Abstract
Background: Evidence suggests that among some occupational groups, there is an elevated risk of kidney cancer. This might, however, derive from a difference in smoking habits across occupational groups. The objective of this study was to determine smoking-adjusted occupational variation in the incidence of kidney cancer in Nordic males.
Material and Methods: The source population for this study consisted of 7.4 million men from Denmark, Iceland, Finland, Norway, and Sweden. Data on occupation were obtained from national censuses conducted in the years 1960–1990. Data on cancer cases came from national cancer registries. A proxy for the occupation-specific smoking prevalence among all Nordic men was calculated based on the occupation-specific smoking prevalence and lung cancer incidence data for Finnish men. Smoking-adjusted standardized incidence ratio (SIRadj) with 95% confidence intervals (95%CI) were calculated for each occupational group.
Results: The highest SIRadj estimates were observed in dentists (1.32, 95%CI 1.06–1.62), journalists (1.20, 95%CI 1.00–1.42), physicians (1.19, 95%CI 1.03–1.36), public safety workers (1.18, 95%CI 1.10–1.26), administrators (1.17, 95%CI 1.13–1.22), military personnel (1.16, 95%CI 1.05–1.28), and religious workers (1.17, 95%CI 1.09–1.26). The lowest SIRadj was observed among forestry workers (0.82, 95%CI 0.76–0.88).
Conclusions: Tobacco smoking plays an important role in the occupational variation in the risk of kidney cancer. The smoking-adjusted incidence of kidney cancer was increased in dentists, physicians, journalists, administrators, and public safety workers.
Introduction
Kidney cancer is a common condition that has a considerable impact on global cancer mortality rates [Citation1]. Its most widely recognized risk factors include obesity, hypertension, and end-stage renal disease [Citation2–4]. Furthermore, there is evidence that tobacco smoking is one of the most important risk factors for the disease [Citation5]. A much-debated question is whether any occupational exposures can contribute to the risk of kidney cancer [Citation6-15].
In the field of occupational exposures, most studies have focused on occupational groups or specific agents. Oftentimes, they have failed to address the potential confounding connected with tobacco smoking. Notably, studies based on big datasets, like whole national populations, tend to be limited by lack of data on smoking habits.
The impact of the occupation-specific prevalence of tobacco smoking on the risk of kidney cancer in particular occupational groups remains unknown. This study aimed to examine the smoking-adjusted occupational variation in the incidence of kidney cancer in Nordic males.
Material and methods
Study population
The population of the Nordic Occupational Cancer Study (NOCCA) (described in detail elsewhere [Citation16]) served as a source population for the presented study. The NOCCA population included all individuals aged 30–64, living in the Nordic countries (Denmark, Iceland, Finland, Norway, and Sweden), who participated in at least one of the national censuses conducted between 1960 and 1990. In total, the NOCCA population included 14.9 million individuals (7.4 million men, and 7.5 million women). In this study, we used data on men only. We did not analyze data for women since smoking among them was less prevalent, and the smoking pattern across occupations changed over time from the most frequent in women with high socioeconomic status to the most frequent among those with low socioeconomic status [Citation17]. Therefore, it would be hard to estimate the sum effect of smoking in the female population.
Data on exposure and outcome
Data on exposure (occupation) were obtained from national censuses. The censuses were held in the following years: 1960, 1970, and 1990 in Sweden; 1960, 1970, and 1980 in Norway; 1970, 1980, and 1990 in Finland; 1970 in Denmark; and 1981 in Iceland. All men aged 30–64 during at least one of the censuses were included in the study. For participants of more than one census, the first registered occupation was used. The data were initially coded using national coding schemes. For the NOCCA study, they were uniformly categorized in 53 occupational categories and an additional category of economically inactive men.
The data on the outcome, namely kidney cancer (International Classification of Diseases, 7th Revision 180), were obtained from national cancer registries in the respective countries. The follow-up took place until the day of emigration, death, or 31st December of the following year: 2003 in Denmark and Norway, 2004 in Iceland, and 2005 in Finland and Sweden; whichever came first.
Data on occupation-specific standardized incidence ratios (SIRs) of male lung cancer were obtained from the publication by Pukkala et al. [Citation16]. The SIR was defined as a ratio of the observed to the expected number of cases, with national incidence rates as a reference.
Since no individual-level data on smoking were available, we used occupational-group-level data. Such data were available from Finnish men only. They came from the annual surveys the health behavior of the Finnish adult population carried by the Finnish National Public Health Institute in 1978–1991 [Citation18]. No comparable data from other Nordic countries were available.
Statistical analysis
Based on the occupation-specific smoking prevalence [Citation18] and lung cancer incidence data for Finnish men [Citation16], we calculated a proxy for the occupation-specific smoking prevalence among all Nordic men.
First, we fitted a regression line Y = 0.05 + 2.48X (r2=0.57; ), where X denoted the occupation-specific smoking prevalence, Y the occupation-specific SIR of lung cancer, and where the intercept of 0.05 represented the risk of lung cancer in nonsmokers [Citation19] (Model A). Due to missing data on smoking prevalence, domestic assistants, economically inactive persons, hairdressers, and tobacco workers did not contribute to the model. The model was validated using a jackknife resampling [Citation20].
Figure 1. Association between smoking prevalence and standardized incidence ratio (SIR) of lung cancer in Finnish males.
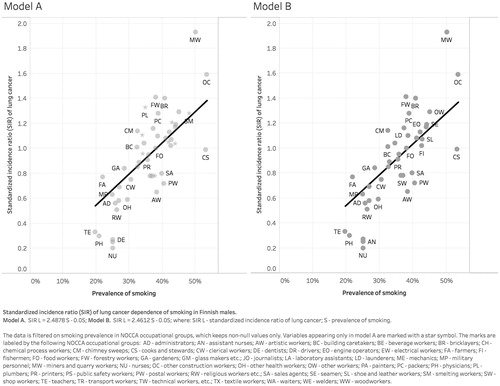
In the second model, we excluded occupations likely to be exposed to lung cancer risk factors other than smoking, e.g., drivers to diesel engine exhaust [Citation21,Citation22]; painters to polycyclic aromatic hydrocarbons [Citation23]; and plumbers to asbestos [Citation24]. We excluded beverage workers, chemical process workers, drivers, electrical workers, painters, plumbers, smelting workers, tobacco workers, and waiters. In the analysis by Haldorsen et al. [Citation25], all of these groups had a smoking adjusted SIR for lung cancer >1.15. The fitted regression line was Y = 0.05 + 2.46X (r2=0.58; ) (Model B). The model was validated using a jackknife resampling [Citation20].
Subsequently, Model B and occupation-specific SIRs of lung cancer for men in the other Nordic countries [Citation16] were used to predict their occupation-specific smoking prevalence, assuming that the association between the occupation-specific smoking prevalence and the occupation-specific risk of lung cancer was similar across the Nordic countries. Using person counts in all occupational groups as weights, we calculated a proxy of national smoking prevalence for all Nordic countries.
The calculation of the occupation-specific smoking-adjusted SIR (SIRadj) of kidney cancer included several steps. First, the national smoking prevalence was subtracted from the smoking prevalence in a given occupational group. Second, the difference was multiplied by the expected number of kidney cancer cases in the given occupation. Third, to calculate the smoking-adjusted expected number of cases, the obtained product was added to/subtracted from the expected number of kidney cancer cases in the given occupation. Finally, SIRadj was calculated as a ratio between the observed number of cases and the smoking-adjusted expected number of cases. The 95% confidence intervals (CI) were calculated assuming a Poisson distribution.
Statistical analysis was performed with Stata/IC 15.0 for Mac (StataCorp LP, College Station, TX, USA).
Results
During the follow-up of 185 million person-years, altogether 50,330 cases of kidney cancer were identified among Nordic men. The highest unadjusted SIRs (>1.15) were observed among waiters (SIR 1.26, 95%CI 1.02–1.53), welders (SIR 1.25, 95%CI 1.14–1.36), cooks and stewards (SIR 1.23, 95%CI 1.05–1.44), public safety workers (SIR 1.16, 95%CI 1.08–1.25), and seamen (SIR 1.16, 95%CI 1.07–1.26) ().
Table 1. The observed number of cases (Obs), crude and smoking-adjusted standardized incidence ratios (SIR) of kidney cancer in Nordic males by occupation.
The highest adjusted SIRs (>1.15) were observed among dentists (SIRadj 1.32, 95%CI 1.06–1.62), journalists (SIRadj 1.20, 95%CI 1.00–1.42), physicians (SIRadj 1.19, 95%CI 1.03–1.36), public safety workers (SIRadj 1.18, 95%CI 1.10–1.26), administrators (SIRadj 1.17, 95%CI 1.13–1.22), military personnel (SIRadj 1.16, 95%CI 1.05–1.28), and religious workers (SIRadj 1.17, 95%CI 1.09–1.26) (). The lowest SIRadj (<0.85) was observed among forestry workers (SIRadj 0.82, 95%CI 0.76–0.88).
In most occupational groups (34 out of 54), the adjusted SIR was closer to 1.0 than the unadjusted SIR (). In the case of 18 occupational groups, SIR changed from above 1.0 to below 1.0, or vice versa.
Discussion
Main research findings
Several studies have indicated that the risk of kidney cancer may be elevated in certain occupations [Citation6, 7, 8, 9, 10, 11, 12, 13Citation6–14]. However, until now, no population-level study controlling for the possible confounding from tobacco smoking has been published. The present study was designed to determine the smoking-adjusted risk of kidney cancer across occupations in Nordic men.
An unexpected finding of this study was the significantly elevated SIRadj among dentists and physicians. To our knowledge, this is the first study reporting such results. Previously, some studies indicated an elevated risk of oral cancer [Citation24] and cutaneous squamous cell carcinoma [Citation25] among dentists, and breast cancer [Citation26] and seminoma [Citation27] among physicians. In contrast to some of these diseases, in the case of kidney cancer, we do not expect that surveillance bias may play an important role. A possible explanation for our findings could be occupational exposure to X-radiation and gamma radiation, classified by the International Agency for Research on Cancer (IARC) as carcinogenic to the human kidney [Citation28]. Further research should be undertaken to obtain a full understanding of these findings.
Another unexpected finding was the elevated SIRadj among journalists, administrators, and religious workers. According to our knowledge, this is the first study reporting such observations. Previously, an increased risk of mouth and pharynx cancer was reported among journalists [Citation24,Citation29,Citation30]. Among administrators and religious workers, elevated risks of ovarian cancer [Citation31], testicular cancer [Citation27,Citation32], skin cancers [Citation25,Citation33], hematological tumors [Citation33,Citation34], and thyroid cancer [Citation33] have been reported. One of the explanations of our findings can be higher body mass index (BMI) observed in these occupational groups, possibly associated with the sedentary nature of their work.
Consistently with previous literature [Citation35–42], we observed an increased risk of kidney cancer among public safety workers. This occupational category included firefighters, police officers, detectives, guards with civil duties, and customs officers. Some of these subgroups are occupationally exposed to diesel fumes, asbestos, and polycyclic aromatic hydrocarbons, previously associated with an increased risk of kidney cancer [Citation43–47].
Another group in which we observed an elevated SIRadj was military workers. In previous literature, an increased risk of certain neoplasms, including prostate cancer, were reported in this occupation [Citation25,Citation48,Citation49]. However, these findings could possibly be attributed to overdiagnosis due to regular screening. In the case of kidney cancer, such a surveillance bias is less likely. The reasons for the observed elevated risk among military workers remain to be investigated.
A possible explanation for some of our findings may be different overweight and obesity prevalence across occupational groups. Extensive research has shown that increased BMI is an independent risk factor for kidney cancer [Citation28]. In a meta-analysis, the estimated pooled risk ratio associated with every 5 kg/m2 increase in BMI was 1.24 (95%CI 1.15–1.34) in men [Citation50].
It is noteworthy that, in Finland, among journalists, military personnel, religious workers, and some of the public safety workers (police officers, guards, customs officers), the fraction of individuals with BMI ≥25 was one of the highest among all occupational groups (fourth quarter). However, among dentists, physicians, and firefighters, the fraction of individuals with BMI ≥25 was one of the lowest (first quarter) [Citation51]. No similar data from other Nordic countries were available.
This study showed how adjustment for a proxy of smoking influenced the SIR of kidney cancer. We noticed changes among tobacco workers, waiters, dentists, nurses, teachers, physicians, seamen, and cooks and stewards. In the case of waiters, seamen, and cooks and stewards, the elevated risk of kidney cancer ceased to be statistically significant. Contrarily, in the case of dentists and physicians the risks became statistically significantly elevated. The largest change was observed for teachers, who, before adjustment for smoking had a risk of kidney cancer below that of other men, but after adjustment had an excess risk.
Strengths and limitations of the study
This is the first study examining smoking-adjusted occupational variation in kidney cancer at the national level. A major advantage of this study was the high completeness and accuracy of cancer registration in Nordic countries [Citation52]. Another strength was the large source population and precise coding of occupations.
A limitation of this study was the fact that the occupational categories were based on the first available census only. This could lead to exposure misclassification, which would bias the observed effects towards the null. However, such a dilution is probably rather small because, at the time of the study, occupational stability was high in the Nordic countries [Citation52,Citation53]. In Finland, for example, overall 85–86% of men had the same occupational branch in 1980–85 as they had in 1975–80, varying from 91% in transport to 82% in administration and manufacture [Citation53].
The study was also limited by the lack of individual-level information on smoking. Residual confounding from smoking is therefore possible.
Conclusions
This study showed that the risk of kidney cancer varies across occupations. Differences in tobacco smoking play an important role in this variation. Smoking-adjusted risk of kidney cancer was increased among dentists, physicians, journalists, administrators, and public safety workers.
Disclosure statement
The authors report no conflict of interest
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Additional information
Funding
References
- IARC. GLOBOCAN 2018 [cited 2019 Nov 20]. Available from: http://globocan.iarc.fr/Default.aspx
- Ildaphonse G, George PS, Mathew A. Obesity and kidney cancer risk in men: a meta-analysis (1992–2008). Asian Pac J Cancer Prev. 2009;10(2):279–286.
- Moore LE, Wilson RT, Campleman SL. Lifestyle factors, exposures, genetic susceptibility, and renal cell cancer risk: a review. Cancer Invest. 2005;23(3):240–255.
- Petejova N, Martinek A. Renal cell carcinoma: review of etiology, pathophysiology and risk factors. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160(2):183–194.
- Hunt JD, van der Hel OL, McMillan GP, et al. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005;114(1):101–108.
- Boffetta P, Fontana L, Stewart P, et al. Occupational exposure to arsenic, cadmium, chromium, lead and nickel, and renal cell carcinoma: a case-control study from Central and Eastern Europe. Occup Environ Med. 2011;68(10):723–728.
- Lohi J, Kyyronen P, Kauppinen T, et al. Occupational exposure to solvents and gasoline and risk of cancers in the urinary tract among Finnish workers. Am J Ind Med. 2008;51(9):668–672.
- Guo J, Kauppinen T, Kyyronen P, et al. Risk of esophageal, ovarian, testicular, kidney and bladder cancers and leukemia among Finnish workers exposed to diesel or gasoline engine exhaust. Int J Cancer. 2004;111(2):286–292.
- Partanen T, Heikkila P, Hernberg S, et al. Renal cell cancer and occupational exposure to chemical agents. Scand J Work Environ Health. 1991;17(4):231–239.
- Mellemgaard A, Engholm G, McLaughlin JK, et al. Occupational risk factors for renal-cell carcinoma in Denmark. Scand J Work Environ Health. 1994;20(3):160–165.
- Michalek IM, Martinsen JI, Weiderpass E, et al. Occupation and risk of cancer of the renal pelvis in Nordic countries. BJU Int. 2019;123(2):233–238.
- Michalek IM, Martinsen JI, Weiderpass E, et al. Occupation and risk of kidney cancer in Nordic countries. J Occup Environ Med. 2019;61(1):41–46.
- Pesch B, Haerting J, Ranft U, et al. Occupational risk factors for renal cell carcinoma: agent-specific results from a case-control study in Germany. MURC Study Group. Multicenter urothelial and renal cancer study. Int J Epidemiol. 2000;29(6):1014–1024.
- Mattioli S, Truffelli D, Baldasseroni A, et al. Occupational risk factors for renal cell cancer: a case-control study in northern Italy. J Occup Environ Med. 2002;44(11):1028–1036.
- Michalek I M, Martinsen J I, Weiderpass E, et al. Heavy metals, welding fumes, and other occupational exposures, and the risk of kidney cancer: A population-based nested case-control study in three Nordic countries. Environ Res. 2019;173:117–123. doi:10.1016/j.envres.2019.03.023.
- Pukkala E, Martinsen JI, Lynge E, et al. Occupation and cancer – follow-up of 15 million people in five Nordic countries. Acta Oncol. 2009;48(5):646–790.
- Pukkala E, Guo J, Kyyronen P, et al. National job-exposure matrix in analyses of census-based estimates of occupational cancer risk. Scand J Work Environ Health. 2005;31(2):97–107.
- Helakorpi S, Patja K, Prättälä R, et al. Health behavior and health among Finnish adult population, spring 2002. Publications of the National Public Health Institute, B12. Helsinki: National Public Health Institute; 2002.
- Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses – United States, 2000–2004. MMWR. 2008;57(45):1226–1228.
- Efron B, Stein C. The jackknife estimate of variance. Ann Stat. 1981;9(3):586–596.
- Haldorsen T, Andersen A, Boffetta P. Smoking-adjusted incidence of lung cancer by occupation among Norwegian men. Cancer Causes Control. 2004;15(2):139–147.
- Kjaerheim K, Martinsen JI, Lynge E, et al. Effects of occupation on risks of avoidable cancers in the Nordic countries. Eur J Cancer. 2010;46(14):2545–2554.
- Tarvainen L, Suojanen J, Kyyronen P, et al. Occupational risk for oral cancer in Nordic countries. Anticancer Res. 2017;37(6):3221–3228.
- Alfonso JH, Martinsen JI, Pukkala E, et al. Occupation and relative risk of cutaneous squamous cell carcinoma (cSCC): a 45-year follow-up study in 4 Nordic countries. J Am Acad Dermatol. 2016;75(3):548–555.
- Haldorsen T, Martinsen JI, Kjaerheim K, et al. Adjustment for tobacco smoking and alcohol consumption by simultaneous analysis of several types of cancer. Cancer Causes Control. 2017;28(2):155–165.
- Katuwal S, Martinsen JI, Kjaerheim K, et al. Occupational variation in the risk of female breast cancer in the Nordic countries. Cancer Causes Control. 2018;29(11):1027–1038.
- Ylonen O, Jyrkkio S, Pukkala E, et al. Time trends and occupational variation in the incidence of testicular cancer in the Nordic countries. BJU Int. 2018;122(3):384–393.
- IARC. IARC monographs on evaluation of carcinogenic risk to humans. Lyon, France: WHO/IARC; 2006.
- Tarvainen L, Kyyronen P, Kauppinen T, et al. Cancer of the mouth and pharynx, occupation and exposure to chemical agents in Finland [in 1971–95]. Int J Cancer. 2008;123(3):653–659.
- Kanerva L, Tarvainen K, Leino T. Toluene sulfonamide-formaldehyde resin allergy simulating nickel dermatitis. Eur J Dermatol. 1995;5(2):149–150.
- Le ND, Leung A, Brooks-Wilson A, et al. Occupational exposure and ovarian cancer risk. Cancer Causes Control. 2014;25(7):829–841.
- Yousif L, Hammer GP, Emrich K, et al. Occupational risk factors for testicular cancer: a registry-based case-control study in Rhineland Palatinate–Germany. Ger Med Sci. 2013;11:Doc16
- Stang A, Martinsen JI, Kjaerheim K, et al. Cancer incidence among priests: 45 years of follow-up in four Nordic countries. Eur J Epidemiol. 2012;27(2):101–108.
- Schenk M, Purdue MP, Colt JS, Hartge P, et al. Occupation/industry and risk of non-Hodgkin’s lymphoma in the United States. Occup Environ Med. 2009;66(1):23–31.
- Kang D, Davis LK, Hunt P, et al. Cancer incidence among male Massachusetts firefighters, 1987–2003. Am J Ind Med. 2008;51(5):329–335.
- Ide CW. Cancer incidence and mortality in serving whole-time Scottish firefighters 1984–2005. Occup Med (Lond). 2014;64(6):421–427.
- Baris D, Garrity TJ, Telles JL, et al. Cohort mortality study of Philadelphia firefighters. Am J Ind Med. 2001;39(5):463–476.
- Glass DC, Del Monaco A, Pircher S, et al. Mortality and cancer incidence among male volunteer Australian firefighters. Occup Environ Med. 2017;74(9):628–638.
- Glass DC, Pircher S, Del Monaco A, et al. Mortality and cancer incidence in a cohort of male paid Australian firefighters. Occup Environ Med. 2016;73(11):761–771.
- Ma F, Lee DJ, Fleming LE, et al. Race-specific cancer mortality in US firefighters: 1984–1993. J Occup Environ Med. 1998;40(12):1134–1138.
- Tsai RJ, Luckhaupt SE, Schumacher P, et al. Risk of cancer among firefighters in California, 1988–2007. Am J Ind Med. 2015;58(7):715–729.
- Kleinman EJ, Christos PJ, Gerber LM, et al. NYPD cancer incidence rates 1995–2014 encompassing the entire world trade center cohort. J Occup Environ Med. 2015;57(10):e101–e13.
- Harrison TR, Muhamad JW, Yang F, et al. Firefighter attitudes, norms, beliefs, barriers, and behaviors toward post-fire decontamination processes in an era of increased cancer risk. J Occup Environ Hyg. 2018;15(4):279–284.
- Baxter CS, Hoffman JD, Knipp MJ, et al. Exposure of firefighters to particulates and polycyclic aromatic hydrocarbons. J Occup Environ Hyg. 2014;11(7):D85–D91.
- Driscoll TR, Carey RN, Peters S, et al. The Australian Work Exposures Study: prevalence of occupational exposure to formaldehyde. Ann Occup Hyg. 2016;60(1):132–138.
- Stec AA, Dickens KE, Salden M, et al. Occupational exposure to polycyclic aromatic hydrocarbons and elevated cancer incidence in firefighters. Sci Rep. 2018;8(1):2476.
- Melius J. Occupational health for firefighters. Occup Med. 2001;16(1):101–108.
- Barry KH, Martinsen JI, Alavanja MCR, et al. Risk of early-onset prostate cancer associated with occupation in the Nordic countries. Eur J Cancer. 2017;87:92–100.
- Laukkala T, Parkkola K, Henriksson M, et al. Total and cause-specific mortality of Finnish military personnel following service in international peacekeeping operations 1990–2010: a comprehensive register-based cohort study. BMJ Open. 2016;6(10):e012146.
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. The Lancet. 2008;371(9612):569–578.
- Helakorpi S, Patja K, Prättälä R, et al. Health behavior and health among Finnish adult population, spring 2002. Publications of the National Public Health Institute, B12. Helsinki: National Public Health Institute; 2002.
- Pukkala E. Biobanks and registers in epidemiologic research on cancer. Methods Mol Biol. 2011;675:127–164.
- Notkola V, Pajunen A, Leino-Arjas P. Occupational mortality by cause in Finland 1971–1991 and occupational mobility. Helsinki: Statistics Finland; 1997.

