Abstract
Aim: Anterior-oblique (AO) proton beams can form an attractive option for prostate patients receiving external beam radiotherapy (EBRT) as they avoid the femoral heads. For a cohort with hydrogel prostate-rectum spacers, we asked whether it was possible to generate AO proton plans robust to end-of-range elevations in linear energy transfer (LET) and modeled relative biological effectiveness (RBE). Additionally we considered how rectal spacers influenced planned dose distributions for AO and standard bilateral (SB) proton beams versus intensity-modulated radiotherapy (IMRT).
Material and methods: We studied three treatment strategies for 10 patients with rectal spacers: (A) AO proton beams, (B) SB proton beams and (C) IMRT. For strategy (A) dose and LET distributions were simulated (using the TOPAS Monte Carlo platform) and the McNamara model was used to calculate proton RBE as a function of LET, dose per fraction, and photon α/β. All calculations were performed on pretreatment scans: inter- and intra-fractional changes in anatomy/set-up were not considered.
Results: For 9/10 patients, rectal spacers enabled generation of AO proton plans robust to modeled RBE elevations: rectal dose constraints were fulfilled even when the variable RBE model was applied with a conservative α/β = 2 Gy. Amongst a subset of patients the proton rectal doses for the planning target volume plans were remarkably low: for 2/10 SB plans and 4/10 AO plans, ≤10% of the rectum received ≥20 Gy. AO proton plans delivered integral doses a factor of approximately three lower than IMRT and spared the femoral heads almost entirely.
Conclusion: Typically, rectal spacers enabled the generation of anterior beam proton plans that appeared robust to modeled variation in RBE. However, further analysis of day-to-day robustness would be required prior to a clinical implementation of AO proton beams. Such beams offer almost complete femoral head sparing, but their broader value relative to IMRT and SB protons remains unclear.
Anterior-oblique (AO) proton beams can form an attractive option for prostate patients receiving external beam radiotherapy (EBRT) as they avoid the femoral heads/hip prostheses. It has previously been suggested that, assuming a fixed relative biological effectiveness (RBE) of 1.1, range-verified AO proton beams could reduce the mean dose to the rectum, anterior rectal wall and penile bulb by a factor of approximately two relative to standard bilateral (SB) proton beam arrangements [Citation1]. Using restricted weightings such beams have already been applied clinically in conjunction with lateral portals by a consortium of three centers which published a report on the treatment of 20 patients [Citation2]. However, if a complete EBRT dose prescription was to be split between two AO proton beams, the distal edges of these beams would necessarily coincide with the boundary between the prostate and the rectum. This prompts two concerns regarding rectal dose: (1) variations in patient anatomy, particularly bladder and rectal filling, might result in proton range-overshoot and (2) increased RBE at the distal edge of each AO field might result in unacceptable hotspots in rectal ‘biological dose’. An emerging trend in prostate therapy that could mitigate both of these concerns is the use of synthetic poly(ethylene glycol) hydrogel, introduced into the retroprostatic space.
Injected hydrogel spacers (SpaceOAR, Augmenix Inc.) typically result in a separation of approximately 1 cm between the rectum and prostate [Citation3]. This separation remains stable over 10–12 weeks [Citation4] enabling substantial rectal dose reductions in the 60–70 Gy region [Citation5]. A multicentre, randomized controlled trial was conducted in 222 men with stage T1 or T2 prostate cancer treated using image-guided intensity-modulated radiotherapy (IMRT) to 79.2 Gy with or without rectal spacers. The spacer group experienced a significant reduction in late rectal toxicity severity (p = 0.044) as well as lower rates of decrease in bowel quality of life at six, 12 and 15 months compared to the control group [Citation6]. Further, the spacer technique has been reported as cost effective [Citation7].
For a cohort of patients without rectal spacers, we recently demonstrated that AO proton beam plans that appeared dosimetrically suitable assuming a fixed RBE of 1.1 no longer fulfilled rectal dose constraints when variable RBE weighted (vRBEw) dose models were applied [Citation8]. In this work we studied the impact of rectal spacers upon AO proton beam plans. We asked whether it was possible to generate AO proton plans robust to end-of-range elevations in linear energy transfer (LET) and modeled RBE, the distal beam edge of the proton beam being positioned within the hydrogel rather than the anterior rectal wall. Given the trend towards prostate hypofractionation, we assessed the impact of large doses per fraction on RBE elevation at the distal edge of a proton SOBP. Additionally, we considered how rectal spacers influenced planned dose distributions for AO and lateral proton beams versus IMRT.
Material and methods
Ten patients with low/intermediate risk prostate cancer were studied, all were treated sequentially using a commercial rectum-prostate spacer system (SpaceOAR; Augmenix, Waltham, MA, USA). SpaceOAR hydrogel plus fiducials were implanted and planning computed tomographies (CTs) plus axial T2-weighted magnetic resonance (MR) images with a limited field of view were acquired 3–5 days later. The MR images were rigidly registered to the planning CT using MIM (MIM Software Inc). The clinical target volume (CTV) was defined as the prostate alone. Endo-rectal balloons were not applied.
Three treatment planning techniques were considered:
A. AO proton beams. Beam angles of ±35° beam angles were selected to avoid the femoral heads, avoid beam overlap on skin surface and reduce bladder dose relative to smaller angular separations.
B. SB proton beams (±90°).
C. Seven-field IMRT. With beam angles of 0°, 60°, 100°, 135°, 225°, 260°, 300°.
Both (A) and (B) were implemented as spot-scanned single field uniform dose (SFUD) proton plans using multi-criteria optimization (MCO) within Astroid, our in-house treatment planning system (TPS). The in-air sigma of the proton spot varied from 12 mm at 60 MeV to 4.6 mm at 230 MeV at isocenter. The IMRT plans (C) were implemented using MCO in the Raystation TPS (Raysearch Laboratories, Sweden).
For each planning technique, (A)–(C), two different strategies for planning target volume (PTV) margins were applied: (1) a 5 mm CTV to PTV expansion, uniform in all directions and (2) no PTV expansion, using the CTV as the sole target. For a summary of our complete planning methodology, please see Supplementary Table 1. Whilst margin-free planning (strategy (2)) is clearly not advisable in current clinical practice, we consider it here the extreme of what might be achievable with gating, increased image guidance/adaptation and, in the case of proton therapy, in vivo range verification.
For all planning modalities our strategy was to minimize the rectal dose, subject to fulfillment of our clinical requirements (as detailed in ). Our MCO objectives and constraints are detailed in Supplementary Table 2. Where inter-modality comparisons of dosimetric statistics were performed, we used the (non-parametric) Wilcoxon signed-rank test for related samples.
Table 1. Clinical dose requirements (rigidly enforced for all plans).
For the AO proton plans dose and dose-averaged LET distributions were calculated using TOPAS (TOol for PArticle Simulations, version 2.0.3) [Citation9]. It has been demonstrated that, relative to Monte Carlo (MC) simulations, proton pencil beam algorithms typically over-estimate the mean dose delivered to deep-seated targets such as the prostate by approximately 2% whilst under-estimating the scattered dose to normal tissues [Citation10]. Consequently, in this study proton plan-specific scaling factors were applied to the MC dose distributions so that the dose received by 90% of a patient’s CTV volume matched that for their IMRT plan.
Voxel-by-voxel, the McNamara model [Citation11] was used to calculate RBE for the AO proton plans as a function of dose per fraction, dose-averaged LET and photon α/β. As proposed by the QUANTEC organ-specific papers, we typically considered a photon α/β of 3 Gy for the rectum [Citation12] and bladder [Citation13], but also tested a range of 2–6 Gy. Evidence suggests that the prostate has a lower photon α/β of approximately 1.5 Gy: here we considered a range of 0.5–4 Gy [Citation14–16]. Our standard fractionation scheme was 44 × 1.8 Gy [Citation17], but two additional regimens drawn from photon practice were also considered in the variable RBE modeling: 20 × 3 Gy [Citation18] and 5 × 7.25 Gy [Citation19]. Where relevant, equivalent uniform dose (EUD) values were calculated assuming a-values of 5, 7 and −10 for the rectum, bladder and prostate, respectively [Citation20]. Integral energy depositions were calculated for the whole body minus the target volume, assuming a body composition of water.
Results
Assuming a fixed proton RBE of 1.1
For a fixed RBE of 1.1 the plans produced according to strategies (A)–(C) were well matched in terms of target coverage, all fulfilling the clinical dose requirements detailed in . and Supplementary Table 3 compare dosimetric data across strategies (A)–(C) for the rectum and bladder. For our implementation of IMRT it was not possible to further spare the rectum at the expense of increased bladder dose. Additional rectal sparing for IMRT could only be achieved by forfeiting clinical requirements, particularly those for target coverage. Similarly, for both proton beam configurations the best achievable rectum and bladder dose-volume histograms (DVH) were largely limited by the target coverage requirements, rather than tradeoffs between rectum and bladder dose.
Figure 1. Dose-volume histogram (DVH) comparison between treatment planning strategies (A)–(C) for all 10 rectum spacer patients. Each solid/dashed line corresponds to a DVH plot for an individual patient; the shaded regions indicate the inter-patient range for each plan type. (a) Plans with uniform 5mm CTV to PTV expansion; (b) plans with no CTV to PTV expansion.
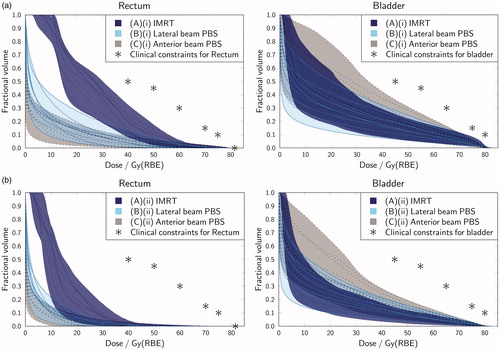
First, we considered plans with a uniform 5 mm CTV to PTV margin (see and Supplementary Table 3a). AO and SB plans both outperformed IMRT in terms of rectal dose statistics, in a manner that was statistically significant for dose levels up to 60 Gy (p < 0.05). For the rectum, the calculated mean rectum EUD values for the AO, SB and IMRT plans were approximately 27 GyRBE, 29 GyRBE and 38 Gy, respectively. demonstrates the inter-patient variation in spacer efficacy. Amongst a subset of patients the proton rectal DVH plots were remarkably low: for 2/10 SB plans and 4/10 AO plans ≤10% of the rectum received ≥20 Gy. For the bladder, the calculated mean EUD values for the AO, SB and IMRT plans were approximately 54 GyRBE, 53 GyRBE and 54 GyRBE, respectively. Although the bladder EUD values were relatively well matched, significant differences in mean bladder dose and bladder fractional volume receiving 30 Gy were evident between the three techniques. In terms of greatest bladder sparing the techniques ranked: (1) SB protons; (2) IMRT; and (3) AO protons.
Similar trends are evident in where no CTV to PTV expansion was applied within any of the techniques. Relative to IMRT, the proton plans maintain a rectal dose advantage at levels ≤30 Gy, but at a slight cost to bladder dose. Further data on these plans is included in Supplementary Table 3.
Overall, regardless of margin choice, if a fixed proton RBE of 1.1 can be assumed then, relative to SB proton beams and IMRT, AO proton beams may deliver improved rectal and femoral head dosimetry at the expense of additional bladder dose. The integral energy deposited was also substantially lower for AO protons than for SB protons and IMRT: calculated mean values were 37.5 J, 49.9 J and 114.0 J, respectively, for the 5 mm PTV plans (Supplementary Table 3a).
Modeling RBE variation for the AO proton beams
For AO proton beams, exemplifies how the highest vRBEw dose values arise at the distal edge of the PTV target (see the color-wash gradient in vRBEw dose). Nonetheless, for nine of 10 cases the rectal spacer provided a barrier sufficient for the rectal maximum dose constraint to be fulfilled for the PTV plans (), even when the McNamara variable RBE model was applied with photon α/β values of 2–6 Gy. In the top panels of , the case where the rectal spacer was least effective is shown: here, at the inferior levels, no gap is created by the spacer between the prostate and the rectum. The lower panels () show a more typical case, where superiorly to inferiorly the spacer forms a buffer for the full length of the prostate PTV. The reader will note that in , application of the McNamara model with an α/β of 3 Gy resulted in modeled biological doses in the target far exceeding the prescription level (79.2 GyRBE). This finding is further reflected in , where the modeled EUD within the CTV is plotted as a function of prostate α/β. The variable RBE model suggests that assuming a fixed RBE of 1.1 leads us to substantially underestimate the biological dose delivered.
Figure 2. Demonstrations of variable RBE weighted (vRBEw) dose distributions for anterior-oblique spot-scanned proton therapy plans with a uniform 5 mm CTV to PTV margin. The planning target volume is contoured in black; the hydrogel, contoured using additional magnetic resonance data, is shown in blue; and the rectum is contoured in pink. The dose distributions overlaid here are for vRBEw dose calculated according to the McNamara model with an α/β of 3 Gy. (a) Sagittal view of case where spacer was least effective; (b) axial view of case where spacer was least effective; (c) sagittal view of more typical case; (d) axial view of more typical case.
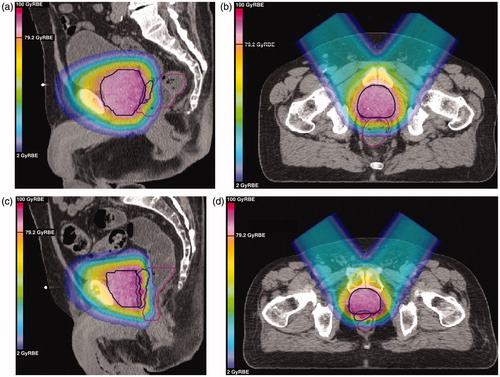
Figure 3. Investigating the McNamara model’s [Citation11] sensitivity to α/β value and fractionation regimen. (a) Plans with uniform 5 mm CTV to PTV expansion: boxplots of the maximum dose to 1cc of the rectum for all 10 patients. The horizontal line shows the constraint that no 1cc of the rectum should receive more than 103% of the prescription dose; (b) plans with uniform 5 mm CTV to PTV expansion: boxplots of the CTV equivalent uniform dose (EUD) for all 10 patients, assuming an EUD a-value of -10 [20]; (c) example application of the McNamara model for various fractionation schemes and α/β values for a simple proton SOBP in water, range = 20 cm, modulation = 10 cm; (d) considering the ratio (vRBEw dose with an α/β of 1.5 Gy)/(vRBEw dose with an α/β of 3 Gy) for the fractionation schemes shown in Figure 3(c).
![Figure 3. Investigating the McNamara model’s [Citation11] sensitivity to α/β value and fractionation regimen. (a) Plans with uniform 5 mm CTV to PTV expansion: boxplots of the maximum dose to 1cc of the rectum for all 10 patients. The horizontal line shows the constraint that no 1cc of the rectum should receive more than 103% of the prescription dose; (b) plans with uniform 5 mm CTV to PTV expansion: boxplots of the CTV equivalent uniform dose (EUD) for all 10 patients, assuming an EUD a-value of -10 [20]; (c) example application of the McNamara model for various fractionation schemes and α/β values for a simple proton SOBP in water, range = 20 cm, modulation = 10 cm; (d) considering the ratio (vRBEw dose with an α/β of 1.5 Gy)/(vRBEw dose with an α/β of 3 Gy) for the fractionation schemes shown in Figure 3(c).](/cms/asset/9eb2aa80-828a-4076-a845-15832e34a6f0/ionc_a_1275781_f0003_c.jpg)
For the standard fractionation scheme considered in this study (44 × 1.8 Gy) the model predicted RBE values exceeding 1.3 at the distal edge of a standard SOBP (for α/β values of 1.5 Gy and 3 Gy), as shown in . However, for a hypofractionated regimen with a dose per fraction of 7 Gy [Citation21] and the same α/β values, the maximum modeled RBE value at the distal edge was <1.2. Consequently, the model predicted that a dose per fraction of 7 Gy could limit biological dose elevation at the beam distal edge to 10%, compared to 20% for the standard fractionation scheme. However, if high values of the ratio:
are taken to indicate therapeutic advantage, that is increased cell kill in the tumor compared to the normal tissue, then the 44 × 1.8 Gy regimen appeared preferable (). This suggests that if we were to optimize intensity-modulated photon therapy (IMPT) plans according to the variable RBE model (applying a variable RBE constraint to rectal dose) then standard fractionation would enable the highest biological dose boosts to the target.
Supplementary Table 4a demonstrates that, when the McNamara model was applied with an α/β of 3 Gy, no significant difference was found between AO and IMRT plans in terms of rectal EUD (for both, matched margin strategies).
Discussion
Assuming a fixed RBE of 1.1, we found that AO protons enabled a greater degree of rectal sparing than SB protons or IMRT. For plans with no CTV to PTV expansion, statistically significant differences between IMRT and AO protons persisted in dose regions up to 30 Gy, whereas for the plans with a uniform CTV to PTV expansion of 5 mm, the improvement persisted to a dose level of 60 Gy. Our findings are consistent with a previous study, where of volumetric modulated arc radiotherapy (VMAT), IMRT and bilateral IMPT, after spacer injection only IMPT managed to decrease the rectal dose at a broad range of dose levels [Citation5].
We demonstrated previously that, for a cohort without rectal spacers, AO proton beams were not robust to modeled elevations in proton RBE: use of such beams could result in unacceptably high rectal doses [Citation8]. Here we show that for cases with rectal spacers, it is typically (9 times of 10) possible to generate AO proton plans with a uniform 5 mm CTV to PTV margin expansion that are robust to variable RBE modeling. That is, when implanted successfully, rectal spacers suitably mitigate the RBE uncertainties associated with AO proton beams. However, imperfect hydrogel insertion could prove problematic in an AO proton beam protocol: in the one case where it was not possible for us to generate a ‘biologically robust’ AO proton plan with a 5 mm PTV, no gap was created between the rectum and the prostate at inferior levels. Clinicians would need to remain mindful that asymmetric/non-homogeneous insertions could lead to hotspots in rectal biological dose.
We did not consider spacer stability, and thus efficacy, over the course of an EBRT treatment. However, clinical data suggest that initial hydrogel volumes are well preserved over 10–12 weeks [Citation4]. A recent photon dosimetric study demonstrated that for CT scans acquired one day, one month and two months post-hydrogel injection, adaptive radiotherapy would lead to only minor improvements in rectal DVH compared to use of a single plan [Citation22]. In this work equal CTV to PTV margins of 5 mm were applied for proton and IMRT plans. Additional analyses would be required to determine whether equal margins would correspond to matched levels of clinical robustness, for example in terms of inter-fractional changes in anatomy and set-up errors [Citation23,Citation24], intra-fractional motion [Citation25], and proton-specific issues such as water-equivalent path length variation [Citation26]. In the long-term, in vivo proton range verification and plan adaptation could also facilitate the application of AO beams, for example using diodes attached to a rectal balloon [Citation27].
It should be noted that whilst a number of clinical studies have reported hydrogel spacers to be safe [Citation3,Citation6], one published case report linked a rectal ulcer to a hydrogel insertion [Citation28] and their use was halted in a recent trial, where two rectal fistulas were presumed due to the gradual accumulation of gel within the confines of the anterior rectal wall, as seen on magnetic resonance imaging during the course of the treatment [Citation29]. However, the latter publication reported that in addition to gel migration, variations in individual patient radiosensitivity could have played a role, on-treatment image guidance was limited to orthogonal x-rays (it did not state whether alignment based on bony anatomy or prostate fiducials), and the in vivo dose to the anterior rectal wall was not known precisely [Citation29]. The quality of the initial gel placement was not described and ultimately the exact source of the fistulas remains unknown.
AO proton beams have generated clinical interest, mainly due to their capacity to spare the femoral heads almost entirely in a manner useful for patients with hip replacements or with previously irradiated hips [Citation2]. Assuming a fixed RBE of 1.1, AO proton beams are also associated with integral doses approximately three times lower than IMRT and offer rectal sparing in the low to medium dose region (<30 Gy) relative to both SB proton beams and IMRT. However, the benefits of AO proton beams come at a cost to increased bladder dose at all levels. As yet there is no consensus as to whether rectal or bladder sparing should be the first priority in prostate radiotherapy. Data from one recent study suggests that bladder sparing should be prioritized: patients with consistent quality of life (QOL) reduction in urinary irritation function were significantly associated with greater mean bladder dose, whereas none of the evaluated rectal dosimetric parameters showed a significant correlation with QOL score change in bowel function [Citation30]. However, the study was retrospective and limited to one year of follow-up for 86 patients treated using stereotactic body radiation therapy. Other work suggests that complete rectal DVHs are important in determining patient-reported outcome [Citation31]. Thus for a cohort with hydrogel spacers, it is not clear whether the additional rectal sparing offered by AO protons (relative to IMRT or SB protons) is likely to prove clinically meaningful or not.
Questions over the clinical desirability of AO proton beams are further complicated by uncertainties in proton RBE. The transition from a fixed RBE value of 1.1 to a variable RBE model resulted in increased target EUD in addition to increased rectal biological dose. Thus, if variable RBE models could be validated in vivo, new possibilities to dose boost the prostate and/or improve normal tissue sparing would arise. Although hypofractionation could further limit potential RBE elevations at the distal edge of AO proton beams, ultimately – if vRBEw dose models were used in plan optimization – standard fractionation should provide the greatest advantage in terms of target relative to normal tissue dose.
In conclusion, typically rectal spacers enabled the generation of anterior beam proton plans that appeared ‘biologically robust’, that is robust to modeled elevations in variable RBE. Although our results do depend on the accuracy of the dose calculation method, the beam characteristics (e.g. the steepness of the distal fall-off) and the accuracy of the RBE model, our parameters can be considered representative. However, as we performed all calculations on-treatment planning CT scans we did not consider inter- and intra-fractional changes in anatomy/set-up: analysis of day-to-day robustness would be required prior to a clinical implementation of AO proton beams, particularly with regards to bladder and rectum filling and thus proton under-/over-shoot. For patients where sparing of the femoral heads is a priority, AO proton beams form an appealing solution. However, given uncertainties in both: (1) proton RBE and (2) the relative importance of bladder versus rectum dose-sparing, the broader value of AO proton beams – relative to SB protons and IMRT – remains unclear.
IONC_1275781_supplementary_tables.docx
Download MS Word (39.7 KB)Acknowledgments
The authors would like to thank Nadya Shusharina for her advice regarding the IMRT treatment planning.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Tang S, Both S, Bentefour H, et al. Improvement of prostate treatment by anterior proton fields. Int J Radiat Oncol Biol Phys. 2012;83:408–418.
- Cuaron J, Harris A, Chon B, et al. Anterior-oriented proton beams for prostate cancer: A multi-institutional experience. Acta Oncol. 2015;54:868–874.
- Hatiboglu G, Pinkawa M, Vallée J-P, et al. Application technique: placement of a prostate-rectum spacer in men undergoing prostate radiation therapy. BJU Int. 2012;110:E647–E652.
- Pinkawa M, Piroth MD, Holy R, et al. Spacer stability and prostate position variability during radiotherapy for prostate cancer applying a hydrogel to protect the rectal wall. Radiother Oncol. 2013;106:220–224.
- Weber DC, Zilli T, Vallee JP, et al. Intensity modulated proton and photon therapy for early prostate cancer with or without transperineal injection of a polyethylen glycol spacer: a treatment planning comparison study. Int J of Radiat Oncol Biol Phys. 2012;84:e311–e318.
- Pieczonka CM, Mariados N, Sylvester, et al. Hydrogel spacer application technique, patient tolerance and impact on prostate intensity modulated radiation therapy: results from a prospective, multicenter, pivotal randomized controlled trial. Urol Pract. 2016;3:141–146.
- Hutchinson R, Sundaram V, Folkert M, et al. Decision analysis model evaluating the cost of a temporary hydrogel rectal spacer before prostate radiation therapy to reduce the incidence of rectal complications. Urol Oncol Elsevier. 2016;34:e19–e26.
- Underwood T, Giantsoudi D, Moteabbed M, et al. Can we advance proton therapy for prostate? considering alternative beam angles and relative biological effectiveness variations when comparing against intensity modulated radiation therapy. Int J of Radiat Oncol Biol Phys. 2016;95:454–464.
- Perl J, Shin J, Schümann J, et al. TOPAS: an innovative proton Monte Carlo platform for research and clinical applications. Med Phys. 2012;39:6818–6837.
- Schuemann J, Giantsoudi D, Grassberger C, et al. Assessing the clinical impact of approximations in analytical dose calculations for proton therapy. Int J Radiat Oncol Biol Phys. 2015;92:1157–1164.
- McNamara AL, Schuemann J, Paganetti H. A phenomenological relative biological effectiveness (RBE) model for proton therapy based on all published in vitro cell survival data. Phys Med Biol. 2015;60:8399–8416.
- Michalski JM, Gay H, Jackson A, et al. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:S123–S129.
- Viswanathan AN, Yorke ED, Marks LB, et al. Radiation dose-volume effects of the urinary bladder. Int J Radiat Oncol Biol Phys. 2010;76:S116–S122.
- Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095–1101.
- Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys. 2001;50:1021–1031.
- Dasu A, Toma-Dasu I. Prostate alpha/beta revisited – an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51:963–974.
- Efstathiou J, Proton Therapy vs. IMRT for Low or Intermediate Risk Prostate Cancer. 2016. [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT01617161 [cited 2016 Jan 5].
- Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060.
- Katz AJ, Santoro M, Diblasio F, et al. Stereotactic body radiotherapy for localized prostate cancer: disease control and quality of life at 6 years. Radiat Oncol. 2013;8:118.
- Trofimov A, Nguyen PL, Coen JJ, et al. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J of Radiat Oncol Biol Phys. 2007;69:444–453.
- Freeman DE, King CR. Stereotactic body radiotherapy for low-risk prostate cancer: five-year outcomes. Radiat Oncol. 2011;6:3.
- Heikkilä V-P. PEG spacer gel and adaptive planning vssingle plan in external prostate radiotherapy—clinical dosimetry evaluation. BJR. 2015;88:20150421–20150422.
- Thörnqvist S, Muren LP, Bentzen L, et al. Degradation of target coverage due to inter-fraction motion during intensity-modulated proton therapy of prostate and elective targets. Acta Oncol. 2013;52:521–527.
- Kubota Y, Kawamura H, Sakai M, et al. Changes in rectal dose due to alterations in beam angles for setup uncertainty and range uncertainty in carbon-ion radiotherapy for prostate cancer. PLoS One. 2016;11:e0153894.
- Tang S, Deville C, McDonough J, et al. Effect of intrafraction prostate motion on proton pencil beam scanning delivery: a quantitative assessment. Int J Radiat Oncol Biol Phys. 2013;87:375–382.
- Thor M, Casares-Magaz O, Muren LP, et al. A method for evaluation of proton plan robustness towards inter-fractional motion applied to pelvic lymph node irradiation. Acta Oncol. 2015;54:1643–1650.
- Hoesl M, Deepak S, Moteabbed M, et al. Clinical commissioning of an in vivo range verification system for prostate cancer treatment with anterior and anterior oblique proton beams. Phys Med Biol. 2016;61:3049–3062.
- Teh AY, Ko HT, Barr G, et al. Rectal ulcer associated with SpaceOAR hydrogel insertion during prostate brachytherapy. BMJ Case Rep. 2014. doi: 10.1136/bcr-2014-206931.
- Habl G, Uhl M, Katayama S, et al. Acute toxicity and quality of life in patients with prostate cancer treated with protons or carbon ions in a prospective randomized phase II study-The IPI trial. Int J Radiat Oncol Biol Phys. 2016;95:435–443.
- Qi XS, Wang JP, Gomez CL, et al. Plan quality and dosimetric association of patient-reported rectal and urinary toxicities for prostate stereotactic body radiotherapy. Radiother Oncol. 2016;121:113–117.
- Thor M, Olsson C, Oh JH, et al. Relationships between dose to the gastro-intestinal tract and patient-reported symptom domains after radiotherapy for localized prostate cancer. Acta Oncol. 2015;54:1326–1334.

