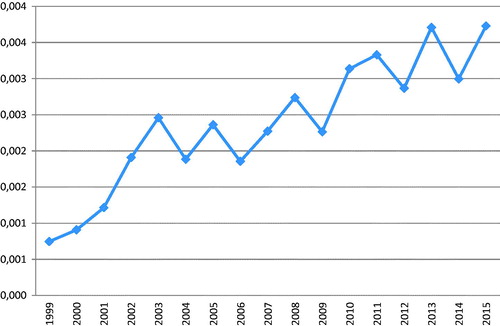
In an editorial many years ago, I referred to a statement I leaned as a new editor of Acta Oncologica during a short course for editors of scientific journals, “tell all the good news about the journal as soon as possible to as many as possible” [Citation1]. The good news then was that the ISI impact factor had steadily risen from a level below 1 to a higher level or 1.9. The good news today is that the impact factor has risen to its highest level ever, or to 3.730 being slightly higher than in 2013. The rise has continued through the years, although with a saw-toothed shape (). I will specially thank all authors contributing the most to the 2015 journal impact factor, i.e. articles published during 2013 and 2014 [Citation2–6], all with more than 15 citations during 2015. For obvious reasons, articles published during the first of those two years, in this case 2013, will have a higher chance to be among those most cited. Although not among the top five mentioned above, several other articles published during 2014 have also contributed substantially to the impact factor for 2015, and will do so also for the coming year 2016 [Citation7–12]. As has been the case during several years [Citation13], articles within radiotherapy and radiophysics are among the most cited, but for 2015, not as dominating as before reflecting the profile of Acta Oncologica as a general oncology journal.
An editorial in the journal in question is of course a natural place for dissemination, but nowadays likely not the most read place. Through the years, I have written several editorials. For obvious reasons, they are not cited frequently, but based upon the number of downloads, they appear to be read frequently. As for all other scientific periodicals, the homepage of the journal is likely much better, since it is more read. That information was updated soon after it was released.
All news within the field of oncology should be and is likely also told rapidly, particularly since they often fulfill an “unmet need”. The companies behind the trials must tell the public including the share-holders about any results as early as they are known if they may have an influence on the stock market. This is sometimes the first information you get about the clinical activity of a drug unless you have participated in the trial. The information is often minimalistic and not very informative besides knowing that the “primary goal of the trial has (not) been met”. Soon after that, the information is given as an abstract to one of the very many meetings, and then, if positive, distributed via lots of channels to almost everyone. The full paper will come later, but more and more quite rapidly since all articles are available early online long before they are published in an issue. Acta Oncologica is in this respect not different from others, although we unfortunately do not belong to the more rapid journals. We have improved, but there are still room for further improvements.
Any good news in pancreatic cancer?
The latest editorial I wrote in this journal, dealing with “Any progress in pancreatic cancer?” was published online in February this year and in an issue in March [Citation14], i.e. not very many months ago before this editorial is written. I wasn't then too positive about the progress during the past years, and still is not, but since then important new knowledge has emerged that I believe already has influenced routines worldwide. The improvements are not particularly large, but a higher chance to become a long-term survivor after radical surgery for a pancreatic adenocarcinoma by a combination of two drugs, gemcitabine and capecitabin, rather than gemcitabine alone is valued by most patients and the medical community in spite of greater toxicity [Citation15]. The study included 732 patients and could report an improved 5-year overall survival of 29% vs 16% (hazard ratio 0.82 (95% confidence interval 0.68 -0.98) p = 0.03) from the adjuvant therapy. Immunotherapy, so successful in certain other “difficult to treat malignancies”, has so far had little success in pancreatic cancer. A recent article opened for targeting focal adhesion kinase (FAK), modifying the uniquely desmoplastic stroma so that the tumor cells may become responsive to immunotherapy with e.g. PD-1 antagonists [Citation16]. It is anticipated that in 2017, i.e. the next year, more deaths will be seen in the EU after pancreatic cancer than after breast cancer, as stated in an article published in this issue of Acta Oncologica [Citation17]. And it will soon become the third most deadly cancer after lung and colorectal cancer in the EU. Much more progress than has been the case is required if this grim development is to be prevented.
Disclosure statement
The author reports no conflicts of interest. The author alone is responsible for the content and writing of this article.
References
- Glimelius B. Acta Oncologica – greater attractiveness. Acta Oncol 2003;42:799.
- Therkildsen C, Bergmann TK, Henrichsen-Schnack T, et al. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: a systematic review and meta-analysis. Acta Oncol 2014;53:852–64.
- Lindegaard JC, Fokdal LU, Nielsen SK, et al. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol 2013;52:1510–19.
- Jones LW, Alfano CM. Exercise-oncology research: past, present, and future. Acta Oncol 2013;52:195–215.
- Maringe C, Walters S, Rachet B, et al. Stage at diagnosis and colorectal cancer survival in six high-income countries: a population-based study of patients diagnosed during 2000-2007. Acta Oncol 2013;52:919–32.
- Leijenaar RT, Carvalho S, Velazquez ER, et al. Stability of FDG-PET radiomics features: an integrated analysis of test-retest and inter-observer variability. Acta Oncol 2013;52:1391–7.
- Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol 2014;53:399–404.
- Hornsby WE, Douglas PS, West MJ, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol 2014;53:65–74.
- Borren A, Groenendaal G, Moman MR, et al. Accurate prostate tumor detection with multiparametric magnetic resonance imaging: dependence on histological properties. Acta Oncol 2014;53:88–95.
- Hamaker ME, Schiphorst AH, ten Bokkel Huinink D, et al. The effect of a geriatric evaluation on treatment decisions for older cancer patients–a systematic review. Acta Oncol 2014;53:289–96.
- Beuselinck B, Karadimou A, Lambrechts D, et al. VEGFR1 single nucleotide polymorphisms associated with outcome in patients with metastatic renal cell carcinoma treated with sunitinib - a multicentric retrospective analysis. Acta Oncol 2014;53:103–12.
- Janson ET, Sorbye H, Welin S, et al. Nordic guidelines 2014 for diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms. Acta Oncol 2014;53:1284–97.
- Glimelius B. A new volume of acta oncologica. Acta Oncol 2011;50:3–5.
- Glimelius B. Any progress in pancreatic cancer? Acta Oncol 2016;55:255–8.
- Neoptolemos JP, Palmer D, Ghaneh P, et al. ESPAC-4: A multicenter, international, open-label randomized controlled phase III trial of adjuvant combination chemotherapy of gemcitabine (GEM) and capecitabine (CAP) versus monotherapy gemcitabine in patients with resected pancreatic ductal adenocarcinoma. 2016 ASCO Annual Meeting. 2016;Abstr LBA4006.
- Jiang H, Hegde S, Knolhoff BL, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 2016;22:851–60.
- Ferlay J, Partensky C, Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol 2016;55:1158–60.