Abstract
Aims: Sweden has a long history of population-based cancer registration. The aim of our study was to assess the validity of DCIS registration in a regional Breast Cancer Quality Register (BCQR) and to analyze trends in incidence, treatment and outcome of DCIS, over a 20-year period.
Material and methods: All patients with a diagnosis of primary DCIS reported in the BCQR of the Uppsala-Örebro healthcare region in Sweden 1992–2012 were included. Three hundred women were randomly selected and their medical records were compared to register data. The study period was divided into four time periods.
Results: A total of 2952 women were registered with a DCIS diagnosis. In the final validation cohort of 295 patients, 23 were found to have either recurrent DCIS or invasive breast cancer and eight had LCIS. The completeness and validity of key variables were 91–99%. Twenty of 31 local recurrences were registered (65%).The proportion of DCIS to all breast cancers was 9.5%. Tumor size increased over time. The frequency of mastectomy increased from 23.0% to 39.0%. The proportion of patients receiving radiotherapy after breast conserving surgery increased from 30.1% to 67.6%. The reported local recurrence rate was 9.7% after 10 years. Reported recurrences after BCS and mastectomy were 12.0 and 7.0%, respectively. The recurrence rate did not differ between women undergoing BCS with or without radiotherapy.
Conclusion: Only 89.5% of reported DCIS was a primary pure DCIS. The completeness of primary treatment and tumor data was high. The proportion of reported local recurrences was disappointingly low, 65%. The proportion of DCIS was stable over time with a trend towards more intensified treatment. The reported recurrence rate was low independent of treatment and can reflect adequate patient selection, but also over treatment. Our results address the necessity to validate register data on a regular basis.
The increased incidence of ductal carcinoma in situ (DCIS) of the breast during the last decades has been attributed mainly to the widespread adoption of population-based mammographic screening programs [Citation1–3]. It has been reported that after the initial rapid increase following the introduction of invitational screening programs, the incidence of DCIS as well as the proportion of patients detected by screening remains stable [Citation2,Citation3]. In Sweden, a nationwide mammography screening service was established between 1974 and 1997.
The Uppsala-Örebro Health Care region in central Sweden has a population of about two million and includes seven counties with both urban and rural areas. The regional Breast Cancer Quality Register (BCQR) was started in 1992. It contains information on tumor characteristics, treatment and follow-up data and is routinely linked to the Swedish Cancer Register (SCR) to which reporting of all tumors, whether invasive or in situ (but not necessarily specified in that aspect), is mandated by law. The overall completeness of the SCR for solid tumors is close to 100% [Citation4] and, for the time period of this study 99–100% of all malignant breast tumors in the SCR were also reported to the BCQR. Since 2008, reporting of the Uppsala-Örebro BCQR has been made to a nationwide breast cancer quality register, a platform held by the Information Network of Cancer (INCA).
Screening was introduced in the Uppsala-Örebro Health Care Region between 1974 and 1993. The invited age group has differed between the seven counties with a starting age from 40 to 50 years and a stopping age from 69 to 74 years [Citation5]. In accordance with national guidelines, all women aged 40–74 have been invited to mammography screening since 2007.
Until the late 1980s the standard treatment for DCIS was mastectomy. Following the introduction of breast conserving surgery (BCS) for the treatment of early-stage invasive breast cancer, BCS with or without radiotherapy (RT) was adopted as the treatment of choice for DCIS. However, this change was implemented without evidence from any randomized controlled trials comparing mastectomy with BCS, even though retrospective studies have not revealed any differences in breast cancer survival between the two different surgical techniques [Citation6]. From the late 1980s to late 1990s four randomized studies on RT after BCS for DCIS were conducted worldwide, and one of these in Sweden. A meta-analysis of these trials has shown a reduced risk of ipsilateral local recurrence by about 50% if BCS is combined with RT [Citation7]. Half of the recurrences were invasive cancers and half new in situ cancers. The individual trials were not designed to detect survival differences and, not surprisingly, the meta-analysis did not show any effect on survival by the addition of RT.
Axillary staging is one of the most important prognostic tools in the management of invasive breast cancer. Since the incidence of lymph node metastasis in pure DCIS is low, the role of sentinel node biopsy (SNB) in DCIS has been intensely debated. It has been suggested that a SNB should be considered in DCIS patients undergoing mastectomy and patients with a high-risk of up-staging to invasive cancer at the final histopathological examination [Citation8].
The aims of the present study were two-fold. First, to assess the validity of DCIS registrations in a population-based regional Breast Cancer Quality Register. Second, to analyze trends in incidence, treatment and outcome of DCIS in Sweden over a 20-year period.
Patients and methods
Definition of study cohort
All patients registered with a diagnosis of a primary DCIS between 1992 and 2012 in the regional Uppsala-Örebro BCQR were included. Patients with pure lobular cancer in situ (LCIS) or patients with an invasive breast cancer event, ipsilateral or contralateral reported within three months from the primary diagnosis date of the DCIS were not included. The study was approved by the Ethics Committee in Stockholm, Sweden (Dnr 2013/1272-31/4).
National and regional guidelines
In 1999, the first Swedish national guidelines on breast cancer included recommendations on RT after surgery for DCIS. The recommendation was to add RT after BCS when the DCIS lesion was grade 3, or grade 1–2 with comedo type necrosis, or DCIS of any grade with a lesion larger than 15 mm or any DCIS with a margin less than 10 mm. In the Uppsala-Örebro regional guidelines, the recommendation from 1992 to 1999 was to perform BCS if possible, and to include the patient in the national randomized study. If the patient was not included, RT was recommended at the discretion of the treating physician. Between 2000 and 2007, the regional guidelines followed the national guidelines with the exception that the size limit was put to 20 mm instead of 15 mm and comedo type necrosis and margins were not included in the recommendations to give RT. From year 2007 and onwards, the regional guidelines adhered to the national guidelines. RT was given in fractions of 2 Gy up to 50 Gy during five weeks. A boost was given according to local guidelines. Tamoxifen has not been recommended, neither in the regional nor the national guidelines. Current Swedish national guidelines recommend a sentinel lymph node biopsy (SNB) to be considered for patients with a DCIS larger than 20 mm and for lesions with high nuclear grade on core needle biopsy [Citation9].
Methods
From the regional BCQR information on date of diagnosis, age at diagnosis, mode of detection, size of DCIS, nuclear grade, type of surgery and planned adjuvant treatment was collected. Information on multifocality or surgical margins was not available in the register. Information on all reported subsequent breast cancer events, such as ipsilateral local recurrences, metastases, contralateral events and survival was also retrieved.
To validate the data on DCIS in the regional breast cancer register, 10% of the included women with DCIS were randomly chosen. Their medical records were reviewed in order to compare clinical data and subsequent breast cancer events with the records in the BCQR
Statistics
The study period was divided into four time periods; 1992–1997, 1998–2002, 2003–2007 and 2008–2012. Tumor size was categorized into <15 mm versus ≥15 mm, corresponding to the cutoff point for adjuvant RT in the current Swedish national guidelines. χ2-tests and Fisher’s exact test were used for testing differences between variables. Cumulative risk for breast cancer events and overall survival were calculated by the Kaplan-Meier method. Relative survival was calculated in Stata 13. Statistics were performed using SAS 9.4 software and R 9.4.
Results
Validation of register data
To verify the completeness and validity of the information in the BCQR, 300 women with a record of a diagnosis of DCIS were randomly selected. We were able to identify and retrieve medical journals for all but five women. For the remaining 295 patients, 23 were found to have either recurrent DCIS or invasive breast cancer, eight had LCIS, and thus, leaving 264 cases of DCIS for the validation of treatment and tumor data (). The overall completeness and validity of variables was good, 91–99%. The validated variables are listed in . In 264 validated DCIS cases there were a total of 31 local recurrences of which 20 were reported (65%). Eighteen of the recurrences were invasive cancer and 13 were DCIS. There was no statistically significant difference between the rates of correctly reported invasive or in situ recurrences and no significant difference in reported recurrences between irradiated and non-irradiated patients. Of 12 cases with distant metastasis, seven events had been reported to the register (58%). The accuracy and completeness of reported recurrences did not change significantly over time.
Figure 1. Selection of DCIS cases validated in the Uppsala-Örebro regional breast cancer register 1992–2012.
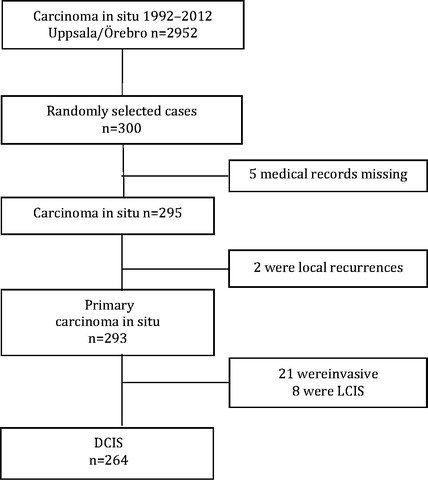
Table 1. Validation of primary DCIS data in the regional breast cancer registry compared to data from medical records in Uppsala-Örebro 1992–2012.
Incidence and mode of detection
A total of 2952 cases of DCIS were registered during the study period. Median age at diagnosis was 58 years and remained constant during the period under study. The incidence of DCIS increased over time, but the proportion of DCIS to all reported breast cancers was stable (). Sixty-eight percent were detected by organized outreach mammography screening (excluding patients with DCIS detected on a mammogram outside of the screening program). There was a trend of increasing tumor size over time, between 1992 and 1997, 36.4% of the lesions were 15 mm or larger compared to 64.8% during 2008–2012. Information on nuclear grade was very limited in the first three time periods; in the period 2008–2012, 51.8% of the tumors were grade 3.
Table 2. Distribution of cases and tumor characteristics for patients registered with DCIS in Uppsala-Örebro 1992–2012 by time period.
Treatment
The mastectomy rate increased over time from 23.2% during the first time period to 39.3% in the period 2008–2012 (). The proportion of women who were treated with adjuvant RT after BCS also increased over time from 30.1% during the first time period to 67.6% in the last time period. Adjuvant hormonal therapy is rarely prescribed to patients with DCIS in Sweden, and is still not recommended in the Swedish national guidelines. The frequency of axillary node clearance declined over time from about 10% to almost none. SNB, however, was not performed before 1998, but then increased rapidly. In the last period, 54.9% of the patients with DCIS underwent a SNB.
Table 3. Distribution of type of surgery and radiotherapy for patients registered with DCIS in Uppsala-Örebro 1992–2012 by time period.
Local recurrences
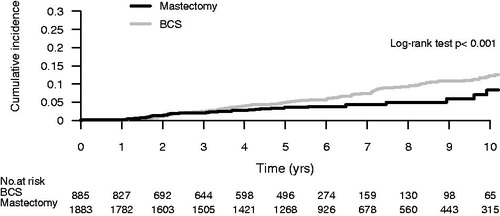
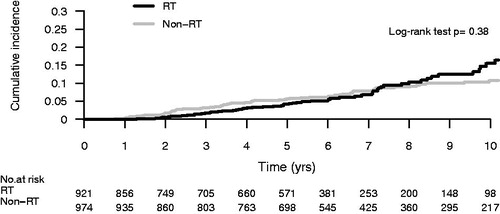
The net probability of a reported local recurrence was 3.5% at five years and 9.7% at 10 years. There were significantly more reported recurrences in the group treated with BCS compared with the mastectomy group, 12.0% versus 7.0% after 10 years, p = 0.02 (). There was no difference in recurrence rate whether adjuvant radiotherapy was added or not after BCS, 11.0% versus 13.0% after 10 years, p = 0.33 ().
Survival
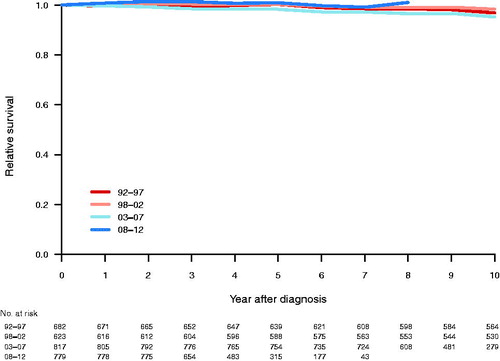
The median follow-up time was 9.7 years. Relative survival was 99.0% and 97.0% at 5 and 10 years respectively, with no clear trend over time ().
Discussion
Our assessment of the validity information on primary DCIS recorded in a population-based register showed that 7% of women with a registration of DCIS actually had an invasive breast cancer. Only 65% of local recurrences and 58% of distant metastasis were reported, a finding which needs to be considered when the otherwise low recurrence rates are discussed. The accuracy of reported surgical treatment and adjuvant RT was very high. The proportion of DCIS to all breast cancers was stable over the study period and there was a trend towards intensified treatment over time with no statistically significant effect on survival or local recurrence rates.
The strength of the study included the population-based setting and a through validation process based on information retrieved from medical records of a random sample of patients representing the full cohort under study. The register has a high overall completeness for primary data, but included a proportion of misclassified patients with invasive cancer and LCIS. For some of these patients, a lesion consisting of both an invasive and an in situ component was registered as two different primary tumors. Today, the guidelines for registering in Sweden are clearer and state that in patients with both an invasive and an in situ component are to be reported as an invasive cancer only. The low number of invasive or LCIS cases falsely reported as DCIS in the register should not seriously bias the data on DCIS incidence and the observed shifts in treatment trends over time. For invasive cancer, the same increase in incidence has been observed resulting in a similar proportion of DCIS over time. In addition, an increase of the proportion of BCS for invasive cancer has been observed during the same period in Sweden. The proportion of patients receiving adjuvant RT after BCS has been high for invasive cancer and therefore a similar increase of adjuvant RT after BCS has not been observed as shown here for DCIS.
A validation of the follow-up data was also performed and to the authors’ knowledge, it is the first report of a validation process of a DCIS registration. The proportion of reported subsequent events was disappointingly low. There is no reason, however, to believe that the reporting differs between treatments given. In the region of the present study, the reported rate of recurrences after BCS is higher in DCIS than in invasive cancer. And furthermore, in a separate validation of 300 cases from the entire regional breast cancer register during the same time period, 89% of all recurrences were reported after any breast cancer (personal communication HH, unpublished data). Following, the integration of the regional BCQR in 2008 into the National Breast Cancer Quality Register, data quality can be more closely monitored and missing data can be requested on a regular basis. The national register will be validated and our results show the need of including follow-up data in such work.
While measurement of tumor size in DCIS is difficult, our data points towards an increasing proportion of larger DCIS lesions. Similar findings have been reported from the US SEER database, where the median tumor size increased significantly from 5 mm in 1992 to 10 mm in 1999 [Citation10]. Whether this represents a true increase in tumor size or a result of the development and improvement of diagnostic tools remains unknown. The more widespread use of large histological tissue sections could be one explanation, but a more technically advanced mammography can also affect how lesions are measured. The nuclear grade of the DCIS lesions was poorly reported in the 1990s. As less than 20% of the cases had information on grade in the first three time periods, it was not possible to analyze the trends over time in the present study.
Management of DCIS varies internationally and between different health care regions within countries [Citation11,Citation12], and there are concerns about both over- and under-treatment. It is difficult to define what the ideal proportion of BCS is when treating DCIS. The increased use of mastectomies in the present report differs from most other population-based studies. This corresponds to a decreased proportion of about 75% BCS to about 60% BCS, which is in contrast with data from other countries during the same period. To some extent, this could be related to the detection of larger lesions and a better accessibility of reconstruction after a mastectomy. However, we have no indication that this should be different in Sweden compared to other countries. Two Dutch studies have shown an increased use of BCS, from around 20% in the early 1990s to 55% in 2003 and 67% in 2010 [Citation11,Citation13]. Studies from the US SEER database also show an increased use of BCS, reaching around 70% in 2008 [Citation10,Citation12,Citation14]. BCS rates in three observational studies from France, Australia and Canada show similar results [Citation15–17]. In a recent report from the International Cancer Screening Network (ICSN), the BCS rate was 67–90% among screen-detected DCIS [Citation18].
The results from the Swedish DCIS trial on BCS with or without RT were initially published in 2006 [Citation19]. The first national guidelines on management of DCIS in Sweden from 1999 were revised in 2007 [Citation9] and in those BCS followed by postoperative RT was recommended as the treatment of choice whenever feasible. Omitting RT can be considered in DCIS with nuclear grade 1 or 2 and smaller than 15 mm and with a surgical margin of more than 10 mm. Mastectomy is recommended for women with multifocal DCIS or with an unfavorable relation between tumor size and breast volume. The implementation of these guidelines fits well with the observed trends in this study. The observations from randomized studies and the guidelines certainly can explain the more intensified use of RT in Sweden [Citation7]. The reports from the US SEER database and the Dutch studies show a similar trend of increased use of RT [Citation10–14] and in the ICSN report, postoperative RT was given to 41–100% of screen-detected DCIS treated by BCS [Citation18].
In the present study, local recurrences were equally common after BCS with or without RT. This can reflect a selection bias by indication, and possibly that the correct group of patients were selected to have RT, meaning that high-risk DCIS plus RT apparently leads to the same outcome as low-risk DCIS without RT. In the SweDCIS study, RT reduced local recurrences by 37.5% after 20 years of follow-up. However, the absolute reduction was 10.0% (95% CI 6.0–14.0) for in situ and only 2.0% (95% CI 3.0–7.0) for invasive recurrences [Citation20]. Not surprisingly, local recurrences were less common after mastectomy compared to after BCS. But still, after 10 years there were 7% with a recurrence after a mastectomy for DCIS. This can also reflect a selection bias and actually indicate that the right patients were selected for BCS and mastectomy, respectively. Overall survival has not been improved by RT as previously shown in the randomized studies [Citation7]. The relative survival of patients with DCIS in the present study was 97% after 10 years and differences by treatment modalities were not possible to detect. The survival might be negatively affected by the small number of invasive breast cancers included in the register.
The declining use of axillary lymph node clearance with a rapidly increasing use of SNB shown is in line with other publications [Citation11,Citation18]. National guidelines, such as the British Association of Surgical Oncology (BASO) and the American Society of Clinical Oncology (ASCO) guidelines recommend the use of SNB for patients planned for a mastectomy or for patients with a high-risk DCIS [Citation21,Citation22]. However, to do a SNB in more than 50% of cases with a final diagnosis of pure DCIS is over-treatment and needs to be addressed. Zetterlund et al. found that in 1273 patients with a postoperative diagnosis of DCIS, a SNB was performed in 59.2% of the patients and only five (0.7%) had a SN metastasis [Citation23]. Morbidity is not neglectable and cost and resources have to be considered as well. Coromilas et al. found an increasing use of SNB in the US in women treated with a mastectomy and they concluded that an additional prospective evaluation is needed to determine if there is a clinical benefit of SNB in women with DCIS [Citation24].
To conclude, 7% of women recorded with a DCIS diagnosis in the regional BCQR in central Sweden were misclassified and had invasive breast cancer. Information in the BCQR on tumor characteristics and treatment data were of high quality whereas follow-up data were of only moderate quality. No similar comprehensive validation of a DCIS registration in a population-based register has been reported earlier, and comparisons with other register data is therefore not possible. The incidence of DCIS increased over time from 1992 to 2012, but the proportion of DCIS to all breast cancer was stable. A trend towards more intensified treatment was observed, but without evidence of decreasing recurrences rates. The recurrence-free survival observed among DCIS patients possibly reflects an adequate choice of therapy for each patient but may also be seen as over-treatment.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ernster VL, Barclay J, Kerlikowske K, et al. Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA 1996;275:913–18.
- Sørum R, Hofvind S, Skaane P, et al. Trends in incidence of ductal carcinoma in situ: the effect of a population-based screening programme. Breast 2010;19:499–505.
- Van Steenbergen LN, Louwman AC, Coebergh JA, et al. Screening caused rising incidence rates of ductal carcinoma in situ of the breast. Breast Cancer Res Treat 2009;115:181–3.
- Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish cancer register: a sample survey for year 1998. Acta Oncol Stockh Swed 2009;48:27–33.
- Olsson S, Andersson I, Karlberg I, et al. Implementation of service screening with mammography in Sweden: from pilot study to nationwide programme. J Med Screen 2000;7:14–18.
- Silverstein MJ, Barth A, Poller DN, et al. Ten-year results comparing mastectomy to excision and radiation therapy for ductal carcinoma in situ of the breast. Eur J Cancer Oxf Engl 1990 1995;31A:1425–7.
- Correa C, McGale P, Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monographs 2010;2010:162–77.
- Van Deurzen CHM, Hobbelink MGG, van Hillegersberg R, et al. Is there an indication for sentinel node biopsy in patients with ductal carcinoma in situ of the breast? A review. Eur J Cancer Oxf Engl 1990 2007;43:993–1001.
- http://www.swebcg.se/Files/Docs/Nationella_riktlinjer130501[1].pdf.
- Baxter NN, Virnig BA, Durham SB, et al. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst 2004;96:443–8.
- Van Steenbergen LN, Voogd AC, Roukema JA, et al. Time trends and inter-hospital variation in treatment and axillary staging of patients with ductal carcinoma in situ of the breast in the era of screening in Southern Netherlands. Breast 2014;23:63–8.
- Punglia RS, Schnitt SJ, Weeks JC. Treatment of ductal carcinoma in situ after excision: would a prophylactic paradigm be more appropriate? J Natl Cancer Inst 2013;105:1527–33.
- Schouten van der Velden AP, Van Dijck JAAM, Wobbes T. Variations in treatment of ductal carcinoma in situ of the breast: a population-based study in the East Netherlands. Eur J Surg Oncol 2007;33:424–9.
- Zujewski JA, Harlan LC, Morrell DM, et al. Ductal carcinoma in situ: trends in treatment over time in the US. Breast Cancer Res Treat 2011;127:251–7.
- Cutuli B, Lemanski C, Fourquet A, et al. Breast-conserving surgery with or without radiotherapy vs mastectomy for ductal carcinoma in situ: French survey experience. Br J Cancer 2009;100:1048–54.
- Kricker A, Armstrong B. Surgery and outcomes of ductal carcinoma in situ of the breast: a population-based study in Australia. Eur J Cancer Oxf Engl 1990 2004;40:2396–402.
- Rakovitch E, Pignol J-P, Chartier C, et al. The management of ductal carcinoma in situ of the breast: a screened population-based analysis. Breast Cancer Res Treat 2007;101:335–47.
- Ponti A, Lynge E, James T, et al. International variation in management of screen-detected ductal carcinoma in situ of the breast. Eur J Cancer Oxf Engl 1990 2014;50:2695–704.
- Emdin SO, Granstrand B, Ringberg A, et al. SweDCIS: radiotherapy after sector resection for ductal carcinoma in situ of the breast. Results of a randomised trial in a population offered mammography screening. Acta Oncol Stockh Swed 2006;45: 536–43.
- Wärnberg F, Garmo H, Emdin S, et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS trial. J Clin Oncol 2014;32:3588–90.
- Association of Breast Surgery at Baso 2009. Surgical guidelines for the management of breast cancer. Eur J Surg Oncol 2009;35:S1–S22.
- Ananthakrishnan P, Giuliano AE. Guidelines for the use of sentinel lymph node biopsy in patients with ductal carcinoma in situ. Breast Dis Year Book Q 2007;18:238.
- Zetterlund L, Stemme S, Arnrup H, et al. Incidence of and risk factors for sentinel lymph node metastasis in patients with a postoperative diagnosis of ductal carcinoma in situ. Br J Surg 2014;101:488–94.
- Coromilas EJ, Wright JD, Huang Y, et al. The influence of hospital and surgeon factors on the prevalence of axillary lymph node evaluation in ductal carcinoma in situ. JAMA Oncol 2015;1:323–32.