Introduction
Aggressive fibromatosis also known as desmoid tumors constitute 3% of soft tissue tumors and 0.03% of all cancers [Citation1]. They most commonly occur in women after childbirth and the gender ratio is 1.5–2.5:1 for females versus males [Citation2]. It is a monoclonal (myo-) fibroblastic proliferation derived from mesenchymal progenitor cells [Citation3]. These tumors commonly develop in the fibrous (connective) tissue of the body that forms tendons and ligaments, usually in the arms, legs or midsection and also the head and neck area. Superficial desmoid tumors tend to be less aggressive than deep desmoids (abdominal, extra abdominal, mesenteric). Desmoid tumors may present sporadically or as a manifestation of a hereditary syndrome called familial adenomatous polyposis (FAP) or familial infiltrative fibromatosis [Citation4,Citation5]. They never metastasize but they might be multifocal. Previously, the primary treatment was surgery but as the recurrence rate often is as high as 50% or more, surgery is now more restricted to non-harmful locations or emergencies. Other therapies include watchful waiting, radiotherapy, antiestrogens (e.g. tamoxifen), non-steroidal anti-inflammatory drugs (NSAIDs) or chemotherapy and tyrosine kinase inhibitors (e.g. imatinib, sorafenib) [Citation6]. Radiotherapy is only advised if all other treatment options have been applied and the clinical condition needs to be solved, i.e., nerve damage, obstruction, etc., this is due to the risk of secondary malignancies [Citation7].
Electrochemotherapy is used in cancer treatment and combines bleomycin and local electric pulses that allow cell permeabilization and free access of bleomycin to its intracellular target. This increases the cytotoxicity of bleomycin [Citation8]. Studies have shown that electrochemotherapy is an effective and cheap treatment of subcutaneous/cutaneous tumors or metastases with a very high response rate in [Citation9–14], even in larger tumors [Citation15]. Electrochemotherapy is a treatment for localized tumors, but due to the dramatic enhancement of cytotoxicity, it works efficiently for a wide range of tumors including planocellular carcinoma, adenocarcinoma, malignant melanoma, sarcoma, renal/transitocellular carcinoma and basocellular carcinoma [Citation16]. Across Europe, it is used in more than 140 cancer centers.
Currently, treatment options are very limited for aggressive fibromatosis and new treatment options are highly needed. We hereby present a case report with women with aggressive fibromatosis treated with electrochemotherapy.
Case report
A 63-year-old woman with FAP and previous colectomy was in December 2012 referred due to severe pain in her right shoulder. An MRI revealed a tumor measuring 7.2 × 2.2 cm in close relation to the right scapula. A biopsy was performed which showed aggressive fibromatosis. Surgical removal was considered to be potentially very extensive, hence the patient began treatment with etodolac 200 mg and exemestane 25 mg daily in March 2013. In August 2013, the MRI showed stable disease, but due to side effects from exemestane, the patient started treatment with letrozole 2.5 mg daily. In June 2014, a Whipple procedure was performed due to high-grade dysplasia in an adenoma in the papilla vateri. As of December 2014, the patient complained about increasing pain in the neck and shoulder and an MRI showed progressive disease. She began treatment with sorafenib 200 mg ×2 daily, but because of side effects, the dose was reduced to 400 mg and 200 mg on alternate days. An MRI in April 2015 showed stable disease, but due to increasing pain, the dose of sorafenib was again increased to 200 mg ×2 daily. The patient was very troubled by diarrhea, exacerbated due to her previous colectomy.
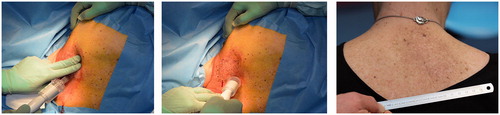
After thorough consideration and discussion of various treatment options and due to skin toxicity, diarrhea and increasing pain, sorafenib was discontinued in October 2015, and the patient was referred for consideration of electrochemotherapy. Her NRS (numeric rating scale) pain score was at that time 7 (on a 0 to 10 scale with 10 being the worst imaginable pain), despite medication with gabapentin and tramadol. The patient was informed that electrochemotherapy was considered experimental treatment in her particular case and consented to treatment. Furthermore, the patient gave written consent to the publication of this case report, including photographs. Before electrochemotherapy, the area of the tumor was measured by a physical examination to be 6.5 × 4.0 × 2.5 cm. The MRI revealed a tumor measuring 7.1 × 2.2 cm. In February 2016, she received her first treatment with electrochemotherapy under general anesthesia with 26,000 international units (IU) of bleomycin (15,000 IU/m2) and 64 pulse sequences of each eight pulses were administered using a square wave pulse generator (Cliniporator, IGEA, Carpi, Italy), and linear array electrodes. The treatment was performed according to the ESOPE protocol [Citation16]. Due to the size of the tumor, the bottom of the tumor was not reached at the first treatment session. shows the application of the electric pulses, which started 8 min after infusion of bleomycin.
Figure 1. Electrochemotherapy treatment and follow-up. The left and middle panels are from the first electroporation procedure. A linear array electrode was used, coupled to a square wave pulse generator. Treatment commenced 8 min after i.v. bleomycin infusion and the tumor was manually palpated after which electrodes were sequentially applied to cover the tumor volume. Right panel: At 1-year follow-up, hyperpigmentation in the treated area is just visible.
Two weeks after treatment, she was reviewed and complained about pain, inflammation and hyperpigmentation of the treated skin area. Six weeks after the first treatment with electrochemotherapy, the MRI revealed slight tumor shrinkage to 6.0 × 2.2 cm. Physical examination revealed tumor shrinkage to 5.0 × 2.5 cm and the patient symptoms had improved with a remarkable reduction in pain. In May 2016, 4 months after the first treatment with electrochemotherapy, the patient experienced a new increase in pain and the tumor had on physical examination increased in size to 6.0 × 4.0 × 3.0 cm. The patient was therefore offered a second treatment with electrochemotherapy which the patient consented to and received in June 2016. An MRI performed in July at follow-up showed impressive regression of the tumor size with shrinkage of the tumor to 5.2 × 2.1 cm and a new MRI in September 2016 showed further regression to 4.0 × 1.8 cm. At 1 year follow-up in March 2017, the patient was doing well with a performance status of 0. She had discontinued gabapentin and tramadol and yet had a marked decrease in pain score to NRS of 2. The MRI from January 2017 showed further regression to 2.9 × 1.7 cm.
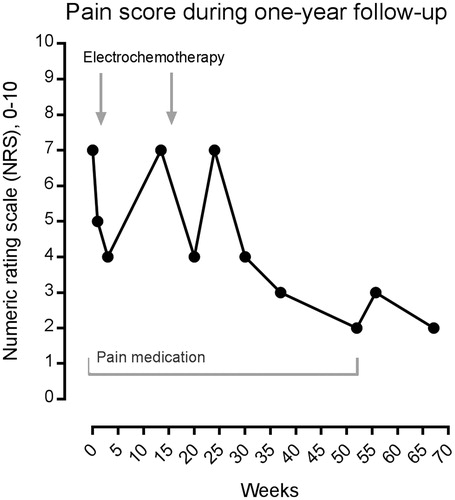
In total, there was tumor reduction with the use of electrochemotherapy from 7.1 × 2.2 cm to 2.9 × 1.7 cm and a reduction in NRS score from 7 to 2. See for MRI and for NRS score.
Figure 2. MRI before, during and after the treatment period. (A) (1 and 2): January 2016; baseline before the first treatment with electrochemotherapy, the tumor is measuring 7.1 × 2.2 cm. (B) (1 and 2): March 2016; after the first treatment with electrochemotherapy, the tumor is measuring 6.0 × 2.2 cm. (C) (1 and 2): January 2017; 1-year follow-up after the second treatment with electrochemotherapy. The tumor is measuring 2.9 × 1.7 cm.
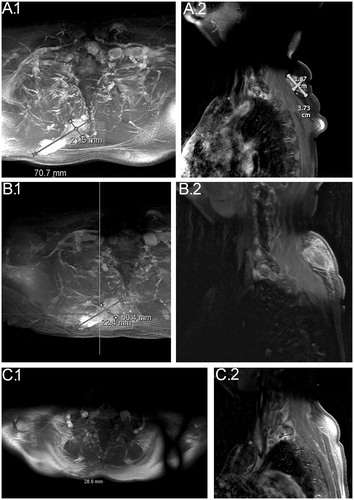
Figure 3. Pain score using the NRS scale, and use of pain medication. Using the numeric rating scale (NRS), from 0 (no pain) to 10 (worst imaginable pain), the patient was asked to describe pain before, during and after electrochemotherapy. Initially, the patient was taking pain medication (gabapentin, tramadol), and continued to do so until her pain score was down to 2, after which this was discontinued (period of pain medication shown on graph). In conclusion, the NRS pain score went from 7 with gabapentin and tramadol to 2 without pain medication after electrochemotherapy.
As the patient is now experiencing a good quality of life, is no longer on pain medication, and there has been no sign of progression after treatment, it has been decided to follow-up the patient without further planned treatment.
Discussion
Electrochemotherapy has proven to be a safe and efficacious therapy for the local control of a wide range of solid tumors. Furthermore, a few studies have also shown very promising results using electrochemotherapy with bleomycin in soft tissue sarcomas [Citation17–19].
In aggressive fibromatosis wide surgical excision is frequently the first line of treatment, though a multidisciplinary approach is often used that includes surgery, chemotherapy and radiation therapy in consideration of the tendency of the disease to recur [Citation7,Citation20,Citation21].
Spontaneous regression is well described in patients with aggressive fibromatosis [Citation6], and indeed is also a possibility here. However, given the history of the patient, and the time-wise correlation between treatment and regression it is considered likely that in this case the response was related to the given treatment with electrochemotherapy.
We were able to locate only one case report from a veterinary case of electrochemotherapy in a dog with aponeurotic fibromatosis. The dog was treated with four courses of electrochemotherapy using the drugs cisplatin and bleomycin. There was complete remission and the dog was still disease-free after 18 months [Citation22].
To our knowledge, electrochemotherapy has never been tested on aggressive fibromatosis in humans before. Our case describes an easily accessible and effective treatment with only minor side effects including pain, inflammation and hyperpigmentation of the treated skin area. Possible reasons for this lack of sufficient response after the first treatment of electrochemotherapy could be the large size of the tumor and the fact that it was not possible to reach the bottom of the tumor with the needle electrode. Although highly speculative, an immune response taking place after the second treatment cannot be ruled out [Citation23,Citation24].
Conclusion
In conclusion, new treatment options are in demand for aggressive fibromatosis and this case report points to electrochemotherapy as a possibility for tumors in proximity to the skin. Electrochemotherapy can be performed over one or a few treatments, and many cancer centers are now able to offer this treatment modality. Based on this observation, investigation as a phase II study is warranted.
Acknowledgments
Rasmus Hvass Hansen and Anders Jaegenø contributed to images.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Micke O, Seegenschmiedt MH. German Cooperative Group on Radiotherapy for Benign Diseases. Radiation therapy for aggressive fibromatosis (desmoid tumors): results of a national Patterns of Care Study. Int J Radiat Oncol Biol Phys. 2005;61:882–891.
- Reitamo JJ, Scheinin TM, Häyry P. The desmoid syndrome. New aspects in the cause, pathogenesis and treatment of the desmoid tumor. Am J Surg. 1986;151:230–237.
- Li M, Cordon-Cardo C, Gerald WL, et al. Desmoid fibromatosis is a clonal process. Hum Pathol. 1996;27:939–943.
- Scott RJ, Froggatt NJ, Trembath RC, et al. Familial infiltrative fibromatosis (desmoid tumours) (MIM135290) caused by a recurrent 3' APC gene mutation. Hum Mol Genet. 1996;5:1921–1924.
- Church J, Xhaja X, LaGuardia L, et al. Desmoids and genotype in familial adenomatous polyposis. Dis Colon Rectum. 2015;58:444–448.
- Kasper B, Ströbel P, Hohenberger P. Desmoid tumors: clinical features and treatment options for advanced disease. Oncologist. 2011;16:682–693.
- ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:iii102–iii112.
- Gothelf A, Mir LM, Gehl J. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev. 2003;29:371–387.
- Spratt DE, Spratt EA, Wu S. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: a meta-analysis. J Clin Oncol. 2014;32(28):3144–3155.
- Matthiessen LW, Chalmers RL, Sainsbury DC, et al. Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 2011;50:621–629.
- Bertino G, Sersa G, De Terlizzi F, et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: results of the treatment of skin cancer. Eur J Cancer. 2016;63:41–52.
- Kunte C, Letulé V, Gehl J, et al. Electrochemotherapy in the treatment of metastatic malignant melanoma: a prospective cohort study by InspECT. Br J Dermatol. 2017;176:1475–1485.
- Campana LG, Testori A, Curatolo P, et al. Treatment efficacy with electrochemotherapy: a multi-institutional prospective observational study on 376 patients with superficial tumors. Eur J Surg Oncol. 2016;42:1914–1923.
- Curatolo P, Quaglino P, Marenco F, et al. Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: a two-center prospective phase II trial. Ann Surg Oncol. 2012;19:192–198.
- Matthiessen LW, Johannesen HH, Hendel HW, et al. Electrochemotherapy for large cutaneous recurrence of breast cancer: a phase II clinical trial. Acta Oncol. 2012;51:713–721.
- Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy – an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. EJC Suppl. 2006;4:3–13.
- Campana LG, Bianchi G, Mocellin S, et al. Electrochemotherapy treatment of locally advanced and metastatic soft tissue sarcomas: results of a non-comparative phase II study. World J Surg. 2014;38:813–822.
- Bonadies A, Elia F, Solivetti FM, et al. Electrochemotherapy of a multirecurrent dermatofibrosarcoma protuberans of the orbital margin: a case report. Anticancer Res. 2015;35:6121–6126.
- Mir LM, Devauchelle P, Quintin-Colonna F, et al. First clinical trial of cat soft-tissue sarcomas treatment by electrochemotherapy. Br J Cancer. 1997;76:1617–1622.
- Berri RN, Baumann DP, Madewell JE, et al. Desmoid tumor: current multidisciplinary approaches. Ann Plast Surg. 2011;67:551–564.
- Rutenberg MS, Indelicato DJ, Knapik JA, et al. External-beam radiotherapy for pediatric and young adult desmoid tumors. Pediatr Blood Cancer. 2011;57:435–442.
- Spugnini EP, Di Tosto G, Salemme S, et al. Electrochemotherapy for the treatment of recurring aponeurotic fibromatosis in a dog. Can Vet J. 2013;54:606–609.
- Falk H, Forde PF, Bay ML, et al. Calcium electroporation induces tumor eradication, long-lasting immunity and cytokine responses in the CT26 colon cancer mouse model. Oncoimmunology. 2017;6:e1301332.
- Falk H, Lambaa S, Johannesen HH, et al. Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with malignant melanoma – a case report. Acta Oncol. 2017;56:1126–1131.

