Introduction
In this case study, we report a remarkable systemic response after treatment with electrochemotherapy and calcium electroporation in a patient with disseminated malignant melanoma.
Electrochemotherapy is an evolving cancer treatment, a non-thermal event where high voltage pulses permeabilize cell membranes in tumor masses and dramatically enhance uptake and cytotoxicity of chemotherapeutic agents. Electrochemotherapy with bleomycin is regularly used for cutaneous and subcutaneous metastases and is an effective treatment with a described high local response in malignant melanomas, and carcinomas [Citation1–7].
Preclinical reports have shown that electrochemotherapy not only possesses the ability to exert a cytotoxic effect on cancer cells, but also has the capability to generate an anticancer immune response, as it induces an immunogenic cell death with release of antigens from the cytosol and antigen presentation on the cell membrane thereby alerting the immune system [Citation8]. Preclinical studies have demonstrated that the local effect of electrochemotherapy is reduced in immunodeficient mice, with lack of T lymphocytes [Citation8,Citation9]. On the contrary, adding interleukin-2 or other pro-inflammatory cytokines has a synergistic effect enhancing the response of electrochemotherapy on both local and distant tumors [Citation10–12]. This indicates that in addition to the local cytotoxic effect from bleomycin, an immune response also plays a part in the effect of electrochemotherapy. Nevertheless electrochemotherapy alone has so far not been reported to be able to exert a treatment effect outside the treatment area [Citation1,Citation13,Citation14].
Here, we present a case with clinically evident and biopsy-verified response in both treated and untreated malignant melanoma lesions, showing that electrochemotherapy and calcium electroporation may in fact give rise to a systemic immune response. This is of particular interest as electrochemotherapy is now being investigated in combination with checkpoint inhibitors [Citation15,Citation16]. This case indicates that electrochemotherapy and maybe calcium electroporation could have a role as immune activator and may be considered in combination with immunotherapeutic treatments.
Case report
An 85-year-old woman with a previous medical history of myxedema and osteoarthritis, but otherwise well, was diagnosed in 2013 with malignant melanoma on her left heel. Pathology showed acral lentiginous melanoma, with a thickness of 7.3 mm, Clarks level 5, with ulceration and dermal mitosis, BRAF mutation negative. She underwent surgery to a 2 cm free margin and had two sentinel nodes removed both with parenchymatous metastases. Inguinal lymph node resection and PET-CT scan did not show any additional sign of dissemination. However, the PET-CT scan revealed pulmonary embolism and a neuroendocrine tumor in her uterus.
Five months after her initial diagnosis of malignant melanoma she relapsed with cutaneous metastases on her left lower limb and two pathologically enlarged lymph nodes in the left inguinal region and pelvis. She was assessed ineligible for isolated limb perfusion (ILP) due to her comorbidities and anticoagulant treatment. In June 2014, she started chemotherapy with temozolamide (standard dose). She tolerated the treatment poorly despite a reduction to 50% dose, and the treatment was discontinued after two series due to pancytopenia, infection and thrombus in her arm. The patient was deemed ineligible for ipilumumab and all treatments were stopped.
After six months without any treatment, she was referred for electrochemotherapy. At the time she had a large number of cutaneous metastases on her left lower limb, extending from above the ligament level to the lower leg, both lateral, medial and anteriorly, with several ulcerated and bleeding lesions (). The two enlarged lymph nodes in the inguinal region and pelvis had shown some regression after treatment with temozolamide, but were still pathologically enlarged. She was treated with electrochemotherapy in general anesthesia according to European Standard Operating Procedures of Electrochemotherapy (ESOPE) [Citation17]. The dose of bleomycin (Baxter A/S, Søborg, Denmark) was 24,000 IE and more than 100 lesions were treated with a total of 407 pulse sequences, applied with hexagonal needle electrodes (Cliniporator, IGEA, Carpi, Italy). Pulses were delivered to the front and back of her thigh, 20 × 25 cm and 30 × 13 cm, respectively, and on the back of her calf 20 × 10 cm (). She underwent one treatment only, and due to the considerable number of lesions, mainly lesions that created discomfort for the patients were treated, the rest of the cutaneous metastases and the lymph nodes remained untreated. The only reported adverse advents were fever the night after treatment and an increase in pain from NRS 7 to NRS 9 (Numerical Rating Scale of pain where 0 = no pain and 10 = severe pain). Already one day after treatment, the bleeding was reduced and the treated lesions appeared pale. In the months after treatment, the treated metastases became necrotic and decreased in size.
Figure 1. Images of left thigh and left calf, over time. (A) February 2015, before electrochemotherapy. Cutaneous metastases extended from the left side of the abdomen to the left calf, with bleeding lesions. (B) March 2015, the day after treatment with electrochemotherapy. The treated areas include the front and back of her thigh, 20 × 25 cm and 30 × 13 cm, respectively, and on the back of her calf 20 × 10 cm. The areas are marked with pen and appear erythematous. The bleeding has stopped and the metastases appear pale. (C) May 2015, two months after treatment with electrochemotherapy, leveling of the cutaneous metastases in the treated area, the lesions appear more pale and as scars, from where exophytic lesions have fallen off, are visible. (D) July 2016, 12 months after retreatment with calcium and electroporation and 16 months after the first electrochemotherapy, complete leveling of all cutaneous metastases and appearance of vitiligo in both treated and untreated area. Biopsies from pigmented lesions were without malignancy (see supplementary Figure 1).
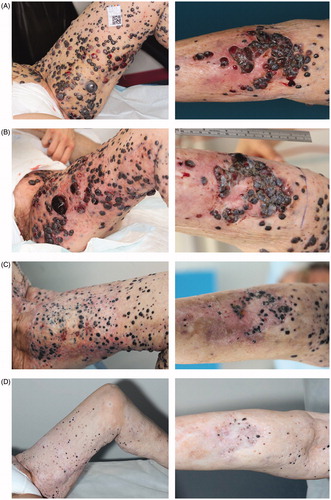
Four months after electrochemotherapy, she had clinical progression in the surrounding skin, despite the continued improvement in the treated area. Two new metastases on the back of her thigh appeared, as well as a new lesion on her back. The latter was surgically removed and the pathology showed an almost complete regression of a heavily pigmented lesion, with dense lymphocytotic infiltration with predominance of T cells and plasma cells.
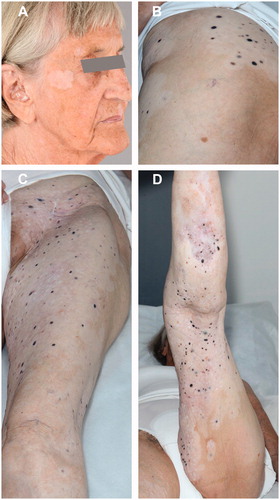
Prior treatments had been harsh for the patient, therefore, she only wanted treatment of two recently progressed metastases on the back of her thigh, while the rest of the lesions, which had not previously received electrochemotherapy, still remained untreated. She was treated according to an experimental protocol comparing calcium electroporation with electrochemotherapy with bleomycin. The treatment was carried out with intratumoral injection of 0.39 ml bleomycin 1000 IE/ml in metastasis number 1 and 0.27 ml calcium chloride (Amgros I/S, København Ø, Denmark) 9 mg/ml in metastasis number 2. The injected volumes were calculated according to ESOPE [Citation17]. Injections of the drugs were immediately followed by a total of eight electric pulse sequences. Both metastases had complete response, verified with biopsies 6 months after the treatment. Nine months after retreatment, the patient had strikingly smooth skin with complete leveling of all the cutaneous metastases. Clinically, the skin lesions had the appearance of regressing melanoma metastases with a completely flat texture and fading pigmentation. There were no new lesions. Vitiligo had developed around the treated as well as untreated lesions on the left lower limb and on both forearms and her right cheek (). Objectively, the patient had recovered with improved performance status, she had gained 10 kg in bodyweight, her analgesic treatment was reduced and she reported significantly improved quality of life.
Figure 2. July 2016. Illustration of widespread vitiligo in treated and untreated areas. (A) Vitiligo in distant untreated area. (B–D) Vitiligo extends beyond the actual treated area.
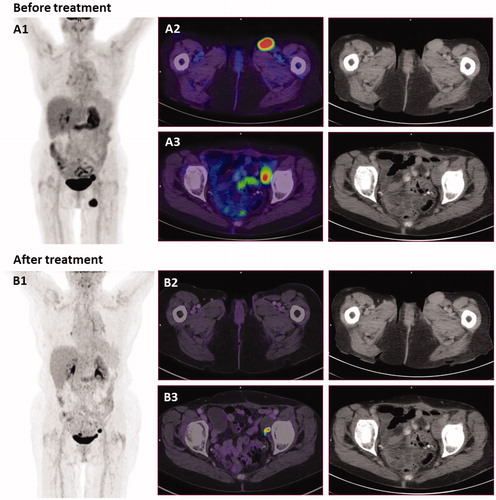
The PET-CT scan, 12 months after retreatment (July 2016), showed no signs of the previous enlarged lymph node in the inguinal region, and the lymph node in pelvis appeared necrotic but had a minimal PET positive spot (). The latter was biopsied and showed necrosis with melanophages. Within the same month, biopsies from pigmented skin lesions in the treated and untreated areas were collected. Biopsies from both areas showed discrete chronic inflammation with no signs of malignant melanoma and a large amount of melanophages in the biopsy from treated area (Supplementary Figure 1).
Figure 3. PET-CT scans and CT-scans before and after treatment. (A) September 2014, before treatment with electrochemotherapy. (A1) MIP image (maximum intensity projection), showing the two metastases. (A2) PET/CT scan shows an enlarged lymph node in the left inguinal region measuring 2.7 cm. (A3) An enlarged necrotic lymph node in the left side of pelvis measuring 2.4 cm. (B) July 2016, 16 months after electrochemotherapy and 12 months after retreatment with calcium electroporation and electrochemotherapy. (B1) MIP image shows disappearance of lymph node in the left inguinal region. (B2) PET/CT scan shows disappearance of lymph node in left inguinal region. (B3) Lymph node in left pelvis is almost completely necrotic, and cannot be measured. This inguinal node was subsequently biopsied and there was no sign of malignancy. Of note, a new PET/CT-scanner was employed for the second scan, thus direct comparison of the SUV values is not strictly possible.
After the retreatment, she received no treatment for her malignant melanoma, but after 17 months her malignant melanoma recurred with a new subcutaneous metastasis on her lower limb and several brain metastases and she underwent irradiation.
Discussion
This patient demonstrated a remarkable response to electrochemotherapy, with biopsy verified complete remission in both treated and untreated cutaneous metastases as well as radiological remission of an inguinal lymph node metastasis. This phenomenon has not been reported before in patients with malignant melanoma after electrochemotherapy alone. The reason this case is different from those previously described is unknown, but the following hypotheses can be discussed.
Unlike previously reported cases, the treatment area was extensive including more than a hundred lesions, which may have released large amounts of cancer antigens as well as danger signals, activating the immune response to an extent that generated a systemic response.
It is also possible that the first major treatment created a strong immune memory and was activated by the retreatment with electrochemotherapy and calcium electroporation 4 months later, creating a significant systemic response.
Spontaneous remission is well known in patients with malignant melanoma. It is most commonly seen in primary lesions, but in a subset of patients with disseminated disease, spontaneous remission has been reported at a frequency of 0.23% [Citation18]. In this case, the patient presented with rampant progression with hundreds of cutaneous metastases up to the time of her first treatment with electrochemotherapy.
The ability of electrochemotherapy to generate immune responses has already been reported from preclinical studies [Citation8,Citation19,Citation20]. We know that electroporation alone has a weak immunogenic effect with release of antigens, which can be strengthened by the addition of bleomycin. It has been shown in murine studies that electrochemotherapy generates cytotoxic T-lymphocytes specific for the treated cancer type, and is able to generate a protective immune memory preventing the growth of tumors when exposed to the same tumor type [Citation8,Citation19,Citation20]. Interestingly, a recent study in patients treated with electrochemotherapy for malignant melanoma showed an increased presence of calrecticulin, which is associated with immunogenic cell death. Furthermore, an increase in the presence of cytotoxic T-cells alongside a decrease in the presence of regulatory T-cells, was observed when comparing post-treatment to pretreatment biopsies [Citation21].
Several observations in this case indicate immunological involvement outside the treated area. Four months after her first extensive treatment and before retreatment, the biopsy from the tumor on her back showed almost complete regression with infiltration of T-lymphocytes and plasma cells. Furthermore biopsies from both treated and untreated lesions on her left lower limb, collected 16 months after her first treatment and 12 months after retreatment, showed complete regression and inflammation, and infiltration of melanophages was seen in the lesions from the treated area (Supplementary Figure 1). In addition, vitiligo appeared around treated as well as untreated lesions and appeared on her forearms and face (). Vitiligo is often reported in advanced metastatic melanoma patients treated with immune-based therapies [Citation22,Citation23] and is associated with an improved clinical response.
The patient was retreated with both calcium electroporation and electrochemotherapy. Calcium electroporation is a novel anticancer treatment where calcium is internalized in cancer cells by electroporation, which leads to acute cell death with ATP depletion and necrosis [Citation24,Citation25]. Preclinical data with calcium electroporation on colon tumor bearing mice have shown to generate a pro-inflammatory cytokine response and a protective immune memory (manuscript submitted). Whether calcium electroporation could have contributed to a reactivation of the immune system in this case is unknown.
Electrochemotherapy is an evolving treatment included in national guidelines, e.g., from NICE (National Institute of Health and Clinical Excellence, UK) [Citation26] and is in several guidelines in treatment of malignant melanoma, cutaneous metastases of any histology, and several clinical trials with electrochemotherapy for internal tumors are progressing. This case represents a new, very interesting perspective to electrochemotherapy as an activator of the immune system with an approach to not only treat local but also distant tumors. Several clinical data on combination of electrochemotherapy and checkpoint inhibitors are emerging [Citation15,Citation16] and show that the combination is feasible with systemic responses, although randomized controlled trials would be needed to determine the possible presence of a synergistic effect.
It also demonstrates how electrochemotherapy is a suitable treatment for fragile, elderly patients. In this case, the patient was 83 years old at diagnosis and had developed extensive disease. Due to comorbidity, she was ineligible for ILP and did not tolerate chemotherapy very well. When referred for electrochemotherapy, her general condition was poor, but her toleration of the treatment was acceptable, with minimal hospitalization (4 days).
With these advantages, electrochemotherapy, and possibly calcium electroporation, would be interesting suggestions in combination with immunostimulating agents for a strategy to enhance response and improve survival in patients with disseminated malignant melanoma.
IONC_1290274_Supplemental_material.zip
Download Zip (6.9 MB)Acknowledgements
We would like to thank the photographers from Rigshospitalet, Copenhagen University Hospital, Tina C. Rasmussen and Trille C. Bohl Skjelborg and photographer from Herlev and Gentofte Hospital, University of Copenhagen, Anders Jaegenoe.
Disclosure statement
Julie Gehl reports a conflict of interest regarding a pending patent on calcium electroporation (PCT/DK2012/050496).
Additional information
Funding
References
- Campana LG, Valpione S, Mocellin S, et al. Electrochemotherapy for disseminated superficial metastases from malignant melanoma. Br J Surg. 2012;99:821–830.
- Heller R, Jaroszeski MJ, Reintgen DS, et al. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer. 1998;83:148–157.
- Kis E, Olah J, Ocsai H, et al. Electrochemotherapy of cutaneous metastases of melanoma—a case series study and systematic review of the evidence. Dermatol Surg. 2011;37:816–824.
- Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy – an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. EJC Suppl. 2006;4:3–13.
- Matthiessen LW, Chalmers RL, Sainsbury DC, et al. Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 2011;50:621–629.
- Matthiessen LW, Johannesen HH, Hendel HW, et al. Electrochemotherapy for large cutaneous recurrence of breast cancer: a phase II clinical trial. Acta Oncol. 2012;51:713–721.
- Spratt DE, Gordon Spratt EA, Wu S, et al. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: a meta-analysis. J Clin Oncol. 2014;32:3144–3155.
- Calvet CY, Famin D, Andre FM, et al. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology. 2014;3:e28131.
- Mir LM, Roth C, Orlowski S, et al. Systemic antitumor effects of electrochemotherapy combined with histoincompatible cells secreting interleukin-2. J Immunother Emphasis Tumor Immunol. 1995;17:30–38.
- Mir LM, Orlowski S, Poddevin B, et al. Electrochemotherapy tumor treatment is improved by interleukin-2 stimulation of the host's defenses. Eur Cytokine Netw. 1992;3:331–334.
- Sersa G, Cemazar M, Menart V, et al. Anti-tumor effectiveness of electrochemotherapy with bleomycin is increased by TNF-alpha on SA-1 tumors in mice. Cancer Lett. 1997;116:85–92.
- Andersen MH, Gehl J, Reker S, et al. Dynamic changes of specific T cell responses to melanoma correlate with IL-2 administration. Semin Cancer Biol. 2003;13:449–459.
- Byrne CM, Thompson JF, Johnston H, et al. Treatment of metastatic melanoma using electroporation therapy with bleomycin (electrochemotherapy). Melanoma Res. 2005;15:45–51.
- Quaglino P, Mortera C, Osella-Abate S, et al. Electrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastases. Ann Surg Oncol. 2008;15:2215–2222.
- Heppt MV, Eigentler TK, Kahler KC, et al. Immune checkpoint blockade with concurrent electrochemotherapy in advanced melanoma: a retrospective multicenter analysis. Cancer Immunol Immunother. 2016;65:951–959.
- Mozzillo N, Simeone E, Benedetto L, et al. Assessing a novel immuno-oncology-based combination therapy: ipilimumab plus electrochemotherapy. Oncoimmunology. 2015;4:e1008842.
- Mir LM, Gehl J, Sersa G, et al. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the Cliniporator (TM) by means of invasive or non-invasive electrodes. EJC Suppl. 2006;4:14–25.
- Kalialis LV, Drzewiecki KT, Klyver H. Spontaneous regression of metastases from melanoma: review of the literature. Melanoma Res. 2009;19:275–282.
- Kuriyama S, Mitoro A, Tsujinoue H, et al. Electrochemotherapy can eradicate established colorectal carcinoma and leaves a systemic protective memory in mice. Int J Oncol. 2000;16:979–985.
- Miyazaki S, Gunji Y, Matsubara H, et al. Possible involvement of antitumor immunity in the eradication of colon 26 induced by low-voltage electrochemotherapy with bleomycin. Surg Today. 2003;33:39–44.
- Di Gennaro P, Gerlini G, Urso C, et al. CD4 + FOXP3+ T regulatory cells decrease and CD3 + CD8+ T cells recruitment in TILs from melanoma metastases after electrochemotherapy. Clin Exp Metastasis. 2016;33:787–798.
- Daud AI, DeConti RC, Andrews S, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26:5896–5903.
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III–IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773–781.
- Frandsen SK, Gibot L, Madi M, et al. Calcium electroporation: evidence for differential effects in normal and malignant cell lines, evaluated in a 3D spheroid model. PLoS One. 2015;10:e0144028.
- Frandsen SK, Gissel H, Hojman P, et al. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012;72:1336–1341.
- National Institute for Health and Care Excellence (NICE). Electrochemotherapy for metastases in the skin from tumours of non-skin origin and melanoma. [Internet]. United Kingdom: National Institute for Health and Excellence; 2013 [cited 2017 Jan 10]. Available from: https://www.nice.org.uk/guidance/ipg446

