response evaluation resulted amongst other changes in new PR criteria: 50% tumor loss in WHO to 30% tumor loss in RECIST [Citation48]. The final response rate is therefore reached using different approaches.
The time of evaluation differs from four weeks and up to two months. Knowing that it is crucial to be able to make comparisons between studies, e.g. regarding response rate, it is essential for future studies that clinical trials are conducted in a consistent manner. In the description of the reaction to electrochemotherapy on mucosal tumors, the healing phase is described as lasting 6–9 weeks after the day of treatment [Citation45]. A response evaluation after four weeks may not deliver the most accurate result, as the tissue is in the middle of the necrotic phase at this point. Studies [Citation30–32] evaluate the effect of the treatment after four weeks, risking a premature evaluation that could falsely give a wrong response before healing of the mucosa is final. An evaluation at the approximate end of the healing phase would probably be more accurate, e.g. after eight weeks or two months.
None of the included studies compared their results to currently used standard treatment regimes for head and neck cancers; neither with respect to response nor length of survival. In the treatment of primary tongue cancer the current standard treatment is surgery with sentinel node/elective neck dissection ± irradiation; a treatment modality with good response and tolerable side effects. Using electrochemotherapy for this patient group without a control group and without performing neck dissection on all patients is therefore questionable.
In Bloom and Goldfarb [Citation31], 25 patients were treated with bleomycin only, injected into the tumor, and were compared to bleomycin + electroporation. It was not stated whether these patients had cutaneous or mucosal tumors. Only one of these 25 patients had a PR, the remaining 24 patients did not respond to bleomycin alone.
The tolerance of electrochemotherapy to mucosal head and neck tumors seems depending on tumor size and location—primary, small tumors tolerate the treatment without serious adverse events whereas recurrent, large tumors display more frequent and severe adverse events. The adverse events mentioned in , are frequently seen in recurrent, palliative, head and neck cancer patients. However, as none of the studies compare with standard palliative chemotherapy regimes, it is not possible to say whether the amount of adverse events are the same or more severe and numerous.
The most severe side effects are reported from the studies treating large, recurrent tumors. Noteworthy are two cases of bleeding from the parapharyngeal area [Citation31] and base of tongue [Citation35]. Both events occurred 6–7 weeks after electrochemotherapy indicating that a large vessel had been exposed by the necrosis of the tumor. Bleeding may very likely have occurred eventually because of tumor progression, but it is possible that the electrochemotherapy has accelerated the process of exposing the vessel. This is – as always with large head and neck cancers – a serious side effect, which patients should be informed of prior to treatment.
Regarding pain, a recent study on the risk for post-treatment pain in the treatment of cutaneous tumors [Citation49], found that pre-operative pain, previous irradiation, large tumor size, and high current values (as seen when treating large necrotic tumors), were predictors of post-operative pain. The advantage of knowing which factors are associated with post-operative pain is that pain treatment may be planned better in advance.
Interestingly, in Study 6 by Landström et al. [Citation35], quality of life was tested using EORTC H&N35 questionnaires. Patients were evaluated at inclusion and 12 months after treatment and were afterwards compared in smokers/non-smokers, tongue cancer/non-tongue cancer and electrochemotherapy with/without radiotherapy. Smokers had worse speech outcome than non-smokers, non-tongue cancer patients had more pain than tongue cancer patients, and electrochemotherapy showed better swallowing and xerostomia outcome than electrochemotherapy combined with radiotherapy.
The current data indicate that electrochemotherapy is possible to perform on mucosal head and neck tumors. In our opinion electrochemotherapy should primarily be reserved for patients with no further curative treatment options left. Nevertheless, implementation of electrochemotherapy and creation of treatment recommendations requires a systematic data collection on a larger sample size. Based on these current findings we propose further studies on mucosal tumors that can elucidate the topic. The studies must cover data with precise inclusion and exclusion criteria, performance status, previous treatment and concomitant therapy. Detailed tumor description including localization, stage (TNM), and histology, technical information regarding preferred electrode choice for different anatomic regions, route of bleomycin administration, precautions regarding anesthesia and caretaking of the patients post-treatment. Furthermore, a systematic recording of side effects using, e.g. Common Terminology Criteria for Adverse Events (CTCAE) and VAS score and a uniform evaluation and follow-up period using, e.g. EORTC H&N35 questionnaires and evaluation approximately two months after treatment, would be valuable. Hopefully, future studies can hereby gather enough information to illuminate which patients will benefit the most from electrochemotherapy on mucosal tumors.
Conclusion
Electrochemotherapy is an established treatment modality for cutaneous metastases and is plausible to be used in the management of recurrent head and neck cancer. The treatment is possible to perform across the mucosa, but serious adverse events have been recorded in the treatment of recurrent, large tumors. In the 11 studies detectable, both small primary and large recurrent tumors had been treated with good response. However, the studies were not immediately comparable and no comparisons were found with existing therapies to estimate safety. Indications and treatment recommendations for electrochemotherapy on mucosal head and neck tumors cannot be applied based upon the current studies. Larger studies, as well as consensus guidelines on electrochemotherapy for head and neck cancer are therefore warranted and foreseen.
Contributions
Christina Caroline Plaschke, Anita Gothelf, Julie Gehl and Irene Wessel planned the article. Christina Caroline Plaschke did literature search and writing of manuscript. Christina Caroline Plaschke, Anita Gothelf and Irene Wessel performed full text assessment of articles. All authors contributed to revision of manuscript and are responsible for the content in this study.
Supplementary table
Download MS Word (38.8 KB)Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Stewart BW, Wild CP. World Cancer Report 2014. Lyon, France: International Agency for Research on Cancer; 2014. pp. 632.
- Mir LM, Orlowski S, Belehradek J, et al. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer 1991;27:68–72.
- Orlowski S, Mir LM. Cell electropermeabilization: a new tool for biochemical and pharmacological studies. Biochim Biophys Acta 1993;1154:51–63.
- Mir LM, Banoun H, Paoletti C. Introduction of definite amounts of nonpermeant molecules into living cells after electropermeabilization: direct access to the cytosol. Exp Cell Res 1988;175:15–25.
- Mir LM. Therapeutic perspectives of in vivo cell electropermeabilization. Bioelectrochemistry 2001;53:1–10.
- Gothelf A, Mir LM, Gehl J. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev 2003;29:371–87.
- Mir LM, Tounekti O, Orlowski S. Bleomycin: revival of an old drug. Gen Pharmacol 1996;27:745–8.
- Tounekti O, Pron G, Belehradek J, et al. Bleomycin, an apoptosis-mimetic drug that induces two types of cell death depending on the number of molecules internalized. Cancer Res 1993;53:5462–9.
- Gehl J, Skovsgaard T, Mir LM. Enhancement of cytotoxicity by electropermeabilization: an improved method for screening drugs. Anticancer Drugs 1998;9:319–25.
- Orlowski S, Belehradek J, Paoletti C, et al. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem Pharmacol 1988;37:4727–33.
- Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy - An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European standard operating procedures of electrochemotherapy) study. Eur J Cancer 2006;4:3–13.
- Matthiessen LW, Chalmers RL, Sainsbury DCG, et al. Management of cutaneous metastases using electrochemotherapy. Acta Oncol 2011;50:621–9.
- Belehradek M, Domenge C, Luboinski B, et al. Electrochemotherapy, a new antitumor treatment. First clinical phase I-II trial. Cancer 1993;72:3694–700.
- Mir LM, Belehradek M, Domenge C, et al. [Electrochemotherapy, a new antitumor treatment: first clinical trial]. C R Acad Sci III Sci Vie 1991;313:613–18.
- Heller R, Jaroszeski MJ, Glass LF, et al. Phase I/II trial for the treatment of cutaneous and subcutaneous tumors using electrochemotherapy. Cancer 1996;77:964–71.
- Glass LF, Pepine ML, Fenske NA, et al. Bleomycin-mediated electrochemotherapy of metastatic melanoma. Arch Dermatol 1996;132:1353–7.
- Heller R, Jaroszeski MJ, Reintgen DS, et al. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer 1998;83:148–57.
- Rols MP, Bachaud JM, Giraud P, et al. Electrochemotherapy of cutaneous metastases in malignant melanoma. Melanoma Res 2000;10:468–74.
- Matthiessen LW, Johannesen HH, Hendel HW, et al. Electrochemotherapy for large cutaneous recurrence of breast cancer: a phase II clinical trial. Acta Oncol 2012;51:713–21.
- Garbay JR, Billard V, Bernat C, et al. Successful repetitive treatments by electrochemotherapy of multiple unresectable Kaposi sarcoma nodules. Eur J Cancer 2006;4:29–31.
- Spratt DE, Gordon Spratt EA, Wu S, et al. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: a meta-analysis. J Clin Oncol 2014;32:3144–55.
- Scala D, Rega D, Ruffolo F, et al. Electrochemotherapy for rectal cancer after neoadjuvant radiotherapy: a case report. Eur J Surg Oncol 2015;41:S13–14.
- Edhemovic I, Brecelj E, Gasljevic G, et al. Intraoperative electrochemotherapy of colorectal liver metastases. J Surg Oncol 2014;110:320–7.
- Frandsen SK, Gissel H, Hojman P, et al. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res 2012;72:1336–41.
- Vásquez JL, Ibsen P, Lindberg H, et al. In vitro and in vivo experiments on electrochemotherapy for bladder cancer. J Urol 2015;193:1009–15.
- Gothelf A, Gehl J. What you always needed to know about electroporation based DNA vaccines. Hum Vaccin Immunother 2012;8:1694–702.
- Gehl J. Gene electrotransfer in clinical trials. Electroporation protocols. In: Li S, Cutrera J, Heller R, et al., editors. Methods in molecular biology. (Clifton, NJ). New York, NY: Springer New York; 2014. pp. 241–6.
- Mir LM, Gehl J, Sersa G, et al. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or noninvasive electrodes. Eur J Cancer Suppl 2006;4:14–25.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41.
- Allegretti JP, Panje WR. Electroporation therapy for head and neck cancer including carotid artery involvement. Laryngoscope 2001;111:52–6.
- Bloom DC, Goldfarb PM. The role of intratumour therapy with electroporation and bleomycin in the management of advanced squamous cell carcinoma of the head and neck. Eur J Surg Oncol 2005;31:1029–35.
- Burian M, Formanek M, Regele H. Electroporation therapy in head and neck cancer. Acta Otolaryngol 2003;123:264–8.
- Campana LG, Mali B, Sersa G, et al. Electrochemotherapy in non-melanoma head and neck cancers: a retrospective analysis of the treated cases. Br J Oral Maxillofac Surg 2014;52:957–64.
- Gargiulo M, Papa A, Capasso P, et al. Electrochemotherapy for non-melanoma head and neck cancers: clinical outcomes in 25 patients. Ann Surg 2012;255:1158–64.
- Landström FJ, Reizenstein J, Adamsson G-B, et al. Long-term follow-up in patients treated with curative electrochemotherapy for cancer in the oral cavity and oropharynx. Acta Otolaryngol 2015;135:1070–8.
- Landström FJ, Reizenstein JA, Nilsson COS, et al. Electrochemotherapy - possible benefits and limitations to its use in the head and neck region. Acta Otolaryngol 2015;135:90–5.
- Mevio N, Bertino G, Occhini A, et al. Electrochemotherapy for the treatment of recurrent head and neck cancers: preliminary results. Tumori 2012;98:308–13.
- Seccia V, Muscatello L, Dallan I, et al. Electrochemotherapy and its controversial results in patients with head and neck cancer. Anticancer Res 2014;34:967–72.
- Skarlatos I, Kyrgias G, Mosa E, et al. Electrochemotherapy in cancer patients: first clinical trial in Greece. In Vivo 2011;25:265–74.
- Tijink BM, De Bree R, Van Dongen GAMS, et al. How we do it: chemo-electroporation in the head and neck for otherwise untreatable patients. Clin Otolaryngol 2006;31:447–51.
- Gargiulo M, Moio M Monda G, et al. Electrochemotherapy: actual considerations and clinical experience in head and neck cancers. Ann Surg 2010;251:773.
- Hofmann GA, Dev SB, Dimmer S, et al. Electroporation therapy: a new approach for the treatment of head and neck cancer. IEEE Trans Biomed Eng 1999;46:752–9.
- Panje WR, Sadeghi N. Endoscopic and electroporation therapy of paranasal sinus tumors. Am J Rhinol 2000;14:187–91.
- Panje WR, Hier MP, Garman GR, et al. Electroporation therapy of head and neck cancer. Ann Otol Rhinol Laryngol 1998;107:779–85.
- Landström FJ, Nilsson COS, Reizenstein JA, et al. Electroporation therapy for T1 and T2 oral tongue cancer. Acta Otolaryngol 2011;131:660–4.
- Campana LG, Bertino G, Rossi CR, et al. The value of electrochemotherapy in the treatment of peristomal tumors. Eur J Surg Oncol 2014;40:260–2.
- Wojcicki M, Kostyrka R, Kaczmarek B, et al. Electrochemical therapy in palliative treatment of malignant dysphagia: a pilot study. Hepatogastroenterology 1999;46:278–84.
- Eisenhauer E. a, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47.
- Quaglino P, Matthiessen LW, Curatolo P, et al. Predicting patients at risk for pain associated with electrochemotherapy. Acta Oncol 2015;54:298–306.