Abstract
Background: Denosumab is a relatively new treatment option for patients with giant-cell tumor of bone (GCTB). The purpose of this study was to report the results for patients treated in Norway.
Materials and methods: Patients treated with denosumab for GCTB were identified from the clinical databases at the Norwegian sarcoma reference centers. Data were retrieved from the clinical databases and supplemented by retrospective review of patient records. Denosumab was given as a subcutaneous injection every 4 weeks with loading doses on day 8 and 15 in cycle 1.
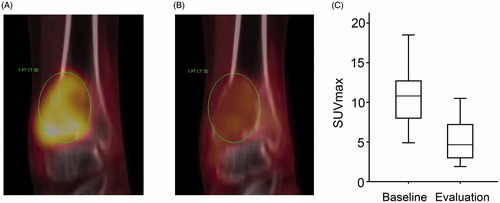
Results: Eighteen patients treated with denosumab for GCTB were identified. Denosumab was given for recurrent disease in seven cases and as first-line treatment in 11 patients, of which 6 received therapy as part of a neoadjuvant/adjuvant strategy and 5 for surgically unsalvageable primary tumor. Ten of 12 patients with unresectable disease are still on denosumab without progression with median treatment duration of 41 months (range 18–60). Two patients discontinued treatment due to osteonecrosis of the jaw and reduced compliance, respectively. In the adjuvant group, four patients experienced disease recurrence after stopping denosumab. In three of six patients, the extent of surgery was reduced due to neoadjuvant therapy. Seventeen of 18 patients underwent response evaluation with 18F-FDG PET/CT at median 4.7 weeks from starting denosumab. Median baseline SUVmax was 11.0 and median SUVmax at evaluation was 4.9 (p < 0.001).
Conclusions: In a nationwide GCTB patient cohort, denosumab was an effective agent and durable responses were observed. Our results do not support the use of adjuvant therapy in routine clinical practice. 18F-FDG PET/CT could be a valuable tool for early response evaluation.
Introduction
Giant-cell tumor of bone (GCTB) is rare, benign, and locally aggressive osteolytic neoplasm. It accounts for 15–20% of benign bone tumors and occurs most frequently between 20 and 50 years of age [Citation1–3]. Histologically, GCTB consists of two cell populations. Mononuclear cells constitute the neoplastic component, and the non-neoplastic population is composed of multinucleated, osteoclastic giant cells. Different classifications have been proposed for GCTB, such as the Campanacci system [Citation4]. There is, however, a poor association with biological behavior and prognosis, and the clinical utility is limited.
Primary treatment for resectable tumors is surgery aiming at local tumor control with minimal morbidity. Surgical treatment options are intralesional curettage or partial or total resection of the involved bone. Curettage may be followed by bone grafting, and adjuvant agents have also been used aiming to reduce the risk of local recurrences after curettage, such as phenol, liquid nitrogen, and polymethylmethacrylate (PMMA) [Citation3]. Local recurrence rates after surgery are reported to be 14–25% [Citation5–7]. Radiotherapy is a treatment option for unresectable GCTB or for tumors where surgery would lead to unacceptable morbidity. Reported local control rates after radiotherapy range from 60 to 90%, but malignant transformation and secondary radiation-induced malignancies are a concern [Citation2,Citation3].
In the past, systemic therapies used for GCTB include bisphosphonates, chemotherapy, interferon-α, dasatinib, and sunitinib [Citation1,Citation2]. The evidence of efficacy of these treatments is mainly based on small, retrospective series, and their role in the management of GCTB is not clear. Recently, denosumab has become a new option for unresectable or locally advanced GCTB. Denosumab is a fully humanized monoclonal antibody against the receptor activator of nuclear factor-κB ligand (RANKL) [Citation8,Citation9]. RANKL is expressed by the neoplastic stromal cells, and the RANKL/RANK signaling pathway is central in the pathogenesis of GCTB [Citation8].
Clinical efficacy and safety of denosumab for GCTB have been evaluated in two phase II studies [Citation10,Citation11]. In the first study, 30 of 35 evaluable patients (86%) responded, defined as lack of radiological progression at 6 months or elimination of at least 90% of giant cells on histological evaluation [Citation11]. The subsequent, larger phase II study included 282 patients in three separate cohorts: surgically unsalvageable, salvageable (surgery planned), and continuation of patients from the previous phase II study [Citation10]. At the time of data analysis, 163 of 169 patients (96%) with unresectable tumors had not progressed, and 98 of 100 patients (98%) with salvageable tumors had no disease progression. Denosumab was well tolerated. Fifty patients (18%) reported grade 3–4 adverse advents, and the most common were hypophosphatemia, anemia, back pain, and pain in extremities [Citation10]. Based on these results, denosumab has been approved by FDA and EMA for treatment of GCTB that is unresectable or where surgical resection is likely to result in severe morbidity.
Even though the two above-mentioned phase II trials have established the clinical efficacy and safety of denosumab in GCTB, few other patient cohorts and series with longer follow-up have been reported. The purpose of this study was to report the results for patients treated in Norway.
Materials and methods
Study cohort
Treatment of GCTB in Norway is centralized to four sarcoma centers: Bergen, Trondheim, Tromsø, and Oslo. Demographical, clinical, and pathology data are recorded prospectively in clinical databases. We identified all patients with GCTB that had started treatment with denosumab before 1 January 2015 in the above-mentioned centers. The date of first denosumab injection was between 5 November 2010 and 20 July 2014. Clinical data were supplemented by retrospective review of patient records. All pathological specimens were evaluated by experienced sarcoma pathologist at the sarcoma centers, but no review of the histological diagnosis within this study was performed. The study is approved by the Data Protection Official at Oslo University Hospital, and written informed consent has been obtained from the patients.
Treatment with denosumab
Denosumab (120 mg) was given as a subcutaneous injection every 4 weeks (with loading doses on day 8 and 15 in cycle 1). Planned duration of neoadjuvant denosumab was 6–12 months, and the duration of adjuvant (postoperative) treatment was 6 months. For patients with a tumor that was considered unresectable, denosumab was given until disease progression or until recommended discontinuation by the treating physician or patient’s decision to discontinue. All patients underwent a routine dental exam before starting treatment, and were advised to take daily supplements containing 500–1000 mg calcium and 400–800 IU vitamin D. Patients attended regular outpatient visits at the participating centers, including standard-of-care radiological imaging (plain x-ray, CT, and MRI), according to the center’s standard practice.
18F-FDG PET/CT
Patients with malignant and benign, locally aggressive bone tumors are often offered routine PET-scans in Norwegian sarcoma centers. For patients with GCTB, 18F-FDG PET/CT was performed before start of denosumab and preferably 4–6 weeks after treatment initiation. 18F-FDG PET/CT was performed using a Siemens Biograph 16 (Siemens Healthcare Global, Munich, Germany) PET/CT scanner. An FDG dose of 370 ± 37 MBq was injected after 6 hours of fasting. SUVmax was calculated by the decay-corrected ratio of the highest voxel activity within a selected volume to the injected dosage corrected for the body weight.
Statistical analysis
SUVmax values were compared using Wilcoxon signed-rank test. All tests were two-sided, and p values <0.05 were considered statistically significant. Statistical analyses were performed using SPSS 21.0 (SPSS, Chicago, IL).
Results
Patients
Eighteen patients treated with denosumab for GCTB in Norway were identified. There were 5 women and 13 men, and median age at diagnosis was 39 years (range 16–63). Twelve patients received denosumab for an unresectable tumor or when surgery would lead to unacceptable morbidity. Of these, five had primary GCTB and seven a recurrent tumor. Six patients received therapy as part of a neoadjuvant/adjuvant strategy. The clinical characteristics are summarized in .
Table 1. Clinical characteristics of the patient cohortTable Footnotea.
Treatment and outcome
Denosumab was generally well tolerated. One patient was diagnosed with osteonecrosis of the jaw 17 months after starting denosumab. Otherwise, no severe side effects were registered. Ten of 12 patients with unresectable disease are still on treatment with denosumab without progression and with median treatment duration of 41 months (range 18–60). Two patients discontinued denosumab: A 60-year-old male developed osteonecrosis of the jaw, and progressive disease was diagnosed three months after stopping denosumab. A 40-year-old male with a sacral tumor decided to stop treatment after an initial response. He suffered increased pain and radiotherapy (60 Gy) was given 15 months after discontinuing denosumab. Six patients with resectable tumors received denosumab in a neoadjuvant/adjuvant setting. Of these, four experienced local recurrence during follow-up. Local recurrences were diagnosed 8, 9, 9, and 13 months after discontinuing denosumab, respectively. Two patients are disease-free 11 and 27 months after stopping treatment.
Surgical procedures
The surgical procedures performed after preoperative denosumab are listed in . A partial or complete resection of the involved bone was performed in two patients, curettage and cementing in two patients, and curettage with bone grafting in two patients. We reviewed radiological imaging to evaluate whether neoadjuvant denosumab led to a less morbid surgical procedure. In three of six patients, surgery was considered less extensive due to preoperative treatment. A 40-year-old male with a tumor in the humerus and a pathological fracture was converted from a prosthetic replacement of the proximal humerus to curettage and cementing, and a 16-year-old male with GCTB in the distal radius and a 25-year-old male with a pelvic tumor were converted from a partial resection of the involved bone to curettage.
Table 2. Surgical procedures performed after neoadjuvant denosumab.
PET/CT
Seventeen of 18 patients underwent 18F-FDG PET/CT response evaluation at median 4.7 weeks from starting denosumab. Median baseline SUVmax was 11.0 (range 6.3–18.5) and median SUVmax at evaluation was 4.9 (range 1.9–14.9; p < 0.001; ). A reduction in SUVmax was seen in 16 of 17 patients (94%), and the mean reduction was 5.6 (range −1.4 to 9.7). In one patient with a baseline SUVmax of 6.3, an increase to 7.7 was observed at evaluation after 4 weeks. However, he experienced reduced pain, CT showed increased mineralization and MRI was consistent with a tumor response. Denosumab was continued, and a new PET-scan at week 12 showed a SUVmax of 4.0. The patient is still on denosumab after 36 months.
Discussion
Denosumab has emerged as a highly effective treatment for GCTB, and interim results from a large phase II study clearly demonstrates the short-term clinical benefit in patients with unresectable tumors and in patients where surgery would lead to severe morbidity [Citation10]. However, several questions are still unanswered, and there are no published data from real-life practice apart from case reports. Here, we report the results from a nationwide cohort with a relatively long follow-up.
No patients progressed while receiving denosumab in our study. This corresponds well with data from the phase II studies, where nearly all patients were progression-free at time of data analysis [Citation10,Citation11]. There are still limited data on long-term disease control, and the duration of response is probably the most important parameter in patients with unresectable tumors. It is a concern that young patients receive a potentially life-long treatment, considering the risk of long-term side effects and a potential risk of malignant transformation. Sarcomatous transformation of GCTB has been reported during treatment with denosumab, but whether the malignancies were caused by denosumab is unclear [Citation12,Citation13]. Nevertheless, these concerns indicate that surgery should still be the preferred option for resectable tumors.
Denosumab was administered 6 months postoperatively in the 26 patients who underwent surgery in the phase II study by Chawla and coworkers [Citation10]. No disease progression was reported after resection, but the follow-up time was relatively short. We adopted the same strategy for adjuvant treatment in our cohort. Four of six patients developed local recurrence, all within 13 months after stopping denosumab. The high rate of local recurrences may be explained by the selection of particularly difficult cases for denosumab treatment, with an expected higher risk of recurrence. Furthermore, curettage after neoadjuvant denosumab may be technically challenging, due to the coarse and thickened trabecular bone often seen after denosumab treatment. Nevertheless, adjuvant denosumab did not prevent local recurrences, and even though the numbers are very small, our findings indicate that more research is needed before adjuvant therapy can be recommended in routine clinical practice.
The risk of local recurrence depends on the surgical procedure. Recurrence rates are lower after complete resection compared with intralesional curettage, and local adjuvants seem to reduce the risk of recurrence after curettage [Citation3]. In our series, two of four patients with local recurrence were treated with intralesional curettage and bone grafting. Many centers advocate the use of cement as an adjuvant agent after curettage. It is postulated that the heat from the warm cement induces death of the remaining neoplastic cells, thus reducing recurrence rates. In a cohort of 294 patients from the Scandinavian Sarcoma Group, filling the cavity with cement seemed to reduce the risk of recurrence compared to bone grafting [Citation14]. However, there are no randomized trials to support the use of local adjuvants compared to curettage alone, or to support the choice of adjuvant agent.
Radiological evaluation criteria in GCTB are not established. Modified RECIST and inverse Choi criteria for CT and MRI and EORTC criteria for 18F-FDG PET/CT have been applied [Citation10,Citation15], and a varying degree of objective responses has been observed using the different criteria. In our study, SUVmax values were reduced in 16 of 17 patients (94%) at first evaluation after median 4.7 weeks, suggesting that 18F-FDG PET/CT is an early and sensitive indicator of tumor response. Similar results were observed in two phase II studies, where 25 of 26 patients (96%) [Citation10] and 14 of 17 patients [Citation15] had a metabolic response according to EORTC criteria. However, whether 18F-FDG PET/CT improves treatment decisions based on clinical observations and conventional radiographic investigations is still unknown. Furthermore, new parameters like SUVpeak, SUL (based on lean body mass), MTV (metabolic tumor volume), and TLG (total lesion glycolysis) have recently been introduced, and these may give a better prediction of treatment response.
Pain reduction after denosumab was registered in the large phase II study, and interim results have been published [Citation16]. More than half of patients with significant pain at baseline had a clinically relevant decrease in pain within two months, but approximately 20% of patients never experienced a significant pain reduction. Although we did not systematically record symptoms, retrospective review of patient records indicated that the majority of patients in our study had reduced pain at first evaluation (median 4.7 weeks after starting denosumab). The vast majority of patients have a clinical treatment benefit (i.e., 96–98% of patients had not progressed [Citation10]), and we propose that a patient with reduced pain at first evaluation has a probability of efficacy of nearly 100%. Whether early response evaluation should be reserved for patients without symptomatic relief could be an issue for further investigation.
Our study has limitations. Certain clinical data were prospectively registered, but a retrospective review of patient records was necessary to supplement the prospective registration. No review of pathology or radiology was performed. However, patients were treated at major sarcoma reference centers, and experienced sarcoma pathologists and radiologists were responsible for the primary evaluation of histological specimens and radiological examinations. The patients were treated in a routine clinical setting, with less rigid follow-up schedules than in clinical trials, which could influence the results. On the other hand, our data reflect very well real-life practice, and since we were able to collect nationwide data we believe that the results are representative of the Norwegian population.
In conclusion, we have shown in a nationwide cohort that denosumab is a highly active treatment in GCTB. No patients progressed while receiving treatment, and durable responses were observed. Four of six patients recurred after stopping adjuvant denosumab, indicating that more research is needed before adjuvant therapy can be recommended in routine clinical practice. 18F-FDG PET/CT could be an early and sensitive tool for response evaluation, but whether it improves clinical decision-making needs further investigation. Even though denosumab represents a major breakthrough for many patients with GCTB, there are still unanswered questions that should be explored in future studies.
Disclosure statement
The authors report no conflict of interest.
References
- Brodowicz T, Hemetsberger M, Windhager R. Denosumab for the treatment of giant cell tumor of the bone. Future Oncol. 2015;11:1881–1894.
- Singh AS, Chawla NS, Chawla SP. Giant-cell tumor of bone: treatment options and role of denosumab. Biologics. 2015;9:69–74.
- van der Heijden L, Dijkstra PD, van de Sande MA, et al. The clinical approach toward giant cell tumor of bone. Oncologist. 2014;19:550–561.
- Campanacci M, Baldini N, Boriani S, et al. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–114.
- O'Donnell RJ, Springfield DS, Motwani HK, et al. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am. 1994;76:1827–1833.
- Turcotte RE, Wunder JS, Isler MH, et al. Giant cell tumor of long bone: a Canadian Sarcoma Group study. Clin Orthop Relat Res. 2002;397:248–258.
- Vult von Steyern F, Bauer HC, Trovik C, et al. Treatment of local recurrences of giant cell tumour in long bones after curettage and cementing. A Scandinavian Sarcoma Group study. J Bone Joint Surg Br. 2006;88:531–535.
- Lacey DL, Boyle WJ, Simonet WS, et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401–419.
- Thomas DM. RANKL, denosumab, and giant cell tumor of bone. Curr Opin Oncol. 2012;24:397–403.
- Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14:901–908.
- Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11:275–280.
- Aponte-Tinao LA, Piuzzi NS, Roitman P, et al. A high-grade sarcoma arising in a patient with recurrent benign giant cell tumor of the proximal tibia while receiving treatment with denosumab. Clin Orthop Relat Res. 2015;473:3050–3055.
- Broehm CJ, Garbrecht EL, Wood J, et al. Two cases of sarcoma arising in giant cell tumor of bone treated with denosumab. Case Rep Med. 2015;2015:767198.
- Kivioja AH, Blomqvist C, Hietaniemi K, et al. Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian Sarcoma Group study of 294 patients followed for a median time of 5 years. Acta Orthop. 2008;79:86–93.
- Ueda T, Morioka H, Nishida Y, et al. Objective tumor response to denosumab in patients with giant cell tumor of bone: a multicenter phase II trial. Ann Oncol. 2015;26:2149–2154.
- Martin-Broto J, Cleeland CS, Glare PA, et al. Effects of denosumab on pain and analgesic use in giant cell tumor of bone: interim results from a phase II study. Acta Oncol. 2014;53:1173–1179.