Introduction
Biphosphonate (BP)-related osteonecrosis of the jaw (BRONJ) was first reported in 2003 [Citation1] and affects approximately 5% of patients with multiple myeloma (MM), breast or prostate metastatic cancer treated with BP [Citation2]. Biphosphonate is a class of drug frequently used for the prevention or treatment of resorptive bone disease, commonly observed in MM [Citation3], bone metastases [Citation4], and osteoporosis [Citation5]. Biphosphonate reduces bone resorption by suppressing osteolytic cell activity, delay the time of onset of skeletal-related events and reduce pain in patients with bone metastases [Citation6,Citation7].
Risk factors for BRONJ are category and dose of BP, among others. High dose (4 mg every 3–4 weeks) of zoledronate, recommended for MM, is more frequently associated with BRONJ than lower dose (5 mg once a year), used for osteoporosis [Citation7]. The BP category more commonly associated with BRONJ is the more potent nitrogen-containing BP (zoledronate, pamidronate, and ibandronate). Besides, BRONJ usually occurs late after beginning of drug exposure, with a median of 11 doses of zoledronate [Citation8]. Other risk factors are tooth extraction, local trauma, pre-existing dental infection, use of corticosteroids, anaemia, diabetes mellitus, and smoking, among others [Citation7]. BRONJ signs and symptoms include exposed necrotic bone, local pain, soft tissue swelling, cellulitis, hypoesthesia/paraesthesia in lower lip or chin, halitosis, and fistula [Citation2,Citation8]. Treatment of BRONJ is conservative for the majority of patients, however, when conservative measures have not been, or are considered unlikely to be, successful, surgery can be indicated to remove the necrotic bone and, in more extensive bone necrosis, to resect and reconstruct the jaw [Citation9,Citation10]. Nevertheless, prognosis of BRONJ remains poor, as only 30% of patients with this condition present favourable resolution [Citation8]. Because of this, new therapies for BRONJ, such as platelet-rich plasma [Citation11] and, possibly, mesenchymal stromal cells (MSCs), are warranted.
Hernigou et al. [Citation12] published the first report of autologous bone marrow cells injection for hip osteonecrosis. Afterwards, the use of MSCs separated from bone marrow and expanded ex vivo became a relatively common therapy for osteonecrosis of the femoral head, with promising results. Andriolo et al. [Citation13] published recently a systematic review in which regenerative therapies, which included MSCs, conferred a significant survival advantage for patients with necrosis of the femoral head compared with the standard therapy of core decompression.
Herein we described two cases of patients with BRONJ successfully treated with autologous MSCs, collected from bone marrow, expanded ex vivo and applied onto the area affected by necrosis. Institutional Review Board approved the study and the patients signed the Informed Consent authorising publication of results.
Methods
Case 1
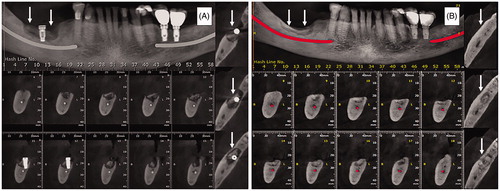
A 68-year-old man was diagnosed with MM and treated with six cycles of cyclophosphamide, bortezomib, and dexamethasone. Besides, he received 12 doses of zoledronic acid (4 mg at 4-week intervals) for bone disease. Two years after diagnosis of MM and after implant installation in jaw, the patient started complaining of local pain. Besides the continuous pain, the patient presented several episodes of local infection, when he was diagnosed as having stage 2 medication-related osteonecrosis on the mandibular body, at right side. Then, bisphosphonate was discontinued. Because the patient had no improvement of osteonecrosis with conservative treatment, which consisted of debridement, washing and antibiotics, it was decided to implant MSCs locally. Briefly, MSCs were obtained from bone marrow, which was harvested from posterior iliac crest (approximately 20 mL). Mononuclear cells were separated using Ficoll-Paque (GE Healthcare, BioSciences, Uppsala, Sweden) 1.077 density gradient centrifugation technique and were suspended in alpha-minimum essential medium (a-MEM—GIBCO, New York, EUA) supplemented with 10% foetal bovine serum (v/v) (FBS) (HyClone, Canada), 1% penicillin/streptomycin (GIBCO, NY), and 2 mM glutamine (GIBCO, New York, EUA). MSCs culture, characterisation and control tests were performed as described previously [Citation14]. MSCs expressed surface markers CD90, CD73, CD105, CD146, CD166 and HLA class I, assessed by flow cytometry after culturing, and did not express CD34, CD14, CD45, CD31 or HLA-DR. Besides, MSCs were shown capable of osteogenic, chondrogenic and adipogenic differentiation in vitro. Cytogenetic analyses (20 metaphases evaluated) of final product showed no alteration. Final product was negative for bacteria, mycoplasm, and endotoxin. MSCs were cryopreserved in passage 3 and kept frozen in vapour phase of liquid nitrogen tank until the moment of use, as previously described [Citation15]. After removal of necrotic bone and dental implant, a total of 0.6 × 106 MSCs was embedded into one small piece of bone substitute (Geistlich Bio-Oss® Collagen) and exposed for 6 days to medium with ascorbic acid, ß-glycerol-phosphate and dexamethasone to induce osteoblastic differentiation [Citation16], before implantation into the cavity formed after removal of necrotic bone. After that, 30 × 106 MSCs (approximately 3 ml solution) were injected around the piece of Bio-Oss, after which the wound was closed. The patient was also treated with amoxicillin + clavulanic acid, initiated 2 days before procedure and maintained for a total of 15 days. The patient presented complete wound healing and adequate bone regeneration, as can be observed in a computerised tomography performed approximately 6 months after cell application (). After cell therapy, the patient had no more local pain or infection. Two months after the patient presented signs of relapse of MM and is now under a second-line treatment for this disease.
Case 2
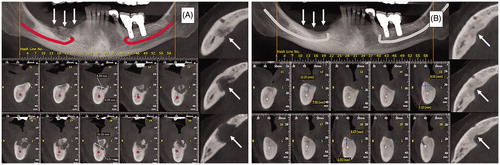
A 66-year-old woman was diagnosed with metastatic breast cancer and treated with 17 doses of zoledronic acid (4 mg at 4-week intervals), after which it was diagnosed stage 2 osteonecrosis of jaw with no triggering event identified. The patient presented local pain and swelling, and pus exudation difficult to control with conservative therapy (oral and intravenous antibiotics, local debridement and washing). MSCs were harvested and expanded as described for patient 1, however, the cells were not exposed to differentiation medium. MSC product presented the same characteristics as that described for patient 1. A total of 110 × 106 MSCs (approximately 3 ml) was injected into the cavity, with no previous removal of necrotic bone. Also, spontaneous elimination of necrotic bone did not occur. As described for patient 1, this patient was also treated with amoxicillin + clavulanic acid, initiated 2 days before procedure and maintained for a total of 15 days. Six months after injection of MSCs it is possible to observe almost complete bone regeneration by computerised tomography (). After cell therapy, the patient presented no more local pain, swelling or infection.
Figure 2. Case 2: (A) Immediate preoperative. (B) Six months after therapy. Arrows indicate the site of bone necrosis and where mesenchymal cells were applied.
Both patients are still under follow-up and show no signs or symptoms in the necrosis area 14 and 12 months after MSCs implant for cases 1 and 2, respectively.
Discussion
BRONJ can be a most severe complication of cancer treatment. It is commonly painful and favours local infection, especially in an immunosuppressed patient, in whom it can be devastating. Management of BRONJ is usually conservative, which consists of washing, debridement, and antibiotics, and aims mainly pain and infection control [Citation17]. This approach is warranted if the necrosis area is relatively small and stable. However, when osteonecrosis affects a large or increasing area, a surgical management is usually recommended. Surgery aims debridement, removal of necrotic bone, smoothing of bony edges, and wound closure [Citation18,Citation19]. Despite a highly variable response rate of 30–90%, many patients present a worsening clinical picture [Citation8,Citation19,Citation20]. Treatment options for patients with BRONJ unresponsive to conventional therapy are limited. One possible option is the use of MSCs, as we believe to have shown herein.
MSCs are a heterogenous cell population that are constituents of organ and tissue stroma. These cells adhere spontaneously to plastic, a property that is useful to separate MSCs from other non-adherent mononuclear cells, express on membrane a typical immunophenotypic profile (CD44, CD73, CD90, CD105) and lack HLA-DR and markers expressed on haemopoietic cells, such as CD45, CD34, and CD14 [Citation21]. Besides, MSCs can differentiate into osteogenic lineage (and chondrogenic and adipogenic) [Citation22], a characteristic that can be explored to regenerate bone necrosis, such as femoral head necrosis. Systematic reviews published recently evaluated cell therapies for this condition. Most papers cited evaluated the effect of autologous bone marrow, which contains a small amount of MSCs, cells injected into femoral head [Citation23]. Some studies showed positive outcomes with the administration of MSCs for this condition [Citation13]. For instance, Ayoama et al. [Citation24] evaluated 10 patients with stage 3 osteonecrosis of femoral head who received locally MSCs obtained from bone marrow. The authors showed increase of bone volume from 56.5 ± 8.5 cm3 to 57.7 ± 10.6 cm3 and improved clinical score (Japan Orthopaedic Association) from 65.6 ± 25.5 points to 87.9 ± 19.0 points [Citation24].
Cella et al. [Citation25] reported a case of BRONJ in a patient with osteoporosis treated with autologous unselected bone marrow cells implanted locally, with total bone healing [Citation22]. Voss et al. [Citation26] treated six patients with BRONJ with autologous MSCs, obtained from bone marrow, mixed with thrombin. The authors reported that all six patients had satisfactory wound healing. However, it is not clear whether MSCs were expanded ex vivo as was the case here, a measure that increases MSCs number, which, theoretically, would favour bone healing. Furthermore, Kaibuchi et al. demonstrated good results with allogeneic MSCs in a canine model of BRONJ [Citation27].
Because of these previous results, our group decided to implant autologous MSCs, expanded ex vivo for 3–4 weeks to improve cell purity and increase their number, into the jaw affected area in two patients with BRONJ refractory to conventional treatment and with worsening clinical picture, with pain, swelling, and recurrent local infection. Herein we have shown that MSCs injection can ameliorate, even heal the wound, a potential devastating complication in patients with cancer. Removal of necrotic bone and of dental implant, in case 1, could have contributed to the favourable results reported here, however, would probably have been insufficient to promote bone healing. Nevertheless, in case 2, necrotic bone was not removed before MSC injection not even occurred spontaneous sequestration. We believe that autologous MSCs administration can be an option for BRONJ refractory to conventional treatment. The patient 1 was treated with Bio-Oss embedded with MSCs and with direct injection of these cells around the implant. It is debateable whether Bio-Oss in itself could have been the main responsible for bone regeneration, however, in case 2, MSCs were injected directly, without Bio-Oss, and bone regeneration was at least as good as that observed in case one, a finding that allow us to conclude that MSCs were the main factor that induced bone regeneration.
In conclusion, we believe that autologous MSCs expanded ex vivo seems to be a valuable alternative treatment option for BRONJ as the two cases reported here presented promising results. Because of these findings, we recommend randomised controlled trial to evaluate the efficacy of this new therapy.
References
- Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117.
- Ryan P, Saleh I, Stassen L. Osteonecrosis of the jaw: a rare and devastating side effect of bisphosphonates. Postgrad Med J. 2009;85:674–677.
- Mhaskar R, Kumar A, Miladinovic B, et al. Bisphosphonates in multiple myeloma: an updated network meta-analysis. Cochrane Database Syst Rev. 2017;12:CD003188.
- O’Carrigan B, Wong MH, Willson ML, et al. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2017;10:CD003474.
- Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393:364–376.
- Fleisch H, Fast D, Rizzoli R, et al. Diphosphonates. Mode of action and clinical applications. Adv Exp Med Biol. 1977;81:279–289.
- Otto S, Pautke C, Van den Wyngaert T, et al. Medication-related osteonecrosis of the jaw: prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat Rev. 2018;69:177–187.
- Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23:1341–1347.
- Nisi M, La Ferla F, Karapetsa D, et al. Conservative surgical management of patients with bisphosphonate-related osteonecrosis of the jaws: a series of 120 patients. Br J Oral Maxillofac Surg. 2016;54:930–935.
- Silva LF, Curra C, Munerato MS, et al. Surgical management of bisphosphonate-related osteonecrosis of the jaws: literature review. Oral Maxillofac Surg. 2016;20:9–17.
- Curi MM, Cossolin GS, Koga DH, et al. Treatment of avascular osteonecrosis of the mandible in cancer patients with a history of bisphosphonate therapy by combining bone resection and autologous platelet-rich plasma: report of 3 cases. J Oral Maxillofac Surg. 2007;65:349–355.
- Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;(405):14–23.
- Andriolo L, Merli G, Tobar C, et al. Regenerative therapies increase survivorship of avascular necrosis of the femoral head: a systematic review and meta-analysis. Int Orthop. 2018;42:1689–1704.
- Dos Santos VT, Mizukami A, Orellana MD, et al. Characterization of human AB serum for mesenchymal stromal cell expansion. Transfus Med Hemother. 2017;44:11–21.
- de Lima Prata K, de Santis GC, Orellana MD, et al. Cryopreservation of umbilical cord mesenchymal cells in xenofree conditions. Cytotherapy. 2012;14:694–700.
- Barba-Recreo P, Del Castillo Pardo de Vera JL, Georgiev-Hristov T, et al. Adipose-derived stem cells and platelet-rich plasma for preventive treatment of bisphosphonate-related osteonecrosis of the jaw in a murine model. J Craniomaxillofac Surg. 2015;43:1161–1168.
- Ristow O, Rückschloß T, Müller M, et al. Is the conservative non-surgical management of medication-related osteonecrosis of the jaw an appropriate treatment option for early stages? A long-term single-center cohort study. J Craniomaxillofac Surg. 2019;47:491–499.
- Ruggiero SL, Dodson TB, Fantasia J, et al. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg. 2014;72 :1938–1956.
- Lopes RN, Rabelo GD, Rocha AC, et al. Surgical therapy for bisphosphonate-related osteonecrosis of the jaw: six-year experience of a single institution. J Oral Maxillofac Surg. 2015;73:1288–1295.
- Abu-Id MH, Warnke PH, Gottschalk J, et al. Bis-phossy jaws” - high and low risk factors for bisphosphonate-induced osteonecrosis of the jaw. J Craniomaxillofac Surg. 2008;36:95–103.
- Noronha NC, Mizukami A, Caliári-Oliveira C, et al. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10:131.
- Valenti MT, Dalle Carbonare L, Mottes M. Osteogenic differentiation in healthy and pathological conditions. IJMS. 2016;18:41.
- Papakostidis C, Tosounidis TH, Jones E, et al. The role of “cell therapy” in osteonecrosis of the femoral head. A systematic review of the literature and meta-analysis of 7 studies. Acta Orthop. 2016;87:72–78.
- Aoyama T, Goto K, Kakinoki R, et al. An exploratory clinical trial for idiopathic osteonecrosis of femoral head by cultured autologous multipotent mesenchymal stromal cells augmented with vascularized bone grafts. Tissue Eng Part B Rev. 2014;20:233–242.
- Cella L, Oppici A, Arbasi M, et al. Autologous bone marrow stem cell intralesional transplantation repairing bisphosphonate related osteonecrosis of the jaw. Head Face Med. 2011;7:16.
- Voss PJ, Matsumoto A, Alvarado E, et al. Treatment of stage II medication-related osteonecrosis of the jaw with necrosectomy and autologous bone marrow mesenchymal stem cells. Odontology. 2017;105:484–493.
- Kaibuchi N, Iwata T, Onizuka S, et al. Allogeneic multipotent mesenchymal stromal cell sheet transplantation promotes healthy healing of wounds caused by zoledronate and dexamethasone in canine mandibular bones. Regen Ther. 2019;10:77–83.