Introduction
Head and neck cancer (HNC) is the sixth most frequent malignancy worldwide among adults [Citation1,Citation2]. However, HNC is infrequent in adolescents and young adults and HNC accounts for approximately 8% of cancers in patients aged 15–24 years [Citation3,Citation4]. Previous studies suggest an increase in the incidence of HNC in young adults [Citation5,Citation6], while information on trends in incidence in adolescents is limited. Further, studies of the general trend of HNC in a nationwide, population-based setting are sparse. We present a nation-wide study addressing the trends in incidence of HNC in adolescents and young adults in Denmark throughout the last four decades.
Methods
The population of Denmark is approximately 5.5 million inhabitants of which 13% are between the ages of 15 to 24 years. All patients aged 15 to 24 years registered with a primary HNC during 1st of January 1978 and 31st of December 2014 in the Danish Cancer Registry (DCR) were included. Information on anatomical cancer location, histology, age at diagnosis, and date of diagnosis was obtained. The DCR has a high degree of completeness [Citation7–10]. A primary head and neck cancer was defined as an onset of malignancy above the clavicle and not involving the skin, orbita, or central nervous system. Only primary HNCs were included as HNC, thus relapse of HNC in the head and neck region was excluded and non of the included patient had more than one HNC. Secondary primary HNCs were also included as a new primary HNC. Patients were divided into eleven groups according to anatomical cancer location: oral cavity, oropharynx, nasopharynx, hypopharynx, sinonasal cavity, middle ear, soft tissue, thyroid, major salivary glands, larynx, and other (ICD-10 codes: DC019-DC119, DC129, DC130-DC149, DC300, DC301, DC310-DC319, DC320-DC329, DC330-DC339, DC490, DC499, DC739, DC755, and DC760). Further, the DCR provides a dual classification code combining morphology (MORPHO-3) and topography (TOPO-3) using the ICD-O-3 code as published by the World Health Organization [Citation11]. Cases were included based on the ICD-10 registration (TOPO-3 codes), and corresponding histology was assessed via the MORPHO-3 codes. Based on histological diagnosis patients were categorized into five histology groups: Squamous cell carcinoma, sarcomas, thyroid carcinomas, salivary gland carcinomas and carcinoma of unspecified origin. We excluded patients with a metastasis in the head and neck region (e.g. not a primary HNC tumor) or hematological disease.
Gender specific average annual percentage change (AAPC) in incidence and gender specific age-adjusted incidence rates (AAIR) were calculated using a single logistic regression model for frequencies by gender, age, and calendar year. Linear terms for age and calendar year by gender were selected in a forward search to identify trends and join points.
When the slope of the whole trend was statistically significant (p < .05), the trend was described as significantly increasing or decreasing [Citation12,Citation13]. AAIR were calculated using logistic regression modeling with stepwise linear gender-specific age and time effects. Calculations were made using SAS University Edition 2017 including the WHO World Standard Population as reference. Age-specific population counts were derived from the Danish National Statistical Database (Statistikbanken) [Citation14]. Descriptive analyses were performed using IBM SPSS 24 (IBM, SPSS, Chicago, IL).
Results
Patient characteristics
In total 424 patients aged 15 to 24 years were diagnosed with HNC in the period 1978–2014. Of the included patients 258 patients had thyroid cancer (60.8%). The median age at diagnosis was 21 years (Q1–3: 16–23) for patients with thyroid cancer. The most frequent location of HNC in adolescents and young adults not including the patient with thyroid cancer was cancer in the nasopharynx (10.4%) followed by salivary gland cancer (9.4%). Their median age at the time of diagnosis for this group was 20 (Q1–3: 17–23).
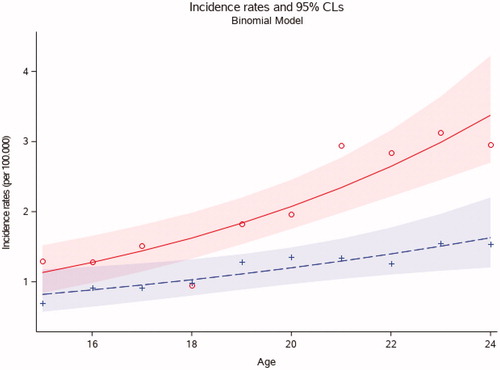
The rate of HNC increased with age, being more frequent in patients aged 20–24 comparing to those aged 15–19 years (p < .01) in both the thyroid cancer patients and in the patients with HNC not including thyroid cancer.
Among patients with HNC the most common histological diagnoses were papillary thyroid carcinomas (48.3%), followed by follicular thyroid carcinomas (6.1%) and squamous cell carcinoma (6.1%).
Incidence
The AAPC for the overall cohort was 3.1 (95% CI 2.2; 4.1). A constant increase in HNC was observed with no significant change in AAPC observed during 1978 to 2014 (p = .3). Stratified according to gender, males had a higher AAPC of 3.4% (95% CI 2.3; 4.6) compared to females with an AAPC of 2.7% (95% CI 1.2; 4.2), however not statistically significant (p = .4).
The AAPC for the cohort of patients with thyroid cancer was 3.5% (95% CI 2.3; 4.7) and the AAPC for patients with head and neck cancer not including thyroid cancer was 2.5% (95% CI 1.1; 4.0) with no significant difference between the two groups (p = .32).
The AAIR of HNC was however significantly higher in females compared to males throughout the time period (p < .01) (). The incidence of HNC among females was 3.7 (95% CI 2.0; 6.2) per 100,000 person-years in 2014 compared to 1.9 (95% CI 0.5; 3.8) per 100,000 person-years among males. Significantly more patients with HNC were in the age group of 20–24 year old compared to 15–19 year olds (p < .01) ().
Figure 1. Age-adjusted incidence rates of head and neck cancer in females and males, aged 15–24 years, in Denmark from 1978 to 2014 (n = 424). The figure shows incidence rates per calendar year. Red and circles represent females. Blue and cross represent males.
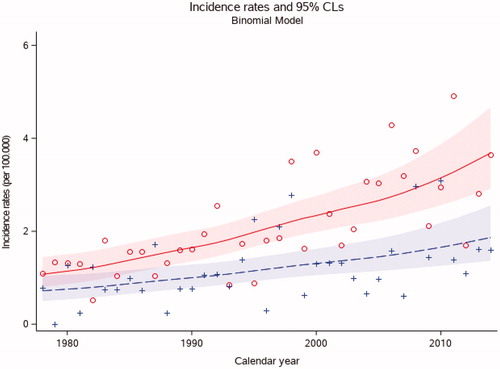
Figure 2. Age-adjusted incidence rates of head and neck cancer in patients, aged 15–24 years, in Denmark from 1978 to 2014 stratified on age at diagnosis (n = 424). The figure shows incidence rates stratified on ages. Each red circle represents the incidence rate for females in each age group and each blue cross represents the incidence rate for males in each age group (15 to 24 years).
The AAIR for patients with thyroid cancer increased from 0.5 per 100,000 person-years in 1978 to 2.2 per 100,000 person-years in 2014, corresponding to four patients with thyroid cancer in 1978 and 16 patients with thyroid cancer in 2014. For the patient with a head and neck cancer, not including thyroid cancer, the AAIR remained stable with an AAIR of 0.4 per 100,000 person-years in 1978 to 0.4 per 100,000 person-years in 2014, corresponding three patients in 1978 and three patients in 2014.
The registration to the DCR became mandatory in 1987. To investigate if the altered registration effected the incidence, the AAPC in the period 1978–1986 were compared to the AAPC of the period 1987–2014. No significant difference (p = .6) between the AAPCs was observed between the two time periods.
Histology
To assess trends in the AAIR, we compared the time periods of 1978–2000 to 2001–2014 stratified on tumor location and histology, and compared the age groups 15–19 years and 20–24 years. Based on MORPHO-3 registration, we observed a significant increase in the incidence for thyroid cancer between 1978–2000 and 2001–2014, with an AAIR of 18.02 in 1978–2000 and 18.42 in 2001–2014 (p < .01), respectively.
Location
Stratified on anatomical location we observed a significant increase in incidence for thyroid and soft tissue malignancies (p < .01) when comparing the time periods 1978–2000 to 2001–2014. When comparing the age groups 15–19 years old to 20–24 years old we found a significant increase in incidence in the 20–24 years old at the locations of thyroid (p < .001) and the oral cavity (p = .02).
Discussion
This nationwide population-based study comprising four decades, describes the trends in incidence of HNC among adolescents and young adults.
We observed a steady increase in HNC among adolescents and young adults during the last 35 years mainly explained by an increase in females diagnosed with papillary thyroid carcinoma. The reason for the increased incidence of HNC in adolescents and young adults remains unclear. We did not find an increase of incidence for squamous cell carcinomas.
Development of secondary malignancies is important to consider; e.g. the late effect of chemo-radiation treatment due to haematological malignancies could take part in the rise of thyroid cancer. Our study group has previously investigated secondary primary cancers in pediatric patients and found that 2.6% of pediatric HNC patients have secondary malignancy, thus a small part of the included HNCs might be secondary maligancies [Citation15]. Further studies to clarify the relation between secondary malignancy and the increase in incidence of HNC this warranted. Furthermore, genetic factors might also play a significant role in the progress of cancers in younger patients. Finally, unknown factors such as impaired ability to repair damaged DNA and an altered ability to metabolize carcinogens may play a role in the development of HNC [Citation5,Citation16]. During the study period indications of benign thyroid surgery and surgical capacity increased, and knowing that the risk of malignancy in thyroid nodules with previous benign or no fine-needle-aspiration are as high as 7.6 and 6.8% in Denmark [Citation17], the increase in benign surgery could contribute to the increase of thyroid cancers. It should also be considered if general medical improvements attribute the rise in incidence; e.g. improvements in diagnostic intensity being detailed histopathological examination, advances within immunohistochemistry and improvements as to imaging modalities increasing the rate of incidentalomas. When addressing incidence of thyroid cancers in the age-group 0–24, we find in line with our findings a significant increase for the total group of thyroid cancers explained by an increase in the group of 18–24 year old with papillary histology [Citation18–21].
Exposure to known risk factors for HNC such as alcohol consumption and tobacco use is short in this age-group and traditionally related to the development of squamous cell carcinomas. This corresponds with the findings that the incidences of squamous cell carcinomas are not increasing in our patient group. This is similar to other reports on the incidence of squamous cell carcinomas in young adults [Citation22–24]. However studies describing an increase in incidence have been published and further studies to clarify this is warranted.
This study is a large, non-selected nationwide study covering the entire Danish population. Denmark provides universal, tax-financed healthcare with free access for all citizens with uniform standards of treatment diminishing referral- and selection-bias, which provides an excellent opportunity to investigate national trends regarding HNC. A strength of this study is thus the non-selected group of patients in a definite geographical area which provides an accurate description of the actual trends in incidence of HNC. A limitation of our study is that classification and registration to the DCR are based on accuracy of clinicians and diagnostic misclassification may occur. Further, anamnestic information on risk factors such as smoking and alcohol exposure was not available which could potentially have aided in the discussion of the causality of the increased incidence of HNC. We did not observe a significant increase in AAPC after 1987 where registration to the DCR became mandatory. Because of the constant AAPC we do not attribute the increase in HNC for this age group to be a registration bias.
In conclusion, we report a description of the increasing incidence of HNC in adolescents and young adults in Denmark between 1978 and 2014 for patients with thyroid cancer. This study highlights the importance of increase awareness of thyroid and soft tissue malignancy in the group of adolescents. Further studies on risk factors for this patient group is necessary.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386.
- Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26(2):123–141.
- Aben KK, Van Gaal C, Van Gils NA, et al. Cancer in adolescents and young adults (15-29 years): a population-based study in the Netherlands 1989-2009. Acta Oncol (Madr). 2012;51(7):922–933.
- Goldstein DP, Irish JC. Head and neck squamous cell carcinoma in the young patient. Curr Opin Otolaryngol Head Neck Surg. 2005;13(4):207–211.
- Annertz K, Anderson H, Biörklund A, et al. Incidence and survival of squamous cell carcinoma of the tongue in Scandinavia, with special reference to young adults. Int J Cancer. 2002;101(1):95–99.
- Majchrzak E, Szybiak B, Wegner A, et al. Oral cavity and oropharyngeal squamous cell carcinoma in young adults: a review of the literature. Radiol Oncol. 2014;48(1):1–10.
- Storm HH, Michelsen EV, Clemmensen IH, et al. The Danish Cancer Registry–history, content, quality and use. Dan Med Bull [Internet]. 1997;44:535–539.
- Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7 Suppl):42–45.
- Jensen A R, Overgaard J, Storm HH. Validity of breast cancer in the Danish Cancer Registry. A study based on clinical records from one county in Denmark. Eur J Cancer Prev. 2002;11(4):359–364.
- Ording AG, Nielsson MS, Frøslev T, et al. Completeness of breast cancer staging in the Danish Cancer Registry, 2004–2009. Clin Epidemiol. 2012;4:11–16.
- Booth JC. International Classification of Diseases for Oncology (ICD-0). Pathol. 1978;10:202–203.
- Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Statist Med. 2000;19(3):335–351.
- Fay MP, Tiwari RC, Feuer EJ, et al. Estimating average annual percent change for disease rates without assuming constant change. Biometrics. 2006;62(3):847–854.
- National Statistical Database (Statistikbanken). [cited 2020 Jan]. Available from: https://www.statistikbanken.dk/statbank5a/default.asp?w=1920
- Jensen JS, Grønhøj C, Kjaer EKR, et al. Second primary cancers in pediatric head and neck cancer survivors in Denmark during 1980-2014: a nationwide study. Int J Pediatr Otorhinolaryngol. 2019;127:109648
- Scully C, Field JK, Tanzawa H. Genetic aberrations in oral or head and neck squamous cell carcinoma (SCCHN): 1. Carcinogen metabolism, DNA repair and cell cycle control. Oral Oncol. 2000;36(3):256–263.
- Gram SB, Rasmussen JH, Feldt-Rasmussen U, et al. Risk of thyroid cancer in 1504 patients referred for thyroid surgery with assumed benign histology. Eur Thyroid J. 2019;8(5):246–255.
- Schmidt Jensen J, Grønhøj C, Mirian C, et al. Incidence and Survival of thyroid cancer in children, adolescents, and young adults in Denmark: a nationwide study from 1980 to 2014. Thyroid. 2018;28(9):1128–1133.
- Mirian C, Grønhøj C, Jensen DH, et al. Trends in thyroid cancer: retrospective analysis of incidence and survival in Denmark 1980-2014. Cancer Epidemiol. 2018;55:81–87.
- Qian ZJ, Jin MC, Meister KD, et al. Pediatric thyroid cancer incidence and mortality trends in the United States, 1973-2013. JAMA Otolaryngol Head Neck Surg. 2019;145(7):617.
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295(18):2164.
- Chow CW, Tabrizi SN, Tiedemann K, et al. Squamous cell carcinomas in children and young adults: a new wave of a very rare tumor? J Pediatr Surg. 2007;42(12):2035–2039.
- Myers JN, Elkins T, Roberts D, et al. Squamous cell carcinoma of the tongue in young adults: increasing incidence and factors that predict treatment outcomes. Otolaryngol Head Neck Surg. 2000;122(1):44–51.
- Modh A, Gayar OH, Elshaikh MA, et al. Pediatric head and neck squamous cell carcinoma: patient demographics, treatment trends and outcomes. Int J Pediatr Otorhinolaryngol. 2018;106:21–25.

