?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Electrochemotherapy (ECT) is an established treatment for primary and secondary cutaneous tumours. The method combines chemotherapy with electroporation, thus increasing the cytotoxic effect of the chemotherapeutic drug. Bleomycin is the drug of choice for ECT, as it is already well established as a treatment for several cancer types and has the largest increase in efficacy after electroporation, enhancing the cytotoxic effect several hundred fold. The response rates of ECT have over the past 30 years been high and consistent. Case based reports point out that the efficacy possibly can be maintained even when the dose of bleomycin is reduced. Consequently in 2018, studies began investigating reducing the bleomycin dose.
Aim
The purpose of this review is to summarise all data published using intravenous bleomycin for cutaneous malignancies and is to our knowledge the first review to examine the use of a reduced bleomycin dose in ECT.
Methods
This study is a systematic review. Fifty-five clinical studies investigating ECT with intravenous bleomycin for patients with cutaneous malignancies were included.
Results
Studies published from 1993 to 2021 investigating the effect of ECT include 3729 patients and indicate a consistent and high response with a mean objective response rate (ORR) of 81.5%. Interestingly, studies using lower doses of bleomycin observe a similar ORR (85.5%), opening the possibility that a lower dose may not be inferior.
Conclusion
This study gives an overview of published studies on ECT with intravenous bleomycin for patients with cutaneous malignancies, including the use of a reduced bleomycin dose, as preparation for a randomised study.
Introduction
Electrochemotherapy (ECT) is a combination of chemotherapy and reversible electroporation, where electric pulses are administered to the tumour after administration of chemotherapy, which permeabilizes cell membranes and thus increases the cytotoxic effect of the chemotherapeutic drug [Citation1,Citation2]. ECT has proven to be highly effective and reliable in the treatment of cutaneous malignancies and is a treatment option when standard treatment is not sufficient or applicable [Citation3–9]. Cutaneous malignancies can be difficult to manage. These tumours can become exuding, bleeding and odorous and may have a large influence on a patient’s quality of life (QOL) [Citation10,Citation11].
Electroporation destabilises the cell membrane and allows different hydrophilic substances to enter the cell [Citation1], such as the cytotoxic drug bleomycin. Bleomycin is favourable for ECT compared to other anticancer drugs, in that it is already established as a treatment for cancer and has the largest increase in efficacy after electroporation, enhancing the cytotoxic effect 300–5000 fold [Citation1,Citation2,Citation12]. ECT is indicated for cutaneous malignancies of any histology, size and quantity, which either are symptomatic or which other treatment modalities have not been possible to treat [Citation9]. Contraindications include pregnancy, allergy or hypersensitivity. ECT is applicable all over the surface of the body and shows high response rates across different tumour subtypes [Citation13]. Often the procedure is performed with general anaesthesia, which requires hospitalisation [Citation9].
Since the first clinical study in 1993, ECT has been reported with a consistently high response rate [Citation3,Citation13,Citation14]. The standard operating procedures (SOP) published in 2006 [Citation4] and updated in 2018 [Citation9] have been helpful in harmonising use of the procedure. The SOP recommends a standard bleomycin dose of 15.000 IU/m2 when administered intravenously, originally based on the treatment for testicular cancer [Citation15].
A study investigating bleomycin pharmacokinetics, suggest that age matters possibly explained by decreases in kidney function and different body compositions regarding water/fat ratio [Citation16]. In 2018 and 2021, two studies therefore attempted to reduce the dose of bleomycin (10.000 IU/m2) when performing ECT in patients older than 65 years old [Citation17–19]. Both studies conclude that ECT with a reduced dose of bleomycin is a feasible treatment option for older patients, with similar efficacy to standard dose treatment (Objective response rate (ORR) from 87 to 100%) and should be considered as a treatment in aged patients with comorbidities [Citation17,Citation18].
The purpose of this review is to provide a systematic overview of published data on ECT using intravenous bleomycin for cutaneous malignancies, as well as examine the possibilities and indications of reducing the bleomycin dose in ECT.
Electrochemotherapy from 1993 to 2021
Standard treatments of cutaneous malignancies include surgery, radiotherapy and chemotherapy [Citation20]. Other treatments include isolated limb perfusion [Citation21], oncolytic viruses (TVEC) [Citation22] and for some diagnoses systemic therapy e.g., with BRAF/MEK [Citation23] inhibitors, hedgehog inhibitors [Citation24] or immune checkpoint inhibitors such as anti-CTLA-4 and anti-PD-1 [Citation25]. Over the previous 30 years, ECT has been implemented for treatment in cases where the standard treatments are not sufficient [Citation4,Citation9,Citation13,Citation14,Citation26,Citation27]. ECT is performed by either intravenous or intratumoural injection of bleomycin, followed by the insertion of an electrode. Different electrodes may be applied depending on tumour localisation and size [Citation9]. This review has limited the scope and does not focus on the electric field or electrode types.
Bleomycin was introduced in the pivotal study by Einhorn in 1977 for the treatment of testicular cancer using combination chemotherapy [Citation15]. Different doses were applied in the first use of ECT [Citation14,Citation26,Citation28] and the standard dose used today was recommended and implemented in the first standard operating procedure (SOP) in 2006 [Citation4]. Since the first SOP was published, several centres have been using this treatment for patients with cutaneous malignancies. Due to the expanded experience within the area, an updated SOP was developed in order to integrate the knowledge obtained from the different centres, recommending the same standard dose of bleomycin [Citation9]. As a result of the consistently high response rates, studies have in 2018 reported the possibility of lowering the dose of bleomycin in ECT [Citation19,Citation29] and suggested that this could be as effective as the standard treatment, especially in patients that have impaired lung or renal function or patients who are candidates for multiple ECT-cycles [Citation29].
The results raised important questions as to what is the optimal drug concentration needed for favourable antitumor effectiveness of ECT and if it is possible to safely decrease the intravenously administered dose of bleomycin without loss of efficacy [Citation19].
Aim of review
We performed a systematic review in order to evaluate the potential role and possibilities of reducing the bleomycin dose in ECT for cutaneous malignancies. Additionally, our aim was to evaluate the ORR found in studies using a standard bleomycin dose compared with studies using a reduced bleomycin dose, in order to investigate if the effect could be similar.
Methods
This review was constructed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation30]. There was no available protocol for this review. An institutional or ethical board approval was not necessary, as all data were retrieved from previously published data. The endpoint of the analysis was overall tumour response after ECT.
A systematic literature search was conducted on the 17 November 2021 from PubMed, Scopus and Cochrane libraries. The following search terms were used: ‘electrochemotherapy’ AND ‘bleomycin’.
Two authors independently performed the process of screening abstracts and full text assessment.
Inclusion criteria
Eligible studies had to be full text in English and describe the use of ECT with intravenous bleomycin in patients with cutaneous malignancies, including more than four patients. Clinical trials and original studies were included.
Exclusion criteria
Papers concerning ECT with intratumoural chemotherapy and treatment of other cancers than cutaneous malignancies or ECT in combination with immunotherapy were not included. In vitro treatments, veterinary studies, general reviews, editorials and letters to the editor were not included.
Data extraction
Full text assessment retrieved the following data: study design, patient number, tumour localisation, tumour size, histology, type of anaesthesia, route of bleomycin administration, choice of electrodes, side effects, length of follow-up and ORR.
Response rate evaluation
The studies included in and , evaluate the response to treatment according to the Response Evaluation Criteria in Solid Tumours (RECIST) criteria [Citation75].
Table 1. Studies performing ECT with standard dose of intravenous bleomycin performed from 1993 to 2021 for treatment of cutaneous malignancies. Studies written in italics are from the InspECT network.
Table 2. Studies performing ECT with a reduced dose of intravenous bleomycin for treatment of cutaneous malignancies.
Results
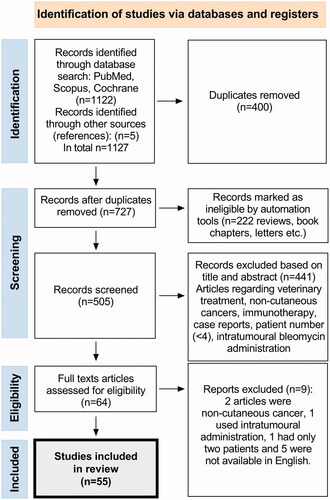
shows the PRISMA flowchart of the study selection. In the initial search, 1127 articles were found. All duplicates were removed (n = 400) and all reviews, books, letters etc. (n = 222) were excluded through automation tools. All abstracts were examined and screened, excluding 441 studies with veterinary treatments, studies treating non-cutaneous tumours, studies solely using intratumoural bleomycin administration, case reports, immunotherapy and studies with a patient number below four. Full text assessments of the remaining 64 articles, resulted in 55 eligible articles published from 1993 to 2021.
The included studies and their characteristics are detailed in and . These studies collectively report 3729 patients in total, who received ECT for cutaneous malignancies. To the best of our ability, studies written by the same author have been thoroughly reviewed in order to note patients presented multiple times. One study mentions that 15 patients were included in preliminary reports, which have already been published [Citation26]. Studies that are listed under the InspECT network are listed in italics. Among these, there can be overlaps between e.g., studies on special cancers and global evaluations.
All studies administer bleomycin intravenously, 25 of these utilise either intravenous or intratumoural administration. All studies provided information regarding choice of electrode and 51 describe response to treatment. The four studies that do not aim to evaluate tumour response are included in the but are excluded from the forest plot in [Citation55,Citation69,Citation71,Citation72]. One of these studies focuses on pain associated to ECT [Citation55], one on mucosal tumours compared to cutaneous tumours [Citation69], one describes the cost-effectiveness of ECT [Citation71] and the last one describes the possibilities of ECT as a neoadjuvant treatment [Citation72].
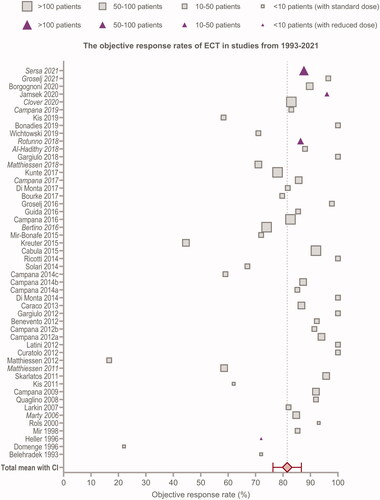
Figure 2. Forest plot of the objective response rates reported in the 55 studies mentioned in and , including the pooled mean with confidence intervals (CI). Squares indicate studies with standard dose of bleomycin and triangles indicate studies with reduced dose of bleomycin. The size of square/triangle is dependent on study size. Studies written in italics are from the InspECT network.
Tumour response
depicts all studies included in this review. These data suggest that despite the many parameters investigated in the studies, the ORR remains high, with a mean of 81.5% [CI 76.3; 87.5] and a standard deviation (SD) of 18.76. Three studies stand out, with an ORR under 55% [Citation28,Citation38,Citation53], without these studies the SD is 11.99. One of these studies is Domenge’s study from 1996, which was one of the first studies conducted and included only seven patients and used electrodes not commercially produced [Citation28]. The other two studies, Matthiessen and Kreuter, include only larger tumours and heavily pre-treated patients, which are well-known parameters that can affect the response [Citation38,Citation53]. Matthiessen and colleagues conclude that evaluation only by clinical examination may lead to an underestimation of the treatment effect in larger tumours. Also, the healing time may be longer in larger tumours and therefore a longer follow-up period would have been beneficial and perhaps lead to better results [Citation38]. Kreuter and colleagues suggest that their study includes patients with markedly advanced disease and larger tumour nodules, which is why they observe a lower ORR [Citation53]. Another five studies stand out with an ORR below 70% [Citation35,Citation36,Citation49,Citation50,Citation68], excluding these studies the SD is 9.03. Three of these studies suggest tumour size causing the more modest response [Citation35,Citation36,Citation49]. One observes an ORR of 86% in tumours <3cm, however when including tumours >3cm the ORR is reduced to 58% [Citation36]. Clover and colleagues also observe highest response rates in smaller tumours (up to 3 cm), but very large tumours (up to 50 cm) still exhibit response at lower levels [Citation13].
Kis et al. observes a CR of 58.3% after one treatment with ECT and 83.3% after two treatments [Citation68], thus indicating the need for multiple treatments in some cases. One study observes an ORR of 67% when including melanoma (MM) and when excluding MM, the ORR increases to 79% [Citation50]. This is however not consistent with studies investigating solely MM, which observe an ORR of 72–100% [Citation31,Citation33,Citation41,Citation45,Citation51,Citation54,Citation76].
Clover and colleagues published the largest study in 2020 involving 987 patients and 2483 tumours [Citation13]. They conclude a slight difference of ORR in tumours with different histology. Basal cell carcinoma (BCC) and Kaposi’s sarcoma (KS) show the highest ORR of 96% and 98% respectively. KS has a high vascularity, which is one of the explanations for the high ORR, due to ECT’s anti-vascular effect. Breast cancer metastases, MM and squamous cell carcinoma (SCC) have similar objective responses of 77%, 82% and 80%, thus concluding a small though significant difference in tumour histology (p < 0.0001). In general non-melanoma tumours show a higher ORR than MM [Citation5,Citation13,Citation77]. Clover and colleagues also observe no significant difference between intravenous administrations and intratumoural injections when treating smaller lesions (<2cm), however when treating larger lesions (>2cm) the complete response rate goes from 48% to 57% using intratumoural and intravenous administration, respectively (p < 0.05) [Citation13]. These data suggest that despite the significant difference between the many parameters investigated in the study, the mean ORR in ECT only differs from 61% to 98% [Citation13].
In summary, most studies observe an ORR between 70 and 100%, indicating a consistent and high response rate of ECT.
Reduced bleomycin dose
This review includes four studies investigating the use of a lower bleomycin dose [Citation17–19,Citation29,Citation74]. These show an ORR of 85.5%, which is similar to the studies using the standard dose of bleomycin. An overview of these studies can be found in , with a total of 77 patients receiving a bleomycin dose below the standard dose.
Side effects
All studies, except seven, describe observed side effects. In general, there are limited side effects and ECT is well tolerated. A common side effect is post-ECT pain. This was mentioned in 31 studies, with a frequency between 5 and 100% in various grades of severity. However, most studies describe the pain being manageable with simple analgesia [Citation34,Citation40,Citation64]. This has also been described in Quaglino’s study from 2015, reporting that 74% of the patients presented with low pain scores, 13% with moderate and 13% with severe pain after treatment [Citation55]. This pain was significantly associated with pain before treatment (p < 0.0001), enabling the possibility for making a pain management strategy before ECT. Hyperpigmentation of the skin is mentioned in 13 out of the 55 studies, with a frequency between 5 and 44% [Citation8,Citation18,Citation29,Citation34,Citation38,Citation42,Citation47,Citation52,Citation53,Citation62,Citation66,Citation67,Citation72]. Only three studies observe serious adverse events (SAEs) [Citation5,Citation8,Citation65] and only one case (sepsis-related death post-treatment) out of the 3729 patients included, was thought related to ECT [Citation8]. No studies reported lung fibrosis, which is a known dose-dependent side effect of bleomycin [Citation78,Citation79]. This can be explained by the low cumulative dose used for ECT, compared with the use of bleomycin for other treatments e.g., testicular cancer [Citation15] and the screening of patients for eligibility with exclusion of patients with lung disease. The same analysis of side effects was observed in the review from Petrelli and colleagues in 2021, which concludes no significant concerns regarding systemic toxicity [Citation80]. Only one study mentions side effects dependent on bleomycin dose and concludes that less side effects are observed when using a reduced bleomycin dose compared to the standard dose [Citation18].
Quality of life
Thirty-six out of the 55 studies mention a reduced QOL being an issue in patients with cutaneous malignancies. In the ESOPE from 2006, QOL is mentioned in their patient monitoring and included in the case report forms [Citation5], however only eight studies have since performed an assessment of QOL using either EORTC, EQ-5D, PGA or SQOLIT questionnaires [Citation8,Citation34,Citation39,Citation47,Citation56,Citation65,Citation69,Citation71]. ECT is often performed as a palliative treatment and it is therefore important to evaluate if the treatment leads to an improvement or deterioration of QOL. Thus, future studies should include this important element.
Discussion
When comparing the 55 studies published from 1993 to 2021 ( and ), we get an overview of the development in ECT over the past 30 years. The ORR has remained high and consistent, which indicates that ECT is a reliable treatment option for patients with cutaneous malignancies. The SOP published in 2006 and updated in 2018 [Citation9] has been helpful in harmonising use of the procedure, according to drug administration, administration time and use of electric pulses. In this review, we have limited the scope to focus solely on drug administration.
It should be noted that 15 studies have evaluated response according to WHO criteria and the remaining studies evaluate according to RECIST criteria. We assume however, that this has a minimal effect on the comparison of tumour response in the studies. Additionally, the time of evaluation differs from four weeks to three months. This can affect the response rates, in that an evaluation after four weeks may not provide the most accurate result. In general however, we can see in and that the response rate does not differ much. This was already reflected in the ESOPE from 2006, which found no difference in success rate among the four different oncological centres using different protocols with a lack of homogeneity [Citation5]. This was also observed in a review from 2018 [Citation81] and in Clover’s study published in 2020 investigating differences in histology and drug administration [Citation13]. These data suggest that despite the many parameters that can influence the response, the differences in ORR are relatively small [Citation13].
In general, studies see a higher response in smaller tumours (<3cm), independent of diagnosis. This can be explained by the insufficient coverage of the electric fields in larger tumours and the reach of applied electrodes, as well as differences in drug distribution within the tumour [Citation47]. Size of tumours may also be indicative of tumour aggressiveness. Additionally, it is thought that previous treatment (such as radiotherapy) can disrupt the vasculature and the tissue can therefore have an impaired blood supply reducing the drug delivery to the tumour [Citation49,Citation82]. Studies mention that irradiated skin can appear hard and fibrotic, making it difficult to insert electrode needles and to reach a deep margin. The measured delivered current is thus seen lower in irradiated tumours compared to non-irradiated, possibly because of difficulties with needle insertion, resulting in a lower response [Citation82].
Compared to other local treatments ECT is a relatively simple procedure that can be performed on any part of the body. ECT is particularly beneficial in cases, where standard treatments are not possible. For example in previously irradiated patients or patients with low performance status and comorbidities. This indicates that patients with SCC or BCC are especially suitable for ECT, since this group includes patients that are immunosuppressed and are therefore not suitable for other treatments e.g., immune checkpoint inhibitors.
Over the past ten years there have been great advances in oncologic treatment with e.g., immune checkpoint inhibitors [Citation25], BRAF/MEK inhibitors [Citation23] and hedgehog inhibitors [Citation24]. These treatments can in combination with ECT give a synergistic response [Citation83]. Hedgehog pathway inhibitors are especially useful for treating BCC, whereas immune checkpoint inhibitors are indicated for treating SCC and MM [Citation84]. Studies have investigated ECT in combination with immune checkpoint inhibitors (e.g., PD-1 inhibitors) or BRAF/MEK inhibitors for patients with MM [Citation83,Citation85,Citation86]. As mentioned in our editorial the use of ECT for limiting surgical resections that require amputation may allow limb-sparing surgery for MM patients and new opportunities for disease management in complex patients [Citation87]. ECT has thus potential both as treatment alone and in combination with other treatments.
ECT is mostly used as a palliative treatment and careful evaluation of patients’ symptoms, patients’ priorities as well as life expectancy must be balanced. Altogether, ECT is a simple procedure, which is reliable and quick to perform. The possibilities of using ECT in other settings are therefore being investigated, e.g., for calcium electroporation [Citation88], endoscopic ECT [Citation89] and as neoadjuvant treatment before conventional treatment [Citation72]. These treatments could expand the therapeutic indications of ECT and possibly increase the overall survival. A study from 2008 published a cost-effective analysis of ECT, confirming a favourable cost-effectiveness ratio, compared to radiotherapy among others [Citation90]. A study published later, suggests however that the electrode price should be reduced for successful implementation of ECT into clinical practice and in order for the treatment to be cost-effective [Citation71].
Since 1993 most studies have used the standard bleomycin dose of 15.000 IU/m2 (see ), with a few exceptions. The first four studies used bleomycin doses of 10 mg/m2 and 15 mg/m2 (corresponding to approximately 15.000 IU/m2 and 22.700 IU/m2) [Citation14,Citation26,Citation28,Citation31]. One of these is Domenge and colleagues from 1996, who conclude that decreasing the dose of bleomycin seems possible, but requires further studies [Citation28]. The standard dose of 15.000 IU/m2 used today, was introduced in the ESOPE study in 2006 [Citation5] and published in the SOP in 2006 [Citation4]. This dose has been used since.
In 2016, Groselj and colleagues investigated the pharmacokinetics of bleomycin in older patients and found a longer therapeutic window for bleomycin injection given in ECT. Based on the pharmacological parameters, they suggest that reducing the dose of bleomycin to 10.000 IU/m2 would give an equally good anti-tumour effect in these patients [Citation58]. Jamsek and colleagues state that the use of standard bleomycin dose in older patients, may lead to prolonged healing time and a more prominent inflammatory response, as a result of exceeded optimal bleomycin concentrations [Citation17]. This study also concludes that a long-term tumour control could be achieved with a lower dose of bleomycin. A single study including six patients receiving a bleomycin dose of 10.000 IU/m2, also concludes that this is an effective treatment [Citation74]. In 2018, Rotunno and colleagues retrieved and analysed data from the InspECT database, examining ECT with reduced bleomycin from 2013 to 2016. The study states that some ECT centres already have adopted the use of a reduced bleomycin dose in patients with impaired renal function or when patients are scheduled for multiple ECT cycles, with the aim to reduce the cumulative dose of bleomycin and associated side effects [Citation29]. The lifetime dose of bleomycin should not exceed 400.000 IU [Citation9], so reducing the dose opens the possibility of giving multiple treatments if necessary. Some clinicians have gone as far as to reduce the dose with 50%. When analysing the different doses of 7.500 IU/m2, 10.000 IU/m2 or 13.500 IU/m2, corresponding to a reduction of 50%, 30% and 10% respectively, a similar ORR is observed (91, 84 and 89%) compared to studies using the standard dose. It is however important to note, that this study only includes 57 patients [Citation29]. It should also be noted that this review includes a total of four studies using a reduced dose of bleomycin. Compared to 50 studies using the standard dose, this results in a much lower power.
Groselj and colleagues suggest that lowering the dose might not be recommended, based on their studies in 2018 and 2021 [Citation16,Citation19]. This is due to differences in plasma concentration and the elimination rate of bleomycin, which are both important parameters. The results indicate that the physiological parameters related to patient age, need to be considered to ensure safe and effective treatment of ECT using intravenous bleomycin [Citation16]. However, it is not possible to give a definite conclusion, since a correlation between the plasma bleomycin and pharmacokinetics of bleomycin in different age groups is needed to confirm this.
In summary, a reduction of the bleomycin dose may benefit the patient by causing fewer side effects and perhaps opening the possibility of treating more patient groups, e.g., patients with comorbidities. Randomised studies with a larger cohort of patients, treated with a reduced dose of bleomycin, have not yet been utilised and are required to evaluate the appropriate indications and clinical significance of reduced bleomycin doses in the use of ECT.
Conclusion
ECT is well established as a treatment for primary and secondary cutaneous malignancies and has been used for the past 30 years. It is a safe and simple palliative procedure with a satisfying response, which is essential for cutaneous malignancies that can be difficult to manage and affect patients QOL. Studies published from 1993 to 2021 investigating the effect of ECT indicate a consistent and high response rate with a mean ORR of 81.5%. However, side effects may be observed, which can be connected to the dose of bleomycin and some patient groups are precluded from treatment due to perceived risks. The few studies that have investigated lowering the bleomycin dose find a similar ORR (85.5%), thus opening the possibility of a non-inferior effect. It will be necessary to conduct a randomised controlled trial, with two groups receiving ECT with different doses of bleomycin, in order to investigate the possibility of reducing the dose whilst retaining efficacy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
References
- Orlowski S, Belehradek J, Jr., Paoletti C, et al. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem Pharmacol. 1988;37(24):4727–4733.
- Gehl J, Skovsgaard T, Mir LM. Enhancement of cytotoxicity by electropermeabilization: an improved method for screening drugs. Anticancer Drugs. 1998;9(4):319–325.
- Gothelf A, Mir LM, Gehl J. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev. 2003;29(5):371–387.
- Mir LM, Gehl J, Sersa G, et al. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systematically or locally and electric pulses delivered by the cliniporator by means of invasive or non-invasive electrodes. Eur J Cancer. 2006;4(11):14–15.
- Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy – an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE. Euro Standard Opera Proced Electrochemother. 2006;4(11):3–13.
- NICE NIfHaCE. Electrochemotherapy for metastases in the skin from tumours of non-skin origin and melanoma; 2013. 1–7.
- NICE NifHaCE. Electrochemotherapy for primary basal cell carcinoma and primary squamous cell carcinoma. 2014. 1–8.
- Bertino G, Sersa G, De Terlizzi F, et al. European research on electrochemotherapy in head and neck cancer (EURECA) project: results of the treatment of skin cancer. Eur J Cancer. 2016;63:41–52.
- Gehl J, Sersa G, Matthiessen LW, et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018;57(7):874–882.
- Bath-Hextall F, Nalubega S, Evans C. The needs and experiences of patients with skin cancer: a qualitative systematic review with metasynthesis. Br J Dermatol. 2017;177(3):666–687.
- Buchhold B, Arnold A, Lutze S, et al. Psychosocial distress and desire for support among inpatients with skin cancer. J Dtsch Dermatol Ges. 2017;15(8):791–799.
- Jaroszeski MJ, Dang V, Pottinger C, et al. Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer Drugs. 2000;11(3):201–208.
- Clover AJP, de Terlizzi F, Bertino G, et al. Electrochemotherapy in the treatment of cutaneous malignancy: outcomes and subgroup analysis from the cumulative results from the pan-European international network for sharing practice in electrochemotherapy database for 2482 lesions in 987 patients (2008–2019). Eur J Cancer. 2020;138:30–40.
- Belehradek M, Domenge C, Luboinski B, et al. Electrochemotherapy, a new antitumor treatment. First clinical phase I–II trial. Cancer. 1993;72(12):3694–3700.
- Einhorn LH, Donohue J. Cis-diamminedichloroplatinum, vinblastine, and bleomycin combination chemotherapy in disseminated testicular cancer. Ann Intern Med. 1977;87(3):293–298.
- Groselj A, Bosnjak M, Krzan M, et al. Bleomycin concentration in patients’ plasma and tumors after electrochemotherapy. A study from InspECT group. Pharmaceutics. 2021;13(9):1324.
- Jamsek C, Sersa G, Bosnjak M, et al. Long term response of electrochemotherapy with reduced dose of bleomycin in elderly patients with head and neck non-melanoma skin cancer. Radiol Oncol. 2020;54(1):79–85.
- Sersa G, Mascherini M, Di Prata C, et al. Outcomes of older adults aged 90 and over with cutaneous malignancies after electrochemotherapy with bleomycin: a matched cohort analysis from the InspECT registry. Eur J Surg Oncol. 2021;47(4):902–912.
- Groselj A, Bosnjak M, Strojan P, et al. Efficiency of electrochemotherapy with reduced bleomycin dose in the treatment of nonmelanoma head and neck skin cancer: preliminary results. Head Neck. 2018;40(1):120–125.
- Spratt DE, Gordon Spratt EA, Wu S, et al. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: a meta-analysis. J Clin Oncol. 2014;32(28):3144–3155.
- Dossett LA, Ben-Shabat I, Olofsson Bagge R, et al. Clinical response and regional toxicity following isolated limb infusion compared with isolated limb perfusion for in-Transit melanoma. Ann Surg Oncol. 2016;23(7):2330–2335.
- Carr MJ, Sun J, DePalo D, et al. Talimogene laherparepvec (T-VEC) for the treatment of advanced locoregional melanoma after failure of immunotherapy: an international multi-institutional experience. Ann Surg Oncol. 2022;29(2):791–801.
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703.
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361(12):1164–1172.
- Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(13):1270–1271.
- Mir LM, Glass LF, Sersa G, et al. Effective treatment of cutaneous and subcutaneous malignant tumours by electrochemotherapy. Br J Cancer. 1998;77(12):2336–2342.
- Sersa G, Cufer T, Cemazar M, et al. Electrochemotherapy with bleomycin in the treatment of hypernephroma metastasis: case report and literature review. Tumori. 2000;86(2):163–165.
- Domenge C, Orlowski S, Luboinski B, et al. Antitumor electrochemotherapy: new advances in the clinical protocol. Cancer. 1996;77(5):956–963.
- Rotunno R, Campana LG, Quaglino P, et al. Electrochemotherapy of unresectable cutaneous tumours with reduced dosages of intravenous bleomycin: analysis of 57 patients from the international network for sharing practices of electrochemotherapy registry. J Eur Acad Dermatol Venereol. 2018;32(7):1147–1154.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
- Rols MP, Bachaud JM, Giraud P, et al. Electrochemotherapy of cutaneous metastases in malignant melanoma. Melanoma Res. 2000;10(5):468–474.
- Larkin JO, Collins CG, Aarons S, et al. Electrochemotherapy: aspects of preclinical development and early clinical experience. Ann Surg. 2007;245(3):469–479.
- Quaglino P, Mortera C, Osella-Abate S, et al. Electrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastases. Ann Surg Oncol. 2008;15(8):2215–2222.
- Campana LG, Mocellin S, Basso M, et al. Bleomycin-based electrochemotherapy: clinical outcome from a single institution’s experience with 52 patients. Ann Surg Oncol. 2009;16(1):191–199.
- Kis E, Olah J, Ocsai H, et al. Electrochemotherapy of cutaneous metastases of melanoma–a case series study and systematic review of the evidence. Dermatol Surg. 2011;37(6):816–824.
- Matthiessen LW, Chalmers RL, Sainsbury DC, et al. Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 2011;50(5):621–629.
- Skarlatos I, Kyrgias G, Mosa E, et al. Electrochemotherapy in cancer patients: first clinical trial in Greece. In Vivo. 2011;25(2):265–274.
- Matthiessen LW, Johannesen HH, Hendel HW, et al. Electrochemotherapy for large cutaneous recurrence of breast cancer: a phase II clinical trial. Acta Oncol. 2012;51(6):713–721.
- Curatolo P, Quaglino P, Marenco F, et al. Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: a two-center prospective phase II trial. Ann Surg Oncol. 2012;19(1):192–198.
- Latini A, Bonadies A, Trento E, et al. Effective treatment of Kaposi’s sarcoma by electrochemotherapy and intravenous bleomycin administration. Dermatol Ther. 2012;25(2):214–218.
- Campana LG, Valpione S, Mocellin S, et al. Electrochemotherapy for disseminated superficial metastases from malignant melanoma. Br J Surg. 2012;99(6):821–830.
- Campana LG, Valpione S, Falci C, et al. The activity and safety of electrochemotherapy in persistent chest wall recurrence from breast cancer after mastectomy: a phase-II study. Breast Cancer Res Treat. 2012;134(3):1169–1178.
- Benevento R, Santoriello A, Perna G, et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: a preliminary report. BMC Surg. 2012;12(Suppl 1):S6.
- Gargiulo M, Papa A, Capasso P, et al. Electrochemotherapy for non-melanoma head and neck cancers: clinical outcomes in 25 patients. Ann Surg. 2012;255(6):1158–1164.
- Caraco C, Mozzillo N, Marone U, et al. Long-lasting response to electrochemotherapy in melanoma patients with cutaneous metastasis. BMC Cancer. 2013;1;13:564.
- Di Monta G, Caraco C, Benedetto L, et al. Electrochemotherapy as "new standard of care" treatment for cutaneous Kaposi’s sarcoma. Eur J Surg Oncol. 2014;40(1):61–66.
- Campana LG, Bianchi G, Mocellin S, et al. Electrochemotherapy treatment of locally advanced and metastatic soft tissue sarcomas: results of a non-comparative phase II study. World J Surg. 2014;38(4):813–822.
- Campana LG, Galuppo S, Valpione S, et al. Bleomycin electrochemotherapy in elderly metastatic breast cancer patients: clinical outcome and management considerations. J Cancer Res Clin Oncol. 2014;140(9):1557–1565.
- Campana LG, Mali B, Sersa G, et al. Electrochemotherapy in non-melanoma head and neck cancers: a retrospective analysis of the treated cases. Br J Oral Maxillofac Surg. 2014;52(10):957–964.
- Solari N, Spagnolo F, Ponte E, et al. Electrochemotherapy for the management of cutaneous and subcutaneous metastasis: a series of 39 patients treated with palliative intent. J Surg Oncol. 2014;109(3):270–274.
- Ricotti F, Giuliodori K, Cataldi I, et al. Electrochemotherapy: an effective local treatment of cutaneous and subcutaneous melanoma metastases. Dermatol Ther. 2014;27(3):148–152.
- Cabula C, Campana LG, Grilz G, et al. Electrochemotherapy in the treatment of cutaneous metastases from breast cancer: a multicenter cohort analysis. Ann Surg Oncol. 2015;22(S3):442–450.
- Kreuter A, van Eijk T, Lehmann P, et al. Electrochemotherapy in advanced skin tumors and cutaneous metastases – a retrospective multicenter analysis. J Dtsch Dermatol Ges. 2015;13(4):308–315.
- Mir-Bonafe JM, Vilalta A, Alarcon I, et al. Electrochemotherapy in the treatment of melanoma skin metastases: a report on 31 cases. Actas Dermosifiliogr. 2015;106(4):285–291.
- Quaglino P, Matthiessen LW, Curatolo P, et al. Predicting patients at risk for pain associated with electrochemotherapy. Acta Oncol. 2015;54(3):298–306.
- Campana LG, Testori A, Curatolo P, et al. Treatment efficacy with electrochemotherapy: a multi-institutional prospective observational study on 376 patients with superficial tumors. Eur J Surg Oncol. 2016;42(12):1914–1923.
- Guida M, Campana LG, Curatolo P, et al. Local treatment with electrochemotherapy of superficial angiosarcomas: efficacy and safety results from a multi-institutional retrospective study. J Surg Oncol. 2016;114(2):246–253.
- Groselj A, Krzan M, Kosjek T, et al. Bleomycin pharmacokinetics of bolus bleomycin dose in elderly cancer patients treated with electrochemotherapy. Cancer Chemother Pharmacol. 2016 May;77(5):939–947.
- Bourke M, Soden D, Clover AJP. Effective treatment of intractable cutaneous metastases of breast cancer with electrochemotherapy: a useful contributor to cutaneous disease control. Breast Cancer Res Treat. 2017;163(2):403–405.
- Di Monta G, Caraco C, Simeone E, et al. Electrochemotherapy efficacy evaluation for treatment of locally advanced stage III cutaneous squamous cell carcinoma: a 22-cases retrospective analysis. J Transl Med. 2017;15(1):82.
- Campana LG, Marconato R, Valpione S, et al. Basal cell carcinoma: 10-year experience with electrochemotherapy. J Transl Med. 2017;15(1):122.
- Kunte C, Letule V, Gehl J, et al. Electrochemotherapy in the treatment of metastatic malignant melanoma: a prospective cohort study by InspECT. Br J Dermatol. 2017;176(6):1475–1485.
- Matthiessen LW, Keshtgar M, Curatolo P, et al. Electrochemotherapy for breast cancer-results from the INSPECT database. Clin Breast Cancer. 2018;18(5):e909–e917.
- Gargiulo M, Serra Mestre JM, Cortese A, et al. Long term effectiveness of electrochemotherapy for the treatment of lower lip squamous cell carcinoma. J Craniomaxillofac Surg. 2018;46(11):1968–1974.
- Al-Hadithy N, Dehnel A, George A, et al. Patient reported outcomes in prospective cohort study of electrochemotherapy. Int J Surg. 2018;52:110–119.
- Wichtowski M, Murawa D, Czarnecki R, et al. Electrochemotherapy in the treatment of breast cancer metastasis to the skin and subcutaneous Tissue – Multicenter experience. Oncol Res Treat. 2019;42(1–2):47–51.
- Bonadies A, Bertozzi E, Cristiani R, et al. Electrochemotherapy in skin malignancies of head and neck cancer patients: clinical efficacy and aesthetic benefits. Acta Derm Venereol. 2019;99(13):1246–1252.
- Kis EG, Baltas E, Ocsai H, et al. Electrochemotherapy in the treatment of locally advanced or recurrent eyelid-periocular basal cell carcinomas. Sci Rep. 2019;9(1):4285.
- Longo F, Perri F, Pavone E, et al. Electrochemotherapy as palliative treatment in patients with advanced head and neck tumours: outcome analysis in 93 patients treated in a single institution. Oral Oncol. 2019;92:77–84.
- Campana LG, Kis E, Bottyan K, et al. Electrochemotherapy for advanced cutaneous angiosarcoma: a European register-based cohort study from the international network for sharing practices of electrochemotherapy (InspECT). Int J Surg. 2019;72:34–42.
- Pirc E, Federici C, Bosnjak M, et al. Early cost-effectiveness analysis of electrochemotherapy as a prospect treatment modality for skin melanoma. Clin Ther. 2020;42(8):1535–1548 e2.
- Fabrizio T, Cagiano L, De Terlizzi F, et al. Neoadjuvant treatment by ECT in cutaneous malignant neoplastic lesions. J Plast Reconstr Aesthet Surg. 2020;73(5):904–912.
- Borgognoni L, Pescitelli L, Gerlini G, et al. Efficacy of electrochemotherapy in the treatment of cutaneous melanoma metastases and rare non-melanoma skin cancer. Anticancer Res. 2020;40(11):6485–6492.
- Heller R, Jaroszeski MJ, Glass LF, et al. Phase I/II trial for the treatment of cutaneous and subcutaneous tumors using electrochemotherapy. Cancer. 1996;77(5):964–971.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
- Rudolf ZS, Cemazar B, Miklavcic M, et al. Electrochemotherapy with bleomycin. The first clinical experience in malignant melanoma patients. Radiol Oncol. 1995;29:229–235.
- Mali B, Jarm T, Snoj M, et al. Antitumor effectiveness of electrochemotherapy: a systematic review and meta-analysis. Eur J Surg Oncol. 2013;39(1):4–16.
- Limper AH. Chemotherapy-induced lung disease. Clin Chest Med. 2004;25(1):53–64.
- Martin WG, Ristow KM, Habermann TM, et al. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin’s lymphoma. J Clin Oncol. 2005;23(30):7614–7620. Oct 20
- Petrelli F, Ghidini A, Simioni A, et al. Impact of electrochemotherapy in metastatic cutaneous melanoma: a contemporary systematic review and Meta-analysis. Acta Oncol. 2021;10:1–12.
- Seyed Jafari SM, Jabbary Lak F, Gazdhar A, et al. Application of electrochemotherapy in the management of primary and metastatic cutaneous malignant tumours: a systematic review and meta-analysis. Eur J Dermatol. 2018;28(3):287–313.
- Falk H, Matthiessen LW, Wooler G, et al. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018;57(3):311–319.
- Dolinsek T, Prosen L, Cemazar M, et al. Electrochemotherapy with bleomycin is effective in BRAF mutated melanoma cells and interacts with BRAF inhibitors. Radiol Oncol. 2016;50(3):274–279.
- Villani A, Potestio L, Fabbrocini G, et al. New emerging treatment options for advanced basal cell carcinoma and squamous cell carcinoma. Adv Ther. 2022;39(3):1164–1178.
- Heppt MV, Eigentler TK, Kahler KC, et al. Immune checkpoint blockade with concurrent electrochemotherapy in advanced melanoma: a retrospective multicenter analysis. Cancer Immunol Immunother. 2016;65(8):951–959.
- Valpione S, Campana LG, Pigozzo J, et al. Consolidation electrochemotherapy with bleomycin in metastatic melanoma during treatment with dabrafenib. Radiol Oncol. 2015;49(1):71–74.
- Bastrup FA, Vissing M, Gehl J. Electrochemotherapy for metastatic cutaneous melanoma. Acta Oncol. 2022;61(5):531–532.
- Vissing M, Ploen J, Pervan M, et al. Study protocol designed to investigate tumour response to calcium electroporation in cancers affecting the skin: a non-randomised phase II clinical trial. BMJ Open. 2021;11(6):e046779.
- Falk Hansen H, Bourke M, Stigaard T, et al. Electrochemotherapy for colorectal cancer using endoscopic electroporation: a phase 1 clinical study. Endosc Int Open. 2020;8(2):E124–E132.
- Colombo GL, Matteo SD, Mir LM. Cost-effectiveness analysis of electrochemotherapy with the cliniporatortrade mark vs other methods for the control and treatment of cutaneous and subcutaneous tumors. Ther Clin Risk Manag. 2008;4(2):541–548.