Abstract
Background: Primary intracranial tumors are relatively uncommon and heterogeneous, which make them challenging to study. We coupled data from unique Swedish population-based registries in order to deeper analyze the most common intracranical tumor types. Patient characteristics (e.g. comorbidities), care process measures like adherence to national guidelines, healthcare resource use and clinical outcome was evaluated.
Materials and methods: A register-based study including several population-based registries for all patients living in Stockholm-Gotland, diagnosed with primary intracranial tumor between 2001 and 2013 was performed. Patient characteristics were captured and investigated in relation to survival, healthcare resource use (inpatient-, outpatient- and primary care) and treatment process.
Results: High-grade glioma and meningioma were the most common tumor types and most patients (76%) were above the age of 40 in the patient population (n = 3664). Older age, comorbidity (Elixhauser comorbidity index) and type of tumor (high-grade glioma) were associated with lower survival rate and increased use of healthcare resources, analyzed for patients living in Stockholm (n = 3031). The analyses of healthcare use and survival showed no differences between males and females, when stratifying by tumor types. Healthcare processes were not always consistent with existing national treatment recommendations for patients with high-grade gliomas (n = 474) with regard to specified lead times, analyzed in the Swedish Brain Tumor Registry, as also observed at the national level.
Conclusions: Age, comorbidity and high-grade gliomas, but not sex, were associated with decreased survival and increased use of healthcare resources. Fewer patients than aimed for in national guidelines received care according to specified lead times. The analysis of comprehensive population-based register data can be used to improve future care processes and outcomes.
Primary intracranial tumors represent a heterogeneous group of disorders emerging in any location of the brain and stemming from a large number of types of cells, ultimately resulting in malignancies of diverse severity and mortality [Citation1]. The long-term survival of high-grade gliomas is poor, with less than 10% five-year survival, whereas the more benign tumor type, meningiomas, has a five-year survival of approximately 90% [Citation2]. Intracranial tumors are relatively uncommon, which makes the disease more difficult to study, and to get acceptable number of patients in both randomized clinical trials (RCTs) and cohort studies. As a result of this, it is extremely important to study these patients in register studies, like the present one, as it enables long-term follow-up of large populations. In addition, population-based data add more information about the disease and treatment effects in general and not only according to a highly selected population included in RCTs [Citation3]. So far, this patient group has not been analyzed in depth in a comprehensive register database with regard to patient characteristics, clinical outcome, healthcare resources and care process. Sweden has unique possibilities to conduct careful and comprehensive analysis of a patient population like this with access to multiple qualitative high coverage registries containing information on cancer diagnosis, treatment, use of healthcare resources and outcome [Citation4,Citation5]. During recent years, new possible treatment options have become available. Whether these advancements have been beneficial for, or even offered to the population in general is unclear but can also be studied in population-based registers. Primary intracranial tumors annually account for around 300 cases in the Stockholm-Gotland region in Sweden and the Swedish Brain Tumor Registry (SBTR) include the majority of those patients. Our aim was to integrate several sources of register data to analyze patient and disease characteristics in relation to the care process, resources used and outcomes for patients with primary intracranial tumors.
Materials and methods
Study population and data sources
This register-based study used data in regional and national databases from 2001 to 2015. The study population constituted of patients in the Stockholm-Gotland region diagnosed with a primary intracranial tumor (ICD-10 C70*, C71* and C72*) between 2001 and 2013 in the Swedish Cancer Registry (SCR) [Citation6]. The Stockholm-Gotland region includes around 23% of the Swedish population (2.3 million inhabitants 2015) [Citation7]. The SCR keeps record of all newly detected tumors in Sweden and has a coverage rate above 95% for malignant tumors of which 99% are morphologically confirmed [Citation8]. Using the SNOMED histopathological classification [Citation9] reported in SCR, tumors were divided into subgroups based on origin and malignancy.
All patients living in or having been treated in the Stockholm-Gotland region were included. Using record linkage through the patients’ personal identification number, data were extracted from the Cause of Death Registry (information on date of death) [Citation10], the patient administrative system (PAS; information on all healthcare visits and procedures in inpatient and outpatient care used in similar studies [Citation11]) and SBTR (cancer treatment in detail and lead times) [Citation2]. For patients with more than one tumor registered in the SCR, the first tumor was used for analyses. The regional Ethical Review Board in Stockholm approved the study protocol (Dnr 2012/1236-31/4).
Subpopulations for analysis
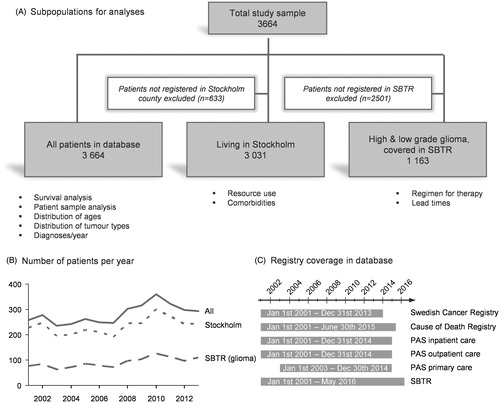
The subpopulations used depending on type of analysis are illustrated in (full data set: n = 3664). Analysis of resource use and comorbidity and was restricted to patients living in the Stockholm region at the time of diagnosis, because PAS data was only available from this region. A third subpopulation consisted of only patients with high- or low-grade glioma, covered in the SBTR. The reporting of the benign meningiomas to the SBTR has increased recently but was inadequate the first years of this study period; hence they were excluded in the third subpopulation. shows the number of patients each year in the subpopulations and show the time period covered by each data source included.
Figure 1. Description of the study population. Three populations were used for different analysis to maximize the possible number of patients for each analysis (A). The numbers of excluded patients for each analysis are given in the white boxes. The number of patients registered in Stockholm, eligible for resource and comorbidity analyses was evenly distributed throughout the whole study period (B). Patients with either high- or low-grade glioma had excellent coverage in the SBTR (almost 100% for high-grade gliomas, data not shown). Data from all sources were available for all patients between 2001 and 2015, except primary care data, available from 2003 (C).
Study variables
The date for the first primary tumor diagnosis, constituted the index date for all analyses. The diagnosis date in SCR corresponded to the date of the first well grounded radiologic suspicion of a brain tumor, and was defined as the date for radiology in the SBTR.
Patient characteristics
A set of sociodemographic and clinical variables were included to study characteristics of the patient population at the time of diagnosis. Four predefined time intervals were used when stratifying patients by diagnosis year. Data on age and sex were taken from SCR. Health profile measures included information on the tumor's histopathological classification (based on SNOMED-codes [Citation9], see Supplementary Table S1), but also on comorbidities (extracted from PAS). For comorbidity analyses the Elixhauser comorbidity index was used [Citation12]. The Elixhauser index consists of a predefined set of 31 comorbidity categories and patients were classified as comorbid in case at least one of these comorbidities (defined in [Citation12]) was present in the PAS (inpatient, outpatient or primary care) during two years before brain tumor diagnosis. The number of comorbid conditions was also calculated, resulting in a score between 0 and 31 for each patient.
Resource use
Information regarding diagnoses and procedures in inpatient, outpatient and primary care was extracted from PAS for the Stockholm region. Inpatient days, outpatient visits and visits in primary care one year before and one year after the date of diagnosis were calculated. Only patients living in Stockholm at the time of diagnosis were included.
Health outcomes
Survival analysis was performed by calculating the number of days from diagnosis until date of death or date of loss to follow-up based on information from the National Cause of Death Register.
Care process: non-surgical cancer treatment and lead times
Analysis of non-surgical cancer treatment based on the SBTR, both registration form and follow-up form (12-month post-diagnosis) was performed. In addition, lead times (in days) between important events in the care process were calculated using date of diagnosis, date of surgery, date of histopathological report and date for start of non-surgical cancer treatment (radiotherapy, chemotherapy or other non-surgical cancer treatments). Lead times were compared to the national guidelines for malignant brain tumors, which includes treatment recommendations based on literature and clinical experience [Citation13]. National guide lines for recommended lead times existed throughout the study period.
Statistical analysis
Analyses were carried out for the entire study population (survival) and for a set of patient subgroups defined in (comorbidities, resource use, cancer treatment and lead times).
Survival over time was stratified by tumor type, year of diagnosis, age, sex or comorbidity status and estimated using Kaplan-Meier analysis. An additional multivariate analysis by Cox proportional hazard model, including age, sex, comorbidity and year of diagnosis was also performed. Resource use as well as lead times was stratified by tumor type, age, sex or comorbidity status. Tests of statistical significance of differences between groups were performed using log-rank test for equality of survivor functions in the survival analyses and using the non-parametric Kruskal–Wallis test in the lead time analyses. For the different components of resource use, normal distributed 95% confidence intervals were estimated.
Statistical analysis was carried out using STATA 13.1 (Stata Corporation, College Station, TX).
Results
Study cohort
In total, the study population included 3664 patients with a primary intracranial tumor diagnosed between 1 January 2001 and 31 December 2013 (). The number of patients diagnosed per year ranged between 236 patients (2003) and 361 patients (2010).
The average age at diagnosis was 51.5 years. Around 76% of the patients were above the age of 40, and 40% above the age of 60 (). In the full analysis population, 47% of the patients were men. The two most common tumor categories were high-grade glioma (34%) and meningioma (32%), representing two substantially different diseases. For patients with glioma covered in the SBTR, 73% were high-grade gliomas and 27% low-grade gliomas.
Table 1. Descriptive data for all subpopulations used in analysis.
In the Stockholm population (n = 3031), 36% of the patients suffered from at least one comorbidity, i.e. had one of the comorbidities in the Elixhauser index definition registered in PAS (inpatient, outpatient or primary care) at least once during two years before diagnosis. The most common comorbidity was hypertension followed by other neurological diseases, depression, other tumor diseases and diabetes (). Of all patients with high-grade glioma, 43% had comorbidities. This was a significantly higher rate of comorbidities compared with patients with low-grade glioma (35%) and meningioma (32%; p ≤ .001).
Table 2. Inpatient days for patients registered in Stockholm (n = 3031) 1 year before and 1 year after diagnosis, and stratified by age, sex, tumor type or comorbidity status.
Survival
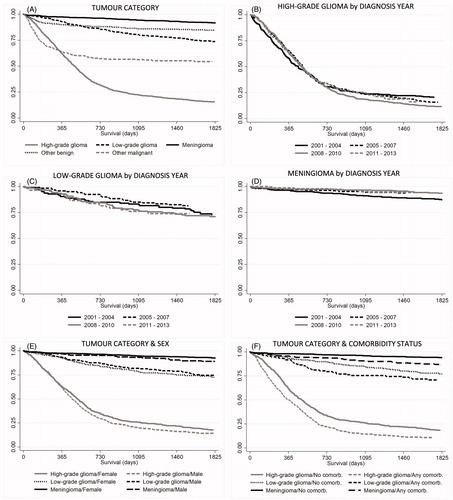
The five-year survival for different subpopulations is shown in . When stratifying patients by tumor category, high-grade glioma (all ages) was associated with the lowest rate of survival (median survival 16 months) whereas meningioma was associated with a statistically significant higher survival (). Noteworthy, the category high-grade glioma includes both grade III (e.g. anaplastic astrocytoma) and grade IV gliomas (e.g. glioblastoma multiforme), which are known to have different survival rate. When analyzing survival by year of diagnosis there was no difference in survival () for neither tumor type if not also considering patient characteristics other than tumor type. A more detailed analysis indicated that the lack of improvement over time was driven by higher proportion older patients and patients with more severe tumors in more recent years. In a separate regression analysis adjusted for age, sex, tumor type and comorbidities, survival had actually improved slightly over the study period (Supplemental Table S3). This will be further investigated in future studies. In the total population, crude values on survival were significantly higher in females than males (data not shown), but there were many more males than females with high-grade gliomas. There were no significant differences between males and females when stratifying patients also by tumor type (). The presence of one or more comorbidities substantially decreased survival () for all tumor types but most strikingly for high-grade glioma patients, where median survival for comorbid patients (all ages) was 11.5 months, compared to 17.7 months for patients with no comorbidity.
Figure 2. Survival over time was estimated using Kaplan-Meier analysis. Patients were grouped by (A) tumor type (p < .001), (B–D) year of diagnosis (n.s.), (E) sex and tumor category (p < .001), (D) comorbidity and tumor category (p < .001). Tests of statistical significance of differences between groups were performed using log-rank test for equality of survivor functions.
A more detailed description of one- and five-year survival stratified by tumor type and stratified by age within tumor types is incorporated in Supplementary Table S4.
Healthcare resource utilization
describes resource use one year before and one year after diagnosis stratified by age, sex, tumor type and comorbidity status. The year before diagnosis the patients had an average of 4.5 inpatient days (median 1 day). As expected, the oldest patients (70+) had highest use of inpatient care (8.0 days). Patients with comorbidities consumed more inpatient care than patients without comorbidities (8.2 days vs. 2.5 days). There were no large differences in inpatient care before diagnosis between tumor types ().
Table 3. Outpatient visits for patients registered in Stockholm (n = 3031) 1 year before and 1 year after diagnosis, and stratified by age, sex, tumor type or comorbidity status.
Table 4. Visits in primary care for patients registered in Stockholm (n = 2362*) 1 year before and 1 year after diagnosis, and stratified by age, sex, tumor type or comorbidity status.
The use of inpatient care was substantially higher for the entire study population during the year after diagnosis (24.1 days) compared with the year before diagnosis (4.5 days). Patients with high-grade gliomas had an average of 47 inpatient days during the year after diagnosis. The increase in inpatient care following diagnosis was significantly less accentuated in patients with more benign tumors like meningiomas with 16 inpatient days during the year following diagnosis and other malignant tumors with 27 inpatient days. The youngest patients had the highest use of inpatient care following diagnosis, despite using relatively little inpatient care before diagnosis. Brain tumor diagnosis resulted in a larger increase in inpatient care for male patients than in female patients, and in patients with comorbidity (average 35.2 days, ).
The average number of outpatient visits the year before diagnosis was 9.7 visits. Older patients and patients with comorbidities had markedly higher use of outpatient care. Patients over the age of 70 had on average 17.1 visits whereas patients with comorbidities had 18.7 visits ().
The number of outpatient visits the year after diagnosis was substantially higher than the year before (average 42.5 visits), especially for certain subgroups such as patients with high-grade glioma (92.7 visits) and patients with comorbidities (58.2 visits, ).
The average number of primary care visits the year before diagnosis was 5.7 visits. The number of visits did not differ markedly between tumor types. However, as expected older patients and patients with comorbidities had a larger number of primary care visits (12.0 and 10.8, respectively) than the average patient (). The year after diagnosis the number of primary care visits did not increase as much as inpatient and outpatient care, and still no significant differences between patients diagnosed with different tumor types were observed. However, patients of older age or with comorbidities had the largest number of primary care visits also after diagnosis ().
Care process: non-surgical cancer treatment
Of all patients with high-grade glioma reported to SBTR, 91% (n = 850) had non-surgical cancer treatment (chemotherapy, radiotherapy or other cancer treatment) planned when registered in the SBTR, whereas it was planned for 79% (n = 305) of low-grade glioma patients (). In the 12-month follow-up form (n = 680), 85% of the high-grade glioma patients, had received chemotherapy and 60% had received radiotherapy. The corresponding data on low-grade glioma patients with follow-up data (n = 242) showed that 32% had received chemotherapy and 62% had received radiotherapy (). In the high-grade glioma cohort 13% of the patients received no non-surgical cancer treatment compared with 52% in the low-grade glioma cohort ().
Table 5. Non-surgical cancer treatment. Planned at time of diagnosis and reported at follow-up (12 months after diagnosis).
Care process: lead times
show the lead time between important events throughout the care process. The analyzed lead times were: (1) days from diagnosis to surgery (); (2) days from surgery to histopathological diagnosis () and (3) days from surgery to start of non-surgical cancer treatment (). Analysis of differences in lead time depending on diagnosis year, age, sex or comorbidity was performed only for patients with high-grade gliomas. Lead time from diagnosis to surgery was substantially different between high-grade gliomas (median 14 days) and low-grade gliomas (median 25 days) as different guidelines for care processes apply for different tumor types (). Accordingly, lead time from surgery to histopathological report and the lead time from surgery to start of non-surgical cancer treatment also differed between high- and low-grade glioma ( and ).
Table 6. Lead time from diagnosis to surgery with comparison to national guidelines. Patients with high-grade glioma stratified by year of diagnosis, age, sex or comorbidity status.
Table 7. Lead time from surgery to histopathological report with comparison to national guidelines. Patients with high-grade glioma stratified by year of diagnosis, age, sex or comorbidity status.
Table 8. Lead time from surgery to start of non-surgical cancer treatment with comparison to national guidelines. Patients with high-grade glioma stratified by year of diagnosis, age, sex or comorbidity status.
The median time from diagnosis to surgery varied between 12 and 14.5 days when stratifying high-grade glioma patients by year of diagnosis and 10 and 17 days when stratifying the same patients by age or comorbidity status. According to national guidelines, 60% of patients should have surgery within 14 days. For many subgroups in this analysis, this directive was not met ().
The median lead time from surgery to histopathological diagnosis varied between five and eight days when stratifying high-grade glioma patients by year of diagnosis (shorter lead times earlier years) but there were only marginal differences when stratifying patients by age, sex or comorbidity status. According to national guidelines 80% of patients should receive the histopathological report within 10 days. During the entire study period the criteria was fulfilled for 77.5% of the patients with high-grade glioma ().
The median lead time from surgery to start of non-surgical cancer treatment varied between 32 and 49.5 days when stratifying patients by year of diagnosis (shorter lead times the later years), but there were marginal differences when stratifying patients by age, sex or comorbidity status (). The national guidelines state that 80% of patients should start non-surgical cancer treatment within 28 days from surgery. However, only 25% of patients in the study population started treatment within the recommended time. Compliance with guidelines improved over time and 34% of patients diagnosed 2011–2013 started non-surgical cancer treatment within 28 days ().
Discussion
To our knowledge, this is the largest population-based comprehensive study capturing patient characteristics, health outcome, healthcare resources and care process measures in patients with primary intracranial tumor.
We present a population including close to complete records of all patients receiving treatment for a primary intracranial tumor with date of diagnosis occurring between 1 January 2001 and 31 December 2013, in the Stockholm-Gotland region. In Sweden, diagnoses are generally well documented in healthcare data bases and the SCR has been extensively validated [Citation8]. The SBTR has unique information on care process and treatment regimens with a close to 100% coverage rate for high-grade gliomas in the Stockholm region. Other tumors, like menigiomas, are however less well reported historically, but have improved since the study period of this paper. The linkage of several registries through the patients’ personal identification number is an advantage of this study. The administrative database in Stockholm covers hospitalizations as well as outpatient visits to specialists and visits in primary care, and was in this study the base for analysis of healthcare resource use and comorbidities. Noteworthy, a few private clinics operate in Stockholm and data from private caregivers are not covered in the database, including meningioma treated by the gamma knife which is only a minor part of all meningiomas. Neither have we included healthcare use outside of the Stockholm county in this study.
The current study focuses on patient characteristics such as health profile at baseline, health outcome (e.g. survival), resource use (inpatient, outpatient and primary care), non-surgical cancer treatment and lead times in the care process, and make relevant analyses between tumor types, age, sex and comorbidity status. A limitation when working with a group of patients with a relatively uncommon diagnosis is the size of the study samples. Indeed, the cohort size for analysis in this study were not optimal for statistical analysis, especially as the cohort has to be stratified by factors such as tumor type, age, sex or comorbidity status.
An important aspect of this study is the analysis of comorbidities and their effect on outcome, which has not been studied extensively previously in intracranial tumor care. Our results show an important impact of comorbidities on survival, resource use and care process in line with previous studies showing similar links between comorbidities and other cancer forms [Citation14–16]. Noteworthy, the more malignant form of tumors were associated to a higher rate of comorbidities, with as much as 42% of patients having one or several comorbidities detected in prediagnostic medical data. The group of patients with meningioma (which may be considered a control group, i.e. patients in the same population but with a benign tumor) had a lower rate of comorbidities (32%). A careful analysis of comorbidities, taking into account potential confounding factors like age, may provide new insights about additional risk factors for the outcome of malignant primary brain tumors.
Our data are in line with previously published studies which show that elderly patients more seldom receive multimodal treatment and have worse survival compared with younger patients [Citation17]. However, which modality, radiotherapy or chemotherapy, that are the most beneficial for the elderly is debated [Citation18]. Surprisingly, the survival analysis performed here showed no improvement of survival over time, but in more recent years patients diagnosed with brain tumor were slightly older, with a higher presence of comorbidities and more severe tumors, within the assigned tumor category. In fact, in this study, the category high-grade glioma includes both grade III (e.g. anaplastic astrocytoma) and grade IV gliomas (e.g. glioblastoma multiforme), known to have different survival rate. A deeper analysis adjusting for age, sex, tumor type and comorbidities indeed indicated an increased survival in more recent years. When selecting a specific age category of patients with high-grade glioma (age 60–69), survival had increased by several months when comparing patients diagnosed earlier to more recently diagnosed patients in concordance with previously published Swedish data by Asklund et al. [Citation19].
The analysis of resource use is a unique feature of this study and it is important to understand the general economic effects of this patient group as they potentially consume large amounts of healthcare resources. Patients with the benign tumor form, meningioma, required less healthcare resources. In fact, most of these patients had no inpatient days, no outpatient visits or primary care visits and were thereby considered a control group to the patients, affected by a primary malignant intracranial tumor. One important insight is that some patients have a large number of visits in primary care during the year prior to tumor diagnosis. This could indicate that the healthcare system has been unable to identify the underlying reason for the patient’s problems. Our data confirm results in a recently published article, that increased consultation for primary care is a risk factor for various types of cancer [Citation20]. Delays in diagnosis intervals could potentially affect mortality [Citation21] and further research of consultation patterns and symptom profiles the year before cancer diagnosis is important to earlier detect the tumor and to optimize the use of healthcare resources. Time to diagnosis could probably be improved by better integration of primary and outpatient care including referral routes, especially for older patients with comorbidities [Citation22]. When analyzing relevant subgroups there were significant differences regarding age, comorbidity and tumor category. Older age, comorbidity and high-grade gliomas were associated with a larger number of inpatient days, outpatient visits and primary care visits. A larger resource use in men compared with women was observed, most likely due to a higher incidence of more severe tumors in men found in this population and other populations [Citation23]. However, no significant difference between sexes was shown when adjusting for different tumor subtypes.
According to our data, a substantial amount of inpatient care is used following diagnosis, especially for younger patients. Rehabilitation with focus on neurological symptoms needs to be further improved in line to improve quality of life and shorten hospital stays. Individualized rehabilitation programs, including both specialized units offering inpatient care and home-based care are wanted.
Analysis of lead times between different events in the care process is crucial for evaluation of the quality of care and compliance to national guidelines [Citation13]. However, there are few conclusive evidence regarding established guidelines for lead times [Citation24]. They indicate 60% of the patients with suspected high-grade glioma should have surgery within two weeks after diagnosis (radiology). In our study, 49% of patients had surgery within 14 days. Another key figure for quality of care is days from surgery to histopathological diagnosis accompanied by a treatment decision. The national guideline states that this should be within 10 days for 80% of the patients [Citation13]. Furthermore, non-surgical cancer treatment (chemo- or radiotherapy) should start within four weeks, for at least 80% of all eligible patients. In our population, a histopathological report was finalized within 10 days for 71% of the patients. However, only 24.5% of the patients started non-surgical cancer treatment within four weeks after surgery. Hence, these crucial lead times do not meet the set goal for good quality of care. Noteworthy, lead time from surgery to start of non-surgical cancer treatment has decreased over the years for high-grade gliomas.
In the high-grade glioma cohort, the significant differences in time from diagnosis to surgery between age groups needs to be further analyzed, as it indicates a potential inequality in healthcare.
Patients with comorbidities had longer lead times, which may partly be explained by the fact that comorbidities are more common in older patients and partly because other time-consuming preparatory procedures have to take place before surgery, for patients with comorbidities. The median time from surgery to histopathological report increased from six to eight days during the whole study period. The ability to comply with guidelines seems to have decreased over time, ultimately restricting optimal patient flows.
In conclusion, studies like ours of comprehensive population-based register data can be of great importance to further improve survival and prevent large unnecessary healthcare costs for these patients. This study was based on data from the Stockholm-Gotland region, and should ideally be compared to other regions in Sweden and even to other countries. Such benchmarking analysis may provide transparency around care process and outcomes and thereby be helpful for identifying areas for improvement.
IONC_1257864_Supplemental_information.docx
Download MS Word (22.5 KB)Acknowledgments
The authors would like to thank Lena Rosenlund for expert advice regarding data in the Swedish Brain Tumor Register.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-Oncology. 2002;4:278–299.
- Asklund T, Malmstrom A, Bergqvist M, et al. Brain tumors in Sweden: data from a population-based registry 1999-2012. Acta Oncol. 2015;54:377–384.
- Grapow MT, von Wattenwyl R, Guller U, et al. Randomized controlled trials do not reflect reality: real-world analyses are critical for treatment guidelines! J Thorac Cardiovasc Surg. 2006;132:5–7.
- Lilja B, Miranda-Télllez J, Ljunggren G, et al. A study on cancer patients in the region of Stockholm by linking data from multiple sources. Adv Pharmacoepidemiol Drug Safety. 2015;4:187.
- Rosen M. National Health Data Registers: a Nordic heritage to public health. Scand J Public Health. 2002;30:81–85.
- National Board of Health and Welfare. The Swedish Cancer Registry (database); 2013.
- Statistics Sweden: longitudinal integration database for health insurance and labour market studies (LISA by Swedish acronym); 2016.
- Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48:27–33.
- Fritz AG. International classification of diseases for oncology: ICD-O. 3rd ed, 1st rev. ed. Geneva: World Health Organization; 2013. p. viii, 242.
- The National Board of Health and Welfare. Causes of Death (database); 2016.
- Carlsson AC, Wandell P, Osby U, et al. High prevalence of diagnosis of diabetes, depression, anxiety, hypertension, asthma and COPD in the total population of Stockholm, Sweden – a challenge for public health. BMC Public Health. 2013;13:670.
- Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- Swedish national care program: high-grade gliomas; 2012. [cited 2012 Aug]. Available from: http://www.cancercentrum.se/globalassets/cancerdiagnoser/hjarna/vardprogram/natvp_hjarntumorer_20120829_final.pdf.
- Islam KM, Jiang X, Anggondowati T, et al. Comorbidity and survival in lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2015;24:1079–1085.
- Iversen LH, Norgaard M, Jacobsen J, et al. The impact of comorbidity on survival of Danish colorectal cancer patients from 1995 to 2006–a population-based cohort study. Dis Colon Rectum. 2009;52:71–78.
- Lemmens VE, Janssen-Heijnen ML, Verheij CD, et al. Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br J Surg. 2005;92:615–623.
- Zarnett OJ, Sahgal A, Gosio J, et al. Treatment of elderly patients with glioblastoma: a systematic evidence-based analysis. JAMA Neurol. 2015;72:589–596.
- Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13:916–926.
- Asklund T, Malmstrom A, Bjor O, et al. Considerable improvement in survival for patients aged 60-84 years with high grade malignant gliomas: data from the Swedish Brain Tumour Population-based Registry. Acta Oncol. 2013;52:1041–1043.
- Ewing M, Naredi P, Nemes S, et al. Increased consultation frequency in primary care, a risk marker for cancer: a case-control study. Scand J Prim Health Care. 2016;34:205–212.
- Torring ML, Frydenberg M, Hansen RP, et al. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: a cohort study in primary care. Eur J Cancer. 2013;49:2187–2198.
- Jensen H, Torring ML, Olesen F, et al. Cancer suspicion in general practice, urgent referral and time to diagnosis: a population-based GP survey and registry study. BMC Cancer. 2014;14:636.
- Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–49.
- Weller M, van den Bent M, Hopkins K, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15:e395–e403.
