Abstract
Background: Neoadjuvant therapy for pancreatic cancer remains controversial. Our aim was to assess differences in survival, disease recurrence and histopathological tumor characteristics between patients treated with neoadjuvant therapy followed by subsequent surgery and patients undergoing upfront surgery.
Material and methods: Out of 399 consecutive pancreatic ductal adenocarcinoma (PDAC) patients operated at Helsinki University Hospital in 2000–2015, 75 borderline resectable patients were treated with neoadjuvant therapy. Resectable propensity scored patients (n = 150) underwent upfront surgery. Neoadjuvant therapy consisted of folfirinox, single gemcitabine or combined with cisplatin, nab-paclitaxel or capecitabine with or without radiation. Survival was calculated with Kaplan–Meier and compared with the Breslow test. Survival was determined from the start of treatment, being the first day of treatment for patients treated with neoadjuvant therapy and the date of surgery for others.
Results: Between 2000 and 2015 median disease-specific survival (DSS) [34 vs. 26 months, p = .016] and disease-free survival (DFS) [22 vs. 13 months, p = .001] were longer in patients treated with neoadjuvant therapy than in those undergoing upfront surgery. Survival differences were not significant in the 2000s but were, in turn, among patients treated in the 2010s with better survival for patients treated with neoadjuvant therapy [DSS 35 vs. 26 months, p = .008 and DFS 25 vs. 13 months, p = .001]. Especially patients with poorly differentiated G3 tumors [DSS 30 vs. 11 months, p = .004 and DFS 21 vs. 7 months, p = .001] and higher stage IIB–III [DSS 34 vs. 20 months, p = .006 and DFS 21 vs. 10 months, p = .001] had longer survival when treated with neoadjuvant therapy.
Conclusions: PDAC patients treated with neoadjuvant therapy had longer DSS and DFS than those undergoing upfront surgery. Neoadjuvant therapy benefits especially borderline resectable patients with higher stage and poorly differentiated tumors.
Introduction
Pancreatic cancer is one of the most fatal cancers with an extremely poor overall five-year survival rate ranging from 5 to 8% [Citation1,Citation2]. Effective treatment regimens are lacking and the fatality of the disease is due to aggressiveness, advanced or metastatic disease at diagnosis and high recurrence rate [Citation3]. Pancreatic ductal adenocarcinoma (PDAC) represents >90% off all exocrine pancreatic malignancies and holds the worst survival [Citation4]. Margin negative surgery combined with oncological treatment is the only curative-intent treatment option with substantially higher survival rate, however, only 15–20% of the patients appear operable [Citation5,Citation6]. Despite advantages in research over the past decades, five-year overall survival rate has not improved drastically [Citation1,Citation3,Citation7], and it has been predicted that PDAC continues to cause even more cancer-related deaths by 2030 due to lack of effective treatments and early detection [Citation8].
Recently, neoadjuvant therapy in the management of PDAC has been a target of avid research. In the best-case scenario, neoadjuvant therapy can downstage locally advanced tumors and increase the likelihood of an R0 resection in borderline resectable cases [Citation9,Citation10]. Neoadjuvant approach to borderline resectable patients can also identify patients who are unlikely to benefit from surgery due to advanced disease [Citation9,Citation11]. However, administration of neoadjuvant therapy with resectable PDAC is still controversial in terms of patient selection [Citation12,Citation13]. With high recurrence rates, it has been postulated that PDAC is most likely a systemic disease at diagnosis [Citation14] and hence, should be treated with neoadjuvant therapy. Furthermore, research shows that patients more likely complete neoadjuvant than adjuvant therapy indicating that systemic therapy before surgery increases the likelihood of multimodal treatment [Citation15,Citation16]. However, it has been debated if administration of neoadjuvant therapy may endanger the possibility of surgery in progressive disease [Citation17]. It is unclear why some patients with PDAC survive longer than others with the same kind of treatment. Recently, PDAC research has focused on personalized medicine and thus, more reliable and adaptable patient-specific prognostic factors and treatment options are needed.
The aim of this study was to compare disease-specific (DSS) and disease-free survival (DFS) and histopathological tumor characteristics in patients treated with neoadjuvant therapy and patients undergoing upfront surgery. We also explored possible sub-populations who would preferably benefit from neoadjuvant therapy over upfront surgery.
Material and methods
Patients
We conducted a search for PDAC patients from the Helsinki University Hospital database and found in total 399 consecutive PDAC patients operated between January 2000 and December 2015 of which 75 borderline resectable patients were treated with neoadjuvant therapy and subsequent surgery. Propensity scored patients with matched age, sex and time of surgery (n = 150) underwent upfront surgery. Patient characteristics and survival data were collected from patient records and the Finnish Population Registry. Cause of death was obtained from Statistics Finland. The study was approved by the Surgical Ethics Committee and the National Supervisory Authority of Welfare and Health. Helsinki University Hospital follows a standardized pancreatic resection procedure [Citation6]. Staging of patients was determined according to the 7th edition of Pancreas cancer staging of American Joint Committee on Cancer (AJCC).
Patient characteristics are presented in . Median follow-up time was 2.1 years. Stage IV patients (n = 2) were excluded from survival analyses. Three T0 patients (4%) were recorded in the neoadjuvant group. Prior to neoadjuvant therapy these patients had histologically confirmed PDAC. One of these patients had pathologically confirmed regional lymph node PDAC metastasis, resulting in two stage 0 complete responses. These two patients with stage 0 disease have lived for 4.8 and 4.2 years with no disease progression observed to date.
Table 1. Patient characteristics of PDAC patients operated in 2000–2015 according to preoperative treatment.
No difference in the administration of postoperative treatment was observed between patient groups. Out of the 48 (65%) patients treated with neoadjuvant therapy who were administered postoperative adjuvant therapy, 35 (73%) completed the given regimen. For those undergoing upfront surgery, 102 (68%) were administered adjuvant therapy and 66 (65%) were able to complete it. Survival analyses according to adjuvant therapy were calculated for all patients receiving adjuvant therapy, including those who were not administered the full adjuvant regimen. No significant differences in postoperative complications, including postoperative mortality, were recorded.
Neoadjuvant therapy and resectability
Neoadjuvant therapy was administered to borderline resectable patients only with the exception of one resectable patient taking part in a clinical trial. There were nine patients whose scans were not available to determine preoperative staging. Borderline resectable was defined as contact with the superior mesenteric vein or the portal vein with no distant metastases. Neoadjuvant therapy regimens consisted of folfirinox, single gemcitabine or combined with cisplatin, capecitabine or nab-paclitaxel. Additional radiotherapy was administered to 29 (39%) patients. Radiotherapy alone was administered to one patient due to comorbidity. Tumor diameter in the axial plane was measured before and after neoadjuvant therapy on contrast-enhanced CT scans or, when unavailable, on MRI scans. The surgical tissue specimen was reviewed to confirm PDAC diagnosis.
Statistics
Fisher’s exact test and linear-by-linear association were used for categorical variables. Mann–Whitney U-test was used for continuous variables and survival was estimated with the Kaplan–Meier method. Survival was compared with the Breslow test. Breslow denotes the early survival differences which in pancreatic cancer are more meaningful due to the dismal five-year survival rate. The main Kaplan–Meier analyses were, in addition, carried out with a landmark analysis; the landmark time was chosen as the median duration of neoadjuvant therapy. Multivariate analyses were carried out by using the Cox proportional hazards method. Tumor grade, stage, LNR, neoadjuvant therapy and adjuvant therapy were included in the multivariate model. The assumption of constant proportional hazard rate over time was tested by adding a time dependent variable for each variable at a time. All variables met the assumption. Multivariate analyses were calculated with a time-dependent factor taking into account the time of surgery from the beginning of treatment to cover guarantee-time bias. Survival was calculated from the start of treatment, which was the first day of treatment for patients treated with neoadjuvant therapy and date of surgery for others, to death due to pancreatic cancer in DSS and disease progression first recorded in DFS. All statistical analyses were calculated with SPSS version 22 (IBM SPSS, New York, NY, USA). A p value <.05 was considered significant and two-tailed tests were used.
Results
Between 2000 and 2015 (n = 223) both median DSS and DFS were significantly longer in patients treated with neoadjuvant therapy than in patients undergoing upfront surgery (). Patients treated in 2000–2009 (n = 91) had no difference in DFS or DSS between compared groups, whereas, patients treated in 2010–2015 (n = 132) showed a significant difference in both DSS and DFS between groups; patients treated with neoadjuvant therapy had both longer median DSS () and DFS () than patients undergoing upfront surgery ().
Figure 1. A. DSS in operated PDAC patients (2010–2015) according to preoperative treatment. Median survival for neoadjuvant therapy was 35 (95% CI 25–44) months and for upfront surgery 26 (95% CI 20–31) months, p = .008. B. DFS in operated PDAC patients (2010–2015) according to preoperative treatment. Median survival for neoadjuvant therapy was 25 (95% CI 13–36) months and for upfront surgery 13 (95% CI 6–21) months, p = .001.
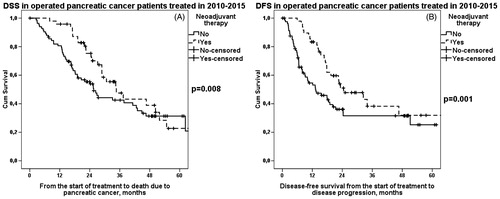
Table 2. DSS and DFS in operated PDAC patients according to preoperative treatment.
Comparing patients treated with neoadjuvant therapy, median DFS was longer in patients treated in 2010–2015 than in patients treated in 2000–2009, whereas, increase in DSS did not reach statistical difference. However, in patients undergoing upfront surgery, no progress in DFS or DSS was observed between patients treated in 2000–2009 and 2010–2015 (Supplement Table 1).
DSS and DFS were first evaluated according to tumor grade, stage and resection margins separately in patients treated with neoadjuvant therapy and those undergoing upfront surgery. DSS and DFS were additionally compared between patients treated with neoadjuvant therapy and patients undergoing upfront surgery according to these prognostic factors (). A significant difference in favor of neoadjuvant therapy was noted in patients with poorly differentiated grade 3 tumor in both median DSS (30 vs. 11 months, p = .004) () and DFS (21 vs. 7 months, p = .001) (). When dividing patients into groups according to stage and lymph node status, 0–IIA and IIB–III, there were no survival differences between groups. However, median DSS (34 vs. 20 months, p = .006) () and DFS (21 vs. 10 months, p = .001) () were recorded to be longer in patients with higher stage (IIB–III) treated with neoadjuvant therapy than upfront surgery.
Figure 2. A. DSS in patients with grade 3 tumor according to preoperative treatment. Patients: n(NEO) = 14, n(US) = 26. Median survival for neoadjuvant therapy 30 (95% CI 17–42) months and for upfront surgery 11 (95% CI 8–15) months, p = .004. NEO: neoadjuvant therapy; US: upfront surgery. B. DFS in patients with grade 3 tumor according to preoperative treatment. Patients: n(NEO) = 14, n(US) = 26. Median survival for neoadjuvant therapy was 21 (95% CI 11–31) months and for upfront surgery 7 (95% CI 5–8) months, p = .001. NEO: neoadjuvant therapy; US: upfront surgery.
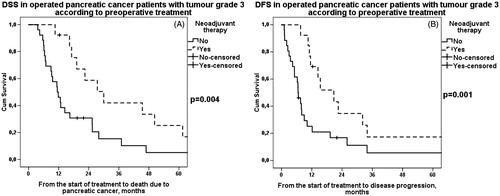
Figure 3. A. DSS in operated stage IIB–III pancreatic cancer patients according to preoperative treatment. Patients: n(NEO) = 35, n(US) = 107. Median survival for neoadjuvant therapy was 34 (95% CI 29–40) months and for upfront surgery 20 (95% CI 14–26) months, p = .006. NEO: neoadjuvant therapy; US: upfront surgery. B. DFS in operated stage IIB–III pancreatic cancer patients according to preoperative treatment. Patients: n(NEO) = 35, n(US) = 107. Median survival for neoadjuvant therapy was 21 (95% CI 12–29) months and for upfront surgery 10 (95% CI 7–13) months, p = .001. NEO: neoadjuvant therapy; US: upfront surgery.
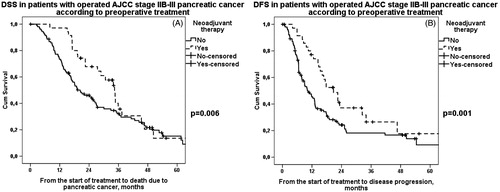
Table 3. DSS and DFS survival in operated PDAC patients according to different prognostic parameters and preoperative treatment.
In the multivariate model including tumor grade, stage, LNR, neoadjuvant and adjuvant therapy, there were significant interactions between tumor grade and neoadjuvant therapy. Therefore, the model was split by tumor grade. After adjusting for other factors, neoadjuvant therapy presented with a protective effect on DSS and DFS in grade 3 patients (HR 0.37; 95% CI 0.17–0.84, p = .018 and 0.40; 95% CI 0.17–0.92, p = .030, respectively). Univariate and multivariate analyses can be seen in Supplement Tables 2 and 3.
Grouping patients according to preoperative and postoperative treatment revealed that patients receiving perioperative treatment had both longest DSS and DFS compared to those treated with neoadjuvant therapy and surgery and those treated with upfront surgery with or without adjuvant therapy (Supplement Table 4). Supplement Table 5 shows main survival results calculated with landmark analysis.
Median time between the start of neoadjuvant therapy and surgery was 4 months (range 2–12 months, IQR 3–6 months). When comparing neoadjuvant regimens in 2000–2009 and 2010–2015, new agents such as folfirinox and nab-paclitaxel had been administered. Also, different agents were combined more often in the 2010s than in the 2000s (Supplement Table 6). All adjuvant therapy regimens are listed in Supplement Table 7.
Discussion
Whereas improvements in surgical techniques and perioperative care have decreased mortality and morbidity after surgery for pancreatic cancer, the overall survival of pancreatic cancer has not improved much during the past decades [Citation1,Citation18]. This study demonstrates that there has been some improvement in the survival of pancreatic cancer. Survival for borderline resectable patients treated with neoadjuvant therapy has improved during the past 15 years. These results offer hope for pancreatic cancer patients since neoadjuvant therapy gives patients with advanced disease a chance at resection and thus, the possibility of longer survival.
Over the studied time period, a comparison between patients treated with neoadjuvant therapy and those undergoing upfront surgery showed that both DSS and DFS were significantly longer in patients treated with neoadjuvant therapy. Interestingly, as there were no significant differences in patients treated in 2000–2009, patients treated in 2010–2015 showed 9 months longer DSS and 12 months longer DFS in patients treated with neoadjuvant therapy. Improvement could be due to more effective systemic treatments. In the 2010s new regimens such as folfirinox and nab-paclitaxel have been used at our institution. In addition, our data showed that nowadays gemcitabine is more often combined with other agents, such as cisplatin. The improvement is emphasized by median DFS increasing significantly from the 2000 to 2010s, from 15 to 25 months. Although survival has not improved for patients undergoing upfront surgery, these results are encouraging for borderline resectable PDAC patients treated with neoadjuvant therapy. Similar findings have been reported before by Cloyd et al. [Citation19], who divided 622 patients treated in 1990–2014 with neoadjuvant therapy and subsequent surgery into four successive time periods. Median overall survival improved drastically from 24 to 43 months. In addition, there was a randomized controlled trial in Korea aiming at 110 patients receiving neoadjuvant therapy. The aim was to compare survival to patients undergoing upfront surgery. However, the trial was ended at interim analysis, since the survival differences were so drastic in favor of neoadjuvant therapy [Citation20].
Locally advanced unresectable pancreatic cancer has been treated with systemic chemotherapy with or without radiation for decades [Citation21,Citation22]. Overall survival has been reported to be 6–11 months [Citation21,Citation22]. Compared to our results, downstaging the disease to resectable with chemo(radio)therapy results in significantly longer survival (median 35 vs. 6–11 months). More aggressive surgery has been advocated as well, but it has been reported that extended pancreatectomy does not guarantee better survival for more advanced disease; mortality and morbidity after surgery increase but survival is not affected [Citation23].
Tumor grade is a known prognostic factor in pancreatic cancer. It has even been postulated to have a stronger impact on survival than tumor size and lymph node positivity [Citation24]. However, there are no studies on the prognostic impact of tumor grade in patients treated with neoadjuvant therapy. Our results suggest that neoadjuvant therapy may be effective especially in patients with poorly differentiated PDAC. This is supported by both longer DSS and DFS in patients with grade 3 tumor treated with neoadjuvant therapy than in patients undergoing upfront surgery; both median DSS and DFS showed a threefold increase in survival. The multivariate analyses also showed that neoadjuvant therapy had a protective effect on both DSS and DFS in patients with grade 3 tumors. Hence, it could be argued that oncologic treatment is more effective in aggressive disease. This is supported by the fact that similar survival differences were not recognized in patients with grade 1 or 2 tumors. However, tumor grade is not usually known prior to treatment due to scarce biopsy material. To achieve patient-specific treatment, more advanced diagnostic techniques are awaited.
Crippa et al. pondered the effectiveness of pancreatic surgery for grade 3 tumor patients [Citation25]. Grade 3 tumor patients had clearly worse DSS (20 vs. 77 months) and DFS (9 vs. 63 months) when compared to grade 1 tumor patients. Also, the study showed that grade 3 tumor patients were most likely to benefit from adjuvant therapy (HR 2.11) [Citation25]. The study recommended neoadjuvant therapy for grade 3 tumor patients. Our results support the recommendation.
Prognostic factors are universal for resectable pancreatic cancer patients and might not be adaptable to patients treated with neoadjuvant therapy. shows that tumor grade; stage and resection margins are prognostic for patients undergoing upfront surgery. However, they do not seem to apply to patients treated with neoadjuvant therapy in the same way.
Stage is widely recognized as a prognostic factor in pancreatic cancer. Lymph node negativity and especially low LNR have been associated with better survival in resectable pancreatic cancer [Citation26]. Here, we divided patients into lymph node negative (0–IIA) and positive groups (IIB–III) according to stage. Here, too, neoadjuvant therapy seems to be effective in aggressive and advanced disease. Median DSS was 14 and DFS 11 months longer in stage IIB–III patients treated with neoadjuvant therapy than upfront surgery. There were no survival differences between lower stage patients.
There is most likely downstaging due to neoadjuvant therapy, which is supported by fewer nodal metastases, smaller tumor size and thus, lower stage. Patients treated with neoadjuvant therapy had a more favorable stage distribution than patients undergoing upfront surgery. This might, in fact, affect survival and multivariate analyses. De Geus et al. analyzed neoadjuvant therapy and subsequent surgery vs. upfront surgery and adjuvant therapy and found that neoadjuvant therapy showed a higher median survival in stage III patients (23 vs. 17 months) but not in early stage patients [Citation13]. However, a larger study of 8026 patients comparing neoadjuvant therapy and upfront surgery found that neoadjuvant therapy has a significant survival benefit (26 vs. 21 months) over upfront surgery in early stage pancreatic cancer [Citation12]. These results are inconclusive and demonstrate the inability to compare different studies due to heterogeneity and different criteria for study inclusion and resectability.
R1 resection is seen as a strong negative prognostic marker in pancreatic cancer and R1 resections are more commonly seen in borderline resectable pancreatic cancer due to contact with nearby blood vessels [Citation27]. In our study, the proportion of R0 and R1 resections did not differ between studied groups. Survival comparison according to R0/R1 status revealed no differences in DSS; however, DFS was longer in both R0 and R1 resected patients who had been treated with neoadjuvant therapy indicating that neoadjuvant therapy delays disease progression.
Grouping patients according to preoperative and postoperative treatment showed that clearly the longest survival was achieved with perioperative treatment and shortest with surgical treatment only. It is, however, unclear whether there is a meaningful difference in survival time between neoadjuvant therapy followed by surgery and surgery followed by postoperative adjuvant therapy. This, quite possibly, is dependent on tumor biology.
We acknowledge the limitations to this study; the study is retrospective, which could indicate selection bias. Also, the neoadjuvant therapy protocol has changed during the past 15 years with the addition of changing imaging and evaluation of resectability protocols. The multivariate results should be considered with caution due to limited number of patients with grade 3 tumors. However, these factors have been minimized by the fact that the propensity matched controls have been treated at the same time. The study does not consider a comparative non-resectable group, nor does it identify the patients with progressive disease during neoadjuvant therapy or those who could not finish their treatment due to other reasons. In Finland, preoperative diagnosis is mostly based on brush cytology, from which PDAC is impossible to diagnose.
In conclusion, this study emphasizes the fact that more aggressive disease and later stage PDAC patients benefit from neoadjuvant therapy; patients with grade 3 tumors presented with three times longer DSS and DFS when treated with neoadjuvant therapy. Survival has improved for borderline resectable PDAC patients treated with neoadjuvant therapy over the past 15 years. Whether to administer neoadjuvant therapy to early stage patients is still controversial. Due to possible selection bias and a vast variety of heterogeneous studies, further prospective and randomized controlled trials are much needed.
Anna_Nurmi_et_al._Supplement_data_manuscript.docx
Download MS Word (31.7 KB)Acknowledgments
We thank Päivi Peltokangas, Olli-Matti Sirviö and Elina Aspiala for their technical assistance.
Disclosure statement
Authors declare no conflicts of interest.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
- Finnish cancer registry [Internet]. Helsinki, Finland. 2017. [cited 2017 April 17]. Available from: http://www.cancer.fi/syoparekisteri/en/statistics/.
- Schneider G, Siveke JT, Eckel F, et al. Pancreatic cancer: basic and clinical aspects. Gastroenterology. 2005;128:1606–1625.
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917.
- Katz MH, Pisters PW, Evans BD, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–848.
- Seppänen H, Juuti A, Mustonen H, et al. The results of pancreatic resections and long-term survival for pancreatic ductal adenocarcinoma: a single-institution experience. Scand J Surg. 2017;106:54–61.
- Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981–990.
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921.
- Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. JCO. 2008;26:3496–3502.
- Roland CL, Yang AD, Katz MH, et al. Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Ann Surg Oncol. 2015;22:1168–1175.
- Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol. 2011;18:619–627.
- Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. JCO. 2016;35:515–522.
- De Geus SW, Eskander MF, Bliss LA, et al. Neoadjuvant therapy versus upfront surgery for resected pancreatic adenocarcinoma: a nationwide propensity score matched analysis. Surgery. 2017;161:592–601.
- Sohal DP, Walsh RM, Ramanathan RK, et al. Pancreatic Adenocarcinoma: treating a systemic disease with systemic therapy. J Natl Cancer Inst. 2014;106:dju011.
- Tzeng CW, Tran Cao HS, Lee JE, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg. 2014;18:16–25.
- Labori KJ, Katz MH, Tzeng CW, et al. Impact of early disease progression and surgical complications on adjuvant chemotherapy completion rates and survival in patients undergoing the surgery first approach for resectable pancreatic ductal adenocarcinoma – a population-based cohort study. Acta Oncol. 2016;55:265–277.
- Desai NV, Sliesoraitis S, Hughes SJ, et al. Multidisciplinary neoadjuvant management for potentially curable pancreatic cancer. Cancer Med. 2015;4:1224–1239.
- Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210.
- Cloyd JM, Katz MH, Prakash L, et al. Preoperative therapy and pancreatoduodenectomy of pancreatic ductal adenocarcinoma: a 25-year single-institution experience. J Gastrointest Surg. 2017;21:164–174.
- Youngmin H, Sun-Whe K, Jinseok H, et al. Multicenter prospective randomized phase II/III study of neoadjuvant chemoradiation with gemcitabine in patients with borderline resectable pancreatic cancer. Oral session presented at: 51st Pancreas Club Annual meeting; 2017 May 5–6; Chicago, IL.
- Niederhuber JE, Brennan MF, Menck HR. The National Cancer Data Base report on pancreatic cancer. Cancer. 1995;76:1671–1677.
- Loehrer PJ, Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with lovally advanced pancreatic cancer: an Eastern cooperative oncology group trial. JCO. 2011;29:4105–4112.
- Hartwig W, Gluth A, Hinz U, et al. Outcomes after extended pancreatectomy in patients with borderline resectable and locally advanced pancreatic cancer. Br J Surg. 2016;103:1683–1694.
- Wasif N, Ko CY, Wainberg Z, et al. Impact of tumor grade on prognosis in pancreatic cancer: should we include grade in AJCC staging? Ann Surg Oncol. 2010;17:2312–2320.
- Crippa S, Partelli S, Zamboni G, et al. Poorly differentiated resectable pancreatic cancer: is upfront resection worthwhile? Surgery. 2012;152:S112–S119.
- Åkenberg D, Ansari D, Andersson R, et al. Re-evaluation of classical prognostic factors in resectable ductal adenocarcinoma of the pancreas. WJG. 2016; 22:6424–6433.
- Katz MH, Crane CH, Varadhachary G. Management of borderline resectable pancreatic cancer. Semin Radiat Oncol. 2014;24:105–112.

