Abstract
Background: Adjuvant chemotherapy following curative resection is the standard treatment for pancreatic adenocarcinoma (PC). Randomized clinical trials using gemcitabine have shown a median overall survival (mOS) of 2 years and a 5-year survival rate of 15-20%. However, the effect of gemcitabine outside these trials is less clear. We examined the effect of postoperative gemcitabine on survival in an unselected cohort of patients receiving curative resection for PC in Denmark during a five-year period.
Material and methods: From 1 May 2011 to 30 April 2016, 731 patients treated with curative resection were identified in the Danish Pancreatic Cancer Database (DPCD). Thirty patients died within 10 weeks postoperatively; 78 received other regimens or preoperative chemotherapy and were excluded. Of the remaining 623 patients, the chemotherapy (CT) group (n = 409, 66%) received gemcitabine within 10 weeks after resection, whereas the non-chemotherapy (NCT) group (n = 214, 34%) did not receive CT within 10 weeks.
Results: CT patients were slightly younger than NCT patients but did not otherwise differ in baseline characteristics. The CT group showed a mOS of 24 months (95% CI; 21–27) and a 5-year survival rate of 22% (95% CI; 17–27); the NCT group had a mOS of 22 months (95% CI; 16–26, p = .27) and a 5-year survival rate of 26% (95% CI; 19–34, p = .66). Most patients (415/623) had lymph node metastases. Of these patients, those in the CT group (n = 280) had significantly longer mOS [20 months (95% CI; 18–24)] than those in the NCT group (n = 135) [14 months (95% CI; 11–17)].
Conclusions: In this national Danish cohort of PC patients undergoing resection between 2011 and 2016, the survival after postoperative gemcitabine was similar to that reported in previous clinical trials. However, the survival advantage of postoperative gemcitabine was limited to patients with lymph node metastases.
Background
Pancreatic adenocarcinoma (PC) is the fourth leading cause of cancer death in both men and women in Europe [Citation1]. However, during the last decade, the overall 5-year survival rate has improved in many European countries. In Denmark, the 5-year survival rate increased from 3.8% between 2000–2004 to 8% between 2010-2014 [Citation2]. This improvement could be due to the implementation of national cancer plans that focused on the centralized and fast-track handling of patients in 2000, 2005 and 2011 [Citation3]. However, the majority of patients with PC are still diagnosed in unresectable locally advanced or metastatic stages. Surgical resection is a potentially curative treatment but is feasible only in 15-20% of patients [Citation4]. In Denmark, nearly 1,000 patients are diagnosed with PC each year [Citation5], but only 150 of these patients undergo curative surgical procedures [Citation6].
The treatment of PC in Denmark is carried out at four surgical and seven oncological centers [Citation6]. Demographic, diagnostic and treatment data are collected and registered prospectively in the Danish Pancreatic Cancer Database (DPCD). The DPCD retrieves data from the Danish Civil Registry, the Danish National Patient Registry and the Danish Pathology Registry. These official national registries have high validity and completeness [Citation7,Citation8].
The impact of adjuvant chemotherapy after PC resection has been evaluated in several randomized clinical trials (RCTs). The following results are given with 95% confidence intervals (95% CI). In the European Study Group for Pancreatic Cancer 1 study (ESPAC-1), a median overall survival (mOS) of 19.7 months (16.4–22.4) with adjuvant 5-fluorouracil (5-FU) was reported, while adjuvant chemoradiation resulted in a mOS of 15.5 months (13.5–17.4) [Citation9]. In the Charité Onkologie 001 study (CONKO-001), adjuvant gemcitabine was superior to observation, with an improved mOS of 22.1 months (18.4–25.8) versus 20.2 months (17–23.4) and a 5-year survival rate of 20.7% (14.7–26.6) versus 10.4% (5.9–15.0) [Citation10,Citation11]. In the ESPAC-3v2 study, adjuvant gemcitabine and adjuvant 5-FU resulted in comparable survival rates, 23 months (21.4–26.4) versus 23.6 months (21.1–25.0), but higher toxicity was reported in the 5-FU arm [Citation12]. Based on these results, adjuvant gemcitabine after PC resection has been recommended for patients in Denmark since 2006 [Citation13].
Reproducing the results from RCTs in general clinical practice is often difficult, as patients are frequently older and have severe comorbidity and poor performance status (PS) [Citation14]. Inclusion criteria thus greatly impacts survival data [Citation15]. Data for real-time registered and unselected PC patients treated in a daily clinical setting are therefore warranted.
Using data from the DPCD, we assessed the effect of postoperative gemcitabine on survival in a national Danish comprehensive cohort of PC patients treated with curative intent surgery from 2011–2016.
Material and methods
Data were collected prospectively from 1 May 2011 to 30 April 2016 and registered in the DPCD. The data input and output protocols have been described elsewhere [Citation16]. Data regarding demographics, the Charlson comorbidity index (CCI), surgical interventions, tumor stage, antineoplastic drugs given and start dates, mortality and survival were retrieved. The CCI was calculated for each patient using the codes for diseases within the last 10 years according to the International Code of Disease, edition 10. The total score of the weighted index of comorbidity has been described elsewhere [Citation17]. No information on the PS of the patients was available.
Patients
Patients were identified by their unique 10-digit civil personal registration number and diagnosis as ICD-10 code C25.*, pancreatic cancer, excluding malignant tumors of the endocrine pancreas (C25.4). Patients curatively resected for ductal adenocarcinoma and associated subtypes according to the WHO criteria were included [Citation18].
Resection, tumor staging and resection margins
During the study period, Danish guidelines recommended upfront resection for patients with T1/T2/T3NXM0 tumors. However, for tumors with vein involvement, vein reconstruction caudal to the tumor should be feasible. For patients with T4NXM0 tumors, a resection upfront was not regularly recommended. For these patients, preoperative chemotherapy or chemo-radiotherapy were the upfront choice of treatment, with a subsequent reevaluation [Citation13]. Pathological tumor and lymph node (pTN) staging was based on histopathological reports according to the AJCC/UICC guidelines, 7th edition [Citation19].
In 2015, the Danish national guidelines regarding the pathological examination of pancreatic resection specimens were updated, recommending a standardized protocol for pancreaticoduodenectomy specimen examination. This protocol uses the precise coding of the minimal distances to the resection margins (RMs) [Citation13,Citation20]. Prior to 2015, variations in the definition and coding of the RM (R-status) were present. Thus, reporting the R-status was not meaningful in this study.
Postoperative chemotherapy
During the study period, Danish guidelines recommended 24 weeks of adjuvant gemcitabine monotherapy starting approximately 4–8 weeks after resection, without consideration of the R-status [Citation13]. Three different gemcitabine schedules were used (data shown in the Supplement Figure 1). The precise intention to treat (adjuvant or palliative) was not recorded in the DPCD. Considering that the patients started gemcitabine treatment within 10 weeks after resection, most should have been treated adjuvantly. However, some patients could have experienced early recurrence. Therefore, we considered gemcitabine treatment to be “postoperative”.
Figure 1. Flowchart showing the inclusion and exclusion of patients registered in the Danish Pancreatic Cancer Database (DPCD) from 2011–2016. The histopathological data for 72 patients were reviewed due to the missing status of the data in the DPCD. *Data shown in the Supplement Table 1. GemPac: Gemcitabine and nab-paclitaxel; FOLFIRINOX: 5-Fluorouracil, leucovorin, irinotecan and oxaliplatin; GemCap: Gemcitabine and capecitabine; GemS1: Gemcitabine and tegafur/gimeracil/oteracil.
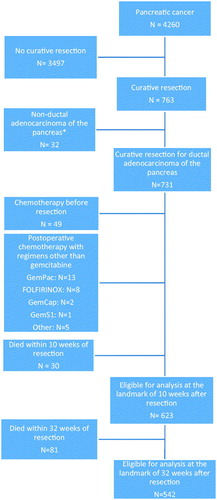
Patients who received preoperative chemotherapy, postoperative treatment other than monotherapy with gemcitabine or died within 10 weeks after resection were excluded.
The overall survival (OS) was calculated and compared between the two groups: patients starting postoperative gemcitabine within 10 weeks after resection (CT group) and patients receiving no chemotherapy of any type within 10 weeks after resection (NCT group).
Subgroup analyses
Subgroup analyses were performed for patients with (pN1) and without (pN0) lymph node metastases in the surgical resection specimen.
The start of gemcitabine treatment after resection was considered potentially important for OS. Therefore, the results of the CT group were further analyzed by two subgroups: patients starting gemcitabine ≤6 weeks and patients starting gemcitabine >6 weeks after surgery.
Completion of the fully scheduled postoperative gemcitabine regimen was also considered a potentially important factor for OS. Patients who received ≥22 weeks of the planned gemcitabine regimen were considered to have completed the fully scheduled postoperative treatment. This definition was chosen because all treatment schedules used included at least 22 weeks of active treatment.
Statistical methods
The direct comparison of time from diagnosis to death of any cause between individuals in the CT and NCT groups and individuals receiving <22 and ≥22 weeks of chemotherapy is problematic, as individuals in the CT group and ≥22 weeks group remain immortal for some time compared to those in the NCT group and <22 weeks group, respectively. This gives them an unfair survival advantage, which might lead to the so-called immortal time bias [Citation21].
An established solution to this problem is to stratify the individuals at a fixed point in time, called a landmark, based on the data available only prior to the defined landmark and remove patients with events or censoring before the landmark from the analysis [Citation22].
The first landmark was set at 10 weeks after surgery. This landmark was used to ensure correct stratification with regards to the start of postoperative chemotherapy.
A second landmark was set at 32 weeks (10 + 22) after the operation. This landmark was used solely to stratify patients receiving postoperative chemotherapy with regards to the length of the treatment.
The OS was calculated from the decided landmark dates to the date of death from any cause or until 10 September 2017. A p-value <.05 was considered statistically significant. A CI of 95% was used. Categorical data were analyzed using Fisher’s exact test, and continuous data were analyzed with the Mann-Whitney U-test. For survival analysis, Kaplan-Meier plots, log-rank tests and Cox proportional hazards regression models were used. A nearest neighbor propensity score matching method was applied to reduce bias in the assessment of the treatment effect on survival. For all statistical analyses, Stata v. 15 (StataCorp LLC, TX, USA) was used.
Ethics
The study was approved by the Danish Data Protection Agency (2008-58-0028) and the Danish Patient Safety Authority (3-3013-1678/1/).
Results
The patients in the NCT and CT groups treated between 2011 and 2013 were compared with those in the corresponding cohorts treated between 2014 and 2016. No differences in the OS were observed (data not shown). For further analyses, all data from 2011–2016 were considered one cohort. Three different gemcitabine schedules were used in the study period. These regimens showed no differences in OS (p = .09) (data shown in Supplement Figure 1). For further analyses, the data for the three gemcitabine schedules were also considered one cohort.
Patient characteristics and clinical outcomes
The characteristics of the PC study cohort are shown in . The resection rate was 17.2%. At the 10 weeks after surgery landmark, 623 patients were still alive and thus eligible for further analysis. The CT group consisted of 409 (66%) patients starting gemcitabine within 10 weeks of resection; the NCT group consisted of 214 (34%) patients who did not receive any chemotherapy within 10 weeks of resection.
The comparison of the two groups is presented in . The mean age (67 years) of the CT group was significantly lower than that of the NCT group (70 years) (p < .01). No difference was observed between the groups regarding gender, comorbidity, tumor stage, resection type or vein resection frequency.
Table 1. Comparison of the variables between patients receiving (CT group) or not receiving (NCT group) postoperative gemcitabine within 10 weeks after resection (landmark of 10 weeks).
After a median follow-up of 34 months (range 16–76), 134 (33%) patients in the CT group and 74 (35%) patients in the NCT group were still alive. The estimated survival curves and data, starting at the 10 weeks after surgery landmark, are shown in and . No significant differences in the mOS and 3-year survival were observed between the two groups. Only the CT subgroup of pN1 patients showed significantly longer survival, with a mOS of 20 months versus 14 months in the NCT subgroup of pN1 patients (p = .01).
Figure 2. OS at the landmark of 10 weeks according to patients receiving (CT group) or not receiving (NCT group) postoperative gemcitabine within 10 weeks after resection.
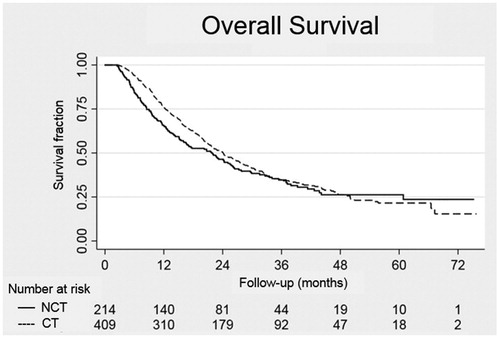
Table 2. Median OS according to postoperative gemcitabine treatment (CT group) or no treatment (NCT group) within 10 weeks after resection in all patients and in the subgroups with and without lymph node metastases, as well as the OS for all patients at 1, 3, and 5 years of follow-up (landmark of 10 weeks).
As shown in , gender, stage, CCI, resection type and vein resection status were significantly associated with survival, whereas age was not. No significant association of postoperative gemcitabine treatment with survival was observed in either the univariate or multivariate analysis. No differences in OS between the time periods 2011–2013 and 2014–2016 were observed (data not shown).
Table 3. Univariate and multivariate Cox regression analyses of the prognostic factors associated with survival (landmark of 10 weeks).
A propensity score analysis matching for age, comorbidity, lymph node status, and resection type between the CT and NCT groups was performed, and there was still no significant effect of postoperative chemotherapy on survival.
No difference was found in the mOS between patients who started gemcitabine ≤6 weeks (228 patients (56%)) and those who started gemcitabine >6 weeks (181 patients (44%)) after surgery or between the subgroups with and without lymph node metastases.
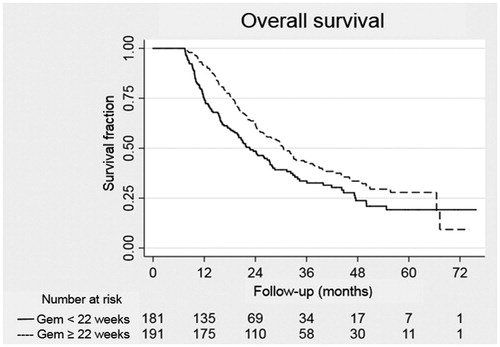
A total of 542 patients were alive at the 32 weeks after surgery landmark and gemcitabine treatment. Between the landmarks at 10 and 32 weeks, 81 patients died. Thirty-seven of these patients had started postoperative chemotherapy and were excluded from the survival analysis. The 191 CT patients who received gemcitabine for at least 22 weeks showed significantly improved survival compared to that of the 181 patients who did not complete the postoperative gemcitabine regimen, p = .01 (see ).
Discussion
In this nationwide, population-based study, 623 patients underwent curative intent resection for ductal adenocarcinoma of the pancreas between 2011 and 2016. The resection rate in our study was 17.2%, which is comparable to the rate in the Netherlands (17.9%) and higher than that in Norway (13%) [Citation23]. The overall resection rate of PC in Denmark increased from 12.0%–17.6% from 2011–2012 to 2013–2014 [Citation23]. Four centers in Denmark perform PC resections, with an annual volume of 13–82 patients. Thus, each center should have sufficient surgical volume, a variable known to influence mortality [Citation24].
The mean age of the whole cohort was similar to that in other literature reports [Citation25]. The mean age (67 years) of the CT group was significantly lower than that of the NCT group (70 years), reflecting the selection of younger patients for CT. The CT group treated with postoperative gemcitabine consisted of 409 (66%) patients. These figures are comparable to those in several other studies reporting initiation rates of 50–70% [Citation26,Citation27]. Severe postoperative complications have previously been described as a reason for omitting adjuvant treatment [Citation28]. These factors, as well as poor PS, patient preference and early recurrence, could explain why 34% of patients did not receive postoperative gemcitabine.
The mOS of 24 months in the CT group was similar to the mOS (22.3–25.5 months) of the gemcitabine-treated cohorts in most previously published RCTs [Citation10,Citation12,Citation29,Citation30]. The mOS of 22 months in the NCT group of patients was slightly longer than the mOSs of 20.2 and 18.4 months reported for the observation groups in previous RCTs [Citation10,Citation29]. Thus, no significant differences in the mOS were observed between the two groups.
The estimated 5-year survival rate of 22% in the CT group was comparable to the 5-year survival rates reported in previous RCTs (16.3–23.6%), which reported patients treated during different time periods, including 1998–2004, 2002–2005, 2000–2007 and 2008–2014 [Citation10–12,Citation29,Citation30]. Hence, over nearly two decades, the estimated 5-year survival rates after adjuvant gemcitabine in PC have not improved. The 5-year survival rate of the NCT group was 26% compared to the 10.4% and 10.6% 5-year survival rates reported for the observation groups in two previous studies [Citation11,Citation29]. Improvements in palliative chemotherapy and best supportive care might explain the higher mOS. However, these factors likely have only a limited effect on the improved 5-year survival rate. In the current study, we did not investigate the impact of subsequent treatment at recurrence.
Several retrospective studies investigating PC cohorts from 2000-2015 have reported improved survival with adjuvant chemotherapy compared to observation [Citation26,Citation31] whereas other studies have not [Citation32,Citation33]. Only one study reported the type of adjuvant chemotherapy as gemcitabine [Citation31]. One study calculated the survival time from the date of resection but did not consider immortal time bias. This approach could contribute to an overestimation of the survival benefit of adjuvant treatment [Citation31]. One study used a landmark Kaplan-Meier OS analysis that excluded patients dying within 6 months of surgery. This study showed no significant difference in OS between patients receiving adjuvant chemotherapy and those receiving no adjuvant treatment, with a 5-year survival rate of 21% versus 17%, respectively [Citation32].
The present study showed no difference in mOS between patients who started chemotherapy within or later than 6 weeks after surgery, in agreement with previous studies [Citation26,Citation34]. Notably, one study suggested that the initiation of adjuvant chemotherapy ≤20 days after PC resection significantly improved 5-year survival compared with that achieved by initiating treatment >20 days after resection [Citation35]. However, the patients in this study received different treatment regimens (gemcitabine and S-1). The results of previous publications and our findings indicate that initiating adjuvant gemcitabine as late as 6–8 weeks after surgery does not influence the survival rate.
The patients receiving complete gemcitabine treatment had significantly longer survival than patients who did not, in line with the results of a previous study reporting the completion of all 6 cycles of adjuvant chemotherapy as an independent prognostic factor for prolonged survival [Citation34].
Lymph node involvement is reported to be the most important prognostic factor in patients with resected PC [Citation36]. In the present study, a significantly better mOS was shown for pN1 patients in the CT group (20 months) than that for those in the NCT group (14 months). This finding is in line with a population-based study from the US, where the effect of adjuvant chemotherapy was most pronounced in patients with poorly differentiated tumors and lymph node metastases [Citation37]. As PC has a tendency to develop early distant metastases, micrometastases after resection could be more frequent in patients in the pN1 subgroup than in patients with stage pN0 [Citation38]. The elimination of micrometastases may explain the effect of gemcitabine in these patients.
Future aspects
Improvements in the effects of adjuvant chemotherapy in PC have been reported recently. Significantly prolonged mOS was shown for combined treatment with gemcitabine and capecitabine compared to gemcitabine monotherapy (28 versus 25 months, respectively) [Citation30]. An impressive treatment effect with modified FOLFIRINOX compared to gemcitabine showed an increased mOS of 54.4 versus 35 months, respectively [Citation15]. Tegafur/gimeracil/oteracil (S-1) compared to gemcitabine has also shown an increased mOS of 45.5 versus 25.5 months, respectively, in Japanese patients [Citation39]. Adjuvant therapy with the combination of gemcitabine and capecitabine was implemented in Denmark in autumn 2016 and then with modified FOLFIRINOX in autumn 2018. Combination chemotherapy improves clinical outcomes but worsens side effects, making the selection of patients with a good PS necessary [Citation15]. Obviously, there will still be a subgroup of patients who can only be offered gemcitabine monotherapy. In light of the new standard therapies, the results of the present study will be useful as a clinical reference in the future for comparing the OS in unselected PC patients receiving new adjuvant combination chemotherapy regimens. Danish national real-time register data will be available in the DPCD during the next three years.
Several randomized trials exploring neoadjuvant/perioperative treatment with FOLFIRINOX, GemPac and other types of combination chemotherapy for resectable PC patients are ongoing [Citation40–42]. By delivering chemotherapy prior to surgical resection, the chance of receiving both modalities might increase. Moreover, neoadjuvant chemotherapy should enable the early treatment of micrometastases and lower the risk for recurrence.
Strengths of this study
This national register study included all Danish patients receiving curative resection for ductal adenocarcinoma of the pancreas during a clearly defined 5-year period. The real-time data collected from the DPCD made it possible to retrieve all treatment data. The relatively large nation-based cohort allowed us to divide the patients into homogenous groups. We used defined landmarks to reduce immortality time bias. The clinical follow-up time was adequate, and no patients were lost to follow-up.
Limitations of this study
This study is a nonrandomized register study, and bias due to imbalances in known and unknown variables influencing survival differences among groups cannot be excluded. Data regarding postoperative complications, reasons for the omission of postoperative gemcitabine, and information about recurrence were not accessible in the DPCD. Furthermore, PS data were not assessable, but PS is known to influence survival [Citation43].
The impact of R-status on OS was not meaningful to analyze in this study. Methods for the pathological handling of surgical PC specimens and the categorization of the R-status vary widely [Citation20,Citation44]. In addition, the R1 rate (microscopic tumor involvement of the RMs) varies from 20% to 80%. Consequently, the impact of R1 on survival differs considerably in published studies [Citation20]. An international consensus definition of the minimum RM clearance in PC is needed.
In conclusion, in a large comprehensive national Danish cohort undergoing curative resection for ductal adenocarcinoma of the pancreas between 2011 and 2016, patients receiving postoperative gemcitabine (66%) had an OS rate comparable to the OS rate in the adjuvant gemcitabine arms in previously published RCTs. Patients not treated with postoperative gemcitabine (34%) had a similar outcome, which was significantly better than that reported in the control arms of previous RCTs. The patients with lymph node metastases, who composed the majority of the patients in this study who were treated with postoperative gemcitabine, fared significantly better than those not treated, whereas no significant difference was found for PC patients without lymph node metastases.
Supplemental Material
Download MS Word (106.4 KB)Acknowledgments
The register data for this study were extracted from the DPCD organized in the Danish Clinical Registries (RKKP). The authors would like to thank the members of The Danish Pancreatic Cancer Group and Professor Ursula G. Falkmer, Head of the Department of Oncology, Aalborg University Hospital, for scientific support.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing.
Additional information
Funding
References
- Malvezzi M, Carioli G, Bertuccio P, et al. European cancer mortality predictions for the year 2016 with focus on leukaemias. Ann Oncol. 2016;27:725–731.
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075.
- Storm HH, Gislum M, Engholm G. Kræftoverlevelse før og efter den danske kræftplan [Cancer survival before and after initiating the Danish Cancer Control plan]. Ugeskr Laeger. 2008;170:3065–3069.
- Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Prim. 2016;2:1–23.
- Engholm G, Ferlay J, Christensen N, et al. NORDCAN – a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49:725–736.
- Dansk Pancreas Cancer Database, Årssrapport 2015/2016 [Internet] [Danish Pancreatic Cancer Database Annual Report 2015/2016]. Available from: http://dpcg.gicancer.dk/Content/Files/Dokumenter/Databaserapporter/dpcd_aarsrapport_2015_2016_officiel_version_f.pdf. Danish.
- Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549.
- Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish National Patient Registry: a review of content. Data Qual Res Potential Clin Epidemiol. 2015;7:449–490.
- Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet. 2001;358:1576–1585.
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277.
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481.
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant Chemotherapy With Fluorouracil Plus Folinic Acid vs Gemcitabine Following Pancreatic Cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–1081.
- Dansk Pancreas Cancer Gruppe, Nationale Kliniske Retningslinier 2015 [Internet] [Danish Pancreatic Cancer Group National Clinical Guidelines 2015]. Danish. Available from: http://dpcg.gicancer.dk/Default.aspx?pID=22
- Sorbye H, Pfeiffer P, Cavalli-Björkman N, et al. Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer. 2009;115:4679–4687.
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395–2406.
- Fristrup C, Detlefsen S, Hansen CP, et al. Danish Pancreatic Cancer Database. Clin Epidemiol. 2016;8:645–648.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon (France): International Agency for Research on Cancer; 2010.
- Sobin LH, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours. 7th edition. New York: International Union Against Cancer (UICC); 2009.
- Verbeke CS. Resection Margins in Pancreatic Cancer. Surg Clin North Am. 2013;93:647–662.
- Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719.
- Mi X, Hammill BG, Curtis LH, et al. Use of the landmark method to address immortal person-time bias in comparative effectiveness research: a simulation study. Stat Med. 2016;35:4824–4836.
- Huang L, Jansen L, Balavarca Y, et al. Resection of pancreatic cancer in Europe and USA: an international large-scale study highlighting large variations. Gut. 2019;68:130–139.
- Tjarda Van Heek N, Kuhlmann KFD, Scholten RJ, et al. Hospital volume and mortality after pancreatic resection: A systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–790.
- Xu J-B, Jiang B, Chen Y, et al. Optimal adjuvant chemotherapy for resected pancreatic adenocarcinoma: a systematic review and network meta-analysis. Oncotarget. 2017;8:81419–81429.
- Bakens MJ, van der Geest LG, van Putten M, et al. The use of adjuvant chemotherapy for pancreatic cancer varies widely between hospitals: a nationwide population-based analysis. Cancer Med. 2016;5:2825–2831.
- Labori KJ, Katz MH, Tzeng CW, et al. Impact of early disease progression and surgical complications on adjuvant chemotherapy completion rates and survival in patients undergoing the surgery first approach for resectable pancreatic ductal adenocarcinoma – A population-based cohort study. Acta Oncol. 2016;55:265–277.
- Wu W, He J, Cameron JL, et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol. 2014;21:2873–2881.
- Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101:908–915.
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;6736:1–14.
- Åkerberg D, Björnsson B, Ansari D. Factors influencing receipt of adjuvant chemotherapy after surgery for pancreatic cancer: a two-center retrospective cohort study. Scand J Gastroenterol. 2017;52:56–60.
- Kagedan DJ, Raju RS, Dixon ME, et al. The association of adjuvant therapy with survival at the population level following pancreatic adenocarcinoma resection. HPB. 2016;18:339–347.
- Parikh AA, Maiga A, Bentrem D, et al. Adjuvant therapy in pancreas cancer: does it influence patterns of recurrence? J Am Coll Surg. 2016;222:448–456.
- Valle JW, Palmer D, Jackson R, et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: Ongoing lessons from the ESPAC-3 study. J Clin Oncol. 2014;32:504–512.
- Murakami Y, Uemura K, Sudo T, et al. Early initiation of adjuvant chemotherapy improves survival of patients with pancreatic carcinoma after surgical resection. Cancer Chemother Pharmacol. 2013;71:419–429.
- Riediger H, Keck T, Wellner U, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg. 2009;13:1337–1344.
- Vanderveen KA, Chen SL, Yin D, et al. Benefit of postoperative adjuvant therapy for pancreatic cancer: A population-based analysis. Cancer. 2009;115:2420–2429.
- Haeno H, Gonen M, Davis MB, et al. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148:362–375.
- Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248–257.
- Clinicaltrails.gov. Neoadjuvant Plus Adjuvant or Only Adjuvant Nab- Paclitaxel Plus Gemcitabine for Resectable Pancreatic Cancer (NEONAX) [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT02047513.
- Clinicaltrails.gov. Randomized Multicenter Phase II/III Study With Adjuvant Gemcitabine Versus Neoadjuvant/Adjuvant FOLFIRINOX for Resectable Pancreas Carcinoma [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT02172976.
- Reni M, Balzano G, Zanon S, et al. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2–3 trial. Lancet Gastroenterol Hepatol. 2018;3:413–423.
- Tas F, Sen F, Odabas H, et al. Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int J Clin Oncol. 2013;18:839–846.
- Delpero JR, Jeune F, Bachellier P, et al. Prognostic value of resection margin involvement after pancreaticoduodenectomy for ductal adenocarcinoma: updates from a French Prospective Multicenter Study. Ann Surg. 2017;266:787–796.