Abstract
Background
A recent overview of cancer survival trends 1990–2016 in the Nordic countries reported continued improvements in age-standardized breast cancer survival among women. The aim was to estimate age-specific survival trends over calendar time, including life-years lost, to evaluate if improvements have benefited patients across all ages in the Nordic countries.
Methods
Data on breast cancers diagnosed 1990–2016 in Denmark, Finland, Iceland, Norway, and Sweden were obtained from the NORDCAN database. Age-standardized and age-specific relative survival (RS) was estimated using flexible parametric models, as was reference-adjusted crude probabilities of death and life-years lost.
Results
Age-standardized period estimates of 5-year RS in women diagnosed with breast cancer ranged from 87% to 90% and 10-year RS from 74% to 85%. Ten-year RS increased with 15–18 percentage points from 1990 to 2016, except in Sweden (+9 percentage points) which had the highest survival in 1990. The largest improvements were observed in Denmark, where a previous survival disadvantage diminished. Most recent 5-year crude probabilities of cancer death ranged from 9% (Finland, Sweden) to 12% (Denmark, Iceland), and life-years lost from 3.3 years (Finland) to 4.6 years (Denmark). Although survival improvements were consistent across different ages, women aged ≥70 years had the lowest RS in all countries. Period estimates of 5-year RS were 94–95% in age 55 years and 84–89% in age 75 years, while 10-year RS were 88–91% in age 55 years and 69–84% in age 75 years. Women aged 40 years lost on average 11.0–13.8 years, while women lost 3.8–6.0 years if aged 55 and 1.9–3.5 years if aged 75 years.
Conclusions
Survival for Nordic women with breast cancer improved from 1990 to 2016 in all age groups, albeit with larger country variation among older women where survival was also lower. Women over 70 years of age have not had the same survival improvement as women of younger age.
Introduction
Breast cancer is the most common cancer in women both globally and in the Nordic countries. In 2019, breast cancer accounted for 21,764 (26%) of incident cancers and 3955 (16%) of cancer deaths in Nordic women [Citation1]. Survival of breast cancer has steadily improved over the last decades and most recent 5-year relative survival ranges from 87% to 90% in the Nordic countries [Citation2]. In 2010, Tryggvadóttir et al. [Citation3] reported breast cancer survival trends in the Nordic countries 1964–2003. They found increasing incidence and stable mortality in the population, yet improved cancer patient survival over calendar time in all Nordic countries, with Denmark exhibiting the lowest survival. In a recent update of the survival trends 1990–2016, we found that improvements in breast cancer survival have continued and that the survival in Denmark is now similar to the other Nordic countries [Citation2].
Since age is a strong determinant of survival in women with breast cancer, it is important to assess if these improvements are similar across age. With aging populations, the proportion of older breast cancer patients will increase in all Nordic countries. Due to improved health among the elderly, the potential for increasing survival should be significant [Citation4].
The aim of the present study was to assess age-specific survival trends over calendar time for women diagnosed with breast cancer to evaluate if improvements have benefited patients across all ages in the Nordic countries. We also present incidence, mortality, and additional measures of survival, e.g., life-years lost and crude probabilities of death, to give a more comprehensive picture of breast cancer survival.
Methods
Data
A population-based cohort of women with breast cancer from Denmark, Finland, Iceland, Norway, and Sweden was extracted from the NORDCAN database, which includes data from the national cancer registries and the national cause of death registries in each country [Citation5]. The Nordic cancer registries are nationwide and based on mandatory reporting with high completeness and validity (Supplementary Table 1) [Citation6,Citation7]. In all countries, the rates of microscopic verification of tumors are high. Similarly, the Nordic cause of death registries is based on mandatory reporting with high timeliness and completeness. However, recording procedures of causes of death as well as autopsy rates have varied between and within countries to some extent over time [Citation7]. From NORDCAN, we included women diagnosed with invasive breast cancer (International Classification of Diseases version 10 [ICD10]: C50) between 1990 and 2016. The IARC multiple primary rules were applied to each national dataset [Citation5]. Women who were diagnosed on the basis of a death certificate alone (DCO) or through incidental autopsy findings were excluded. Traceback of death certificate initiated (DCI) cases is undertaken in all Nordic countries except Sweden, where no cases are added through information from death certificates. We further excluded women aged <18 years at diagnosis. Only the first breast cancer registration per woman was included, i.e., subsequent primary tumors at the same site were excluded (Supplementary Table 2).
In our cohort, TNM stage (tumor size, lymph node involvement, and distant metastases) was available from Denmark, Iceland, Norway, and Sweden for patients diagnosed 2004–2016, and registered according to the UICC Manual of Clinical Oncology edition 6 and 7. In Denmark the highest values of T, N, and M from clinical and pathological reports within four months of diagnosis were used. Iceland and Norway also reported a combination of clinical and pathological TNM, while Sweden mainly reported clinical TNM. Due to the country differences in coding and proportions of missing, stage was not comparable across countries. Thus stage is only presented descriptively and not included in the survival analyses (Supplementary Table 3).
The women were followed for death and emigration until the end of 2017, except in Finland where the end of follow-up was 2016. For Iceland, no emigration data was available. From the national statistics office in each country, we obtained tabulated population mortality rates.
Statistical analysis
Breast cancer incidence and mortality rates per 100,000 women in the population were estimated in five age groups and 5-year diagnosis windows, using the Nordic population distribution in the year 2000 for age-standardization (Supplementary Table 4).
Relative survival (RS), which estimates survival among patients in the absence of death from other causes, was estimated using flexible parametric relative survival models. Within the models, country-specific population mortality rates stratified by age, sex, and calendar year were used to obtain expected mortality rates. We fitted separate models for each country, with time since diagnosis as the underlying time-scale. The models included age at diagnosis and calendar year as covariates. Age-specific and age-standardized estimates of relative survival were obtained through regression standardization as a function of calendar year using postestimation [Citation8]. An adapted version of the International Cancer Survival Standard 1 (ICSS1) weights was used as age standard (Supplementary Table 4). We estimated 1-, 5- and 10-year RS for each country across calendar time. Age-standardized absolute survival differences were estimated as percentage point (pp) differences in 10-year RS between 1990 and 2005, 5-year RS between 1990 and 2010, and 1-year RS between 1990 and 2015 from the models with 95% confidence intervals (CI).
The flexible parametric relative survival models used restricted cubic splines with 5 degrees of freedom (df) for the log cumulative baseline excess hazard over time since diagnosis [Citation9]. We included age and calendar year at diagnosis as continuous non-linear effects using restricted cubic splines with 3 df, and with 2 df for their two-way interaction. A three-way interaction between age, calendar year, and time-since-diagnosis was included as time-dependent splines with 3 df for each time-dependent effect (i.e., relaxing the proportional excess hazards assumption). For Iceland, we used simplified models excluding the three-way interaction and 2 df for the time-dependent effects. To improve model stability, 96% of the age distribution was modeled continuously while individuals outside the 2nd and 98th percentile of age had their age reassigned to those percentile limits and were assumed to have the same relative survival, i.e., winsorizing [Citation10].
To obtain recent survival estimates we utilized a period analysis approach, where the period window was 2013–2017 for Denmark, Norway, and Sweden, 2012–2017 for Iceland and 2013–2016 for Finland. We included covariates for age and the interaction between time-since-diagnosis and age, as above, without the need to incorporate calendar year. Net probability of death was calculated as one minus relative survival. In addition, the crude probability of all-cause death and death due to breast cancer at 5 and 10 years after diagnosis, and the average number of life-years lost per patient, were estimated from the period analysis. The number of life-years lost per patient was calculated as the difference between the life expectancy of a person in the general population (by sex, age, and calendar year) and the predicted life expectancy of a patient with cancer (by sex and age), obtained through extrapolation of expected and relative survival [Citation11,Citation12]. To ensure comparability, the net and crude probabilities of death were age-standardized using the adapted ICSS1 weights. For crude probabilities of death and life-years lost, differences in background populations may also affect the comparability. Hence, to ensure comparability of crude probabilities and life-years lost, we incorporated the average background mortality in the Nordic countries in these predictions, rather than country-specific population mortality rates (‘reference-adjusted’) [Citation13]. For comparison, we also present non-age-standardized estimates calculated using country-specific background mortality.
As a validation of our models, we compared the parametric estimates to Pohar Perme estimates and found them to be in good agreement. All analyses were performed in Stata [Citation14] using commands stpm2 and standsurv to obtain parametric estimates, and strs for non-parametric estimates [Citation15]. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Ethical approval was granted by the Swedish Ethical Review Authority (ethical approval 2017/641-31/1, amendment 2019-01913) and study permission from the National Institute of Health and Welfare in Finland (approval THL/870/5.05.00/2014, amendment 2019).
Results
In total, 446,936 women were diagnosed with breast cancer between 1990 and 2016 in the Nordic countries with mean age ranging from 61.1 to 63.3 years (Supplementary Table 3). Stage information from 2004 and onwards varied between the countries due to coding practices, and the proportion missing stage ranged from 3.5% in Iceland and Norway to 8.8% in Sweden. Within each country, the proportion of women with stages II–IV was higher in ages ≥70 years compared to ages 18–69 years. The proportion missing stage information was also higher in ages ≥70 years, except in Sweden.
Age-specific incidence and mortality (time trends)
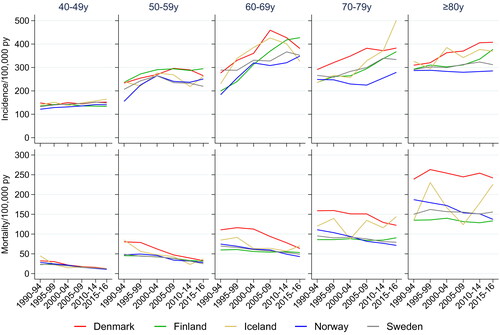
Breast cancer incidence increased over time in ages 60 years and above with largest increases in ages 60–69 years, in particular in Denmark (, top panel). The trends differed somewhat across countries in women aged 80 years or above, where incidence rates increased in Denmark, Finland, and Iceland and were stable in Norway and Sweden. Breast cancer mortality decreased in women below 80 years in Denmark and Norway, and was stable in the other countries (, bottom panel). Among women aged 80 years or above, the mortality was stable in all countries except Norway where the mortality decreased.
Age-standardized and age-specific relative survival (time trends)
During the study period, 1-, 5- and 10-year age-standardized RS improved continuously in all countries (, Supplementary Table 5). In 1990, RS was lowest in Denmark and highest in Sweden; however, the differences in RS between countries diminished over time. The survival improvement in Denmark was particularly pronounced, with a 5-pp increase in age-standardized 1-year RS, and 16 and 18 pp increases in age-standardized 5- and 10-year RS (Supplementary Table 6). Using the most recent data, the age-standardized period estimate of 5-year RS was lowest in Denmark and Iceland (87%), and highest in Finland and Sweden (90%) (, Supplementary Table 5). For long-term survival, period estimates of age-standardized 10-year RS were lowest in Iceland (74%) and highest in Finland (85%).
Figure 2. Time trends in age-standardized and age-specific 1-, 5-, and 10-year relative survival for women diagnosed with breast cancer in the Nordic countries 1990–2016. *Age-standardized estimates of 5- and 10-year relative survival for the latest available period marked with diamonds. Period window 2013–2017 (2012–2017 for Iceland, 2013–2016 for Finland). Estimates with 95% CI in Supplementary Tables 5 and 7.
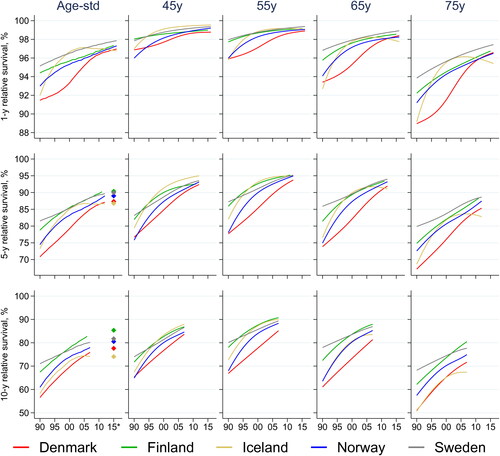
The pattern was similar across all age groups, with substantial improvements in 5- and 10-year RS over time (, Supplementary Table 7). However, older women had substantially lower 5-year RS ranging from lowest 67% (Denmark, age 75 years) and 59% (Denmark, age 85 years) in 1990 to the highest 88% (Sweden, age 75 years) and 79% (Finland, age 85 years) in 2010. For comparison, in women aged 55 years, the 5-year RS in 1990 ranged from 78% (Denmark) to 87% (Sweden), while in 2010 the range was 93% (Denmark) to 95% (Finland, Iceland, Sweden).
Crude probabilities of death and life-years lost (most recent period)
The age-standardized and reference-adjusted crude probability of death due to breast cancer within 5 years from diagnosis ranged from 9% in Finland and Sweden to 12% in Denmark and Iceland (). The age-standardized and reference-adjusted 10-year crude probability of death due to breast cancer ranged from 13% in Finland to 21% in Iceland, and the 10-year crude probability of death due to any cause ranged from 33% in Finland to 39% in Iceland. To quantify the impact of these survival differences on the individual level, we estimated that the age-standardized and reference-adjusted average number of life-years lost due to breast cancer ranged from 3.3 years in Finland to 4.6 years in Denmark. For comparison, non-age-standardized estimates using country-specific population mortality rates are also provided (Supplementary Table 8), indicating that the difference in background mortality rates between countries was small, and ‘reference’ adjustment did not change the overall findings.
Table 1. Period estimates of age-standardized 5- and 10-year net and crude probability of death and life-years lost for women diagnosed with breast cancer in the Nordic countries.
Age-specific relative survival, crude probabilities of death and life-years lost (most recent period)
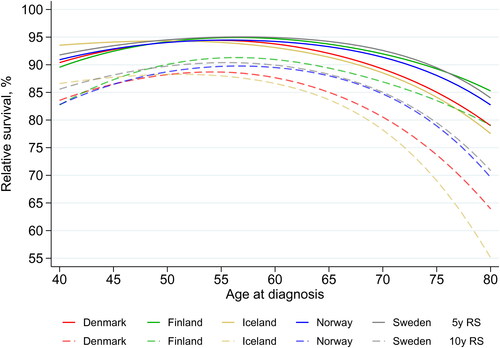
To quantify the effect of age in more detail, the most recent age-specific period estimates of 5- and 10-year RS are presented in and Supplementary Table 7. In all countries, both 5- and 10-year RS peaked around 55 years and decreased in older ages, with most pronounced reductions in Iceland and Denmark. In women diagnosed at age 40 years, 5-year RS ranged between 90% in Finland and 94% in Iceland. In women aged 75 years at diagnosis, the 5-year RS ranged from 84% (Iceland) to 89% (Finland, Sweden). Estimates of 10-year RS in women above age 50 years were lowest in Iceland and highest in Finland, where 10-year RS was 91% at age 55 years and 84% at age 75 years. Compared to women aged 55 years, women aged 75 years had significantly lower 5- and 10-year RS in all Nordic countries.
Figure 3. Period estimates of 5-year (solid lines) and 10-year (interrupted lines) relative survival (RS) for women diagnosed with breast cancer by age at diagnosis. Period window 2013–2017 (2012–2017 for Iceland, 2013–2016 for Finland). Estimates with 95% CI in Supplementary Table 7. RS: relative survival.
To account for the competing risk from other cause-mortality across age, age-specific crude probabilities of death due to breast cancer were estimated (Supplementary Table 9). The 5-year crude probability of breast cancer death was lowest in women aged 55 years (ranged from 5.0% to 5.9% across countries). In women aged 40 years, the 5-year crude probability of breast cancer death ranged from 6.4% to 10.4%, while among women 75 years, the range was 10.0–14.3%. The 10-year crude probabilities of breast cancer death were higher, although with similar age patterns as the 5-year. Of note is that women aged 75 years in Finland had the lowest 10-year crude probability of breast cancer death of 14.9% compared to the other countries (range 18.1–27.3%), where the highest crude probability was found in Iceland.
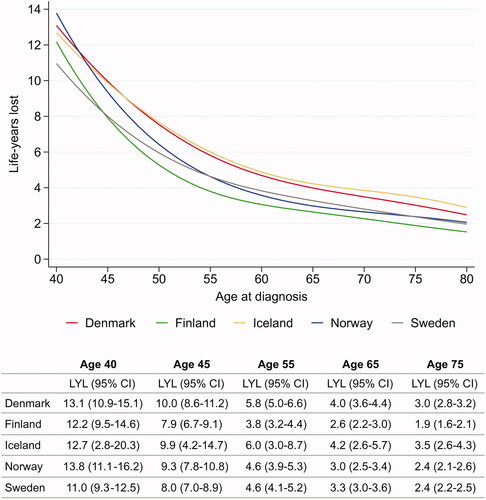
As expected, the average number of life-years lost due to breast cancer was highest in the youngest patients, ranging from 11.0 (Sweden) to 13.8 years (Norway) in women aged 40 years (). The number of life-years lost decreased sharply until age 55 years after which the reduction was less in higher ages. Women aged 75 years at breast cancer diagnosis lost from 1.9 years (Finland) to 3.5 years (Iceland).
Figure 4. Period estimates of average life-years lost for women diagnosed with breast cancer by age at diagnosis. Period window 2013–2017 (2012–2017 for Iceland, 2013–2016 for Finland). Estimates based on average background mortality in the Nordic countries (reference-adjusted). LYL: life-years lost; CI: confidence interval.
Discussion
In this comparison of recent survival trends for women diagnosed with breast cancer in the Nordic countries, we found continued improvements in both short- and long-term survival across all age groups and countries between 1990 and 2016. This indicates that all patients, regardless of age, have benefited from the survival improvements. The improvement in Denmark was particularly pronounced, where age-standardized 5- and 10-year relative survival was almost in level with the other countries at end of 2017. Despite improvements over time, women 70 years or above had a persistently lower survival compared to younger ages. However, younger women lost on average more life-years due to cancer than older women.
In 2010, Tryggvadóttir et al. [Citation3] reported increasing trends in breast cancer survival in the Nordic region 1964–2003, where the survival disadvantage in Denmark was particularly pronounced close to diagnosis. The authors discussed possible explanations for this survival disadvantage as more advanced stage and higher grade at diagnosis, and less screening-detected cancers. Since then Denmark, as the first Nordic country, has launched a national cancer plan in 2000 including several subsequent updates, and implemented accelerated cancer patient pathways already in 2007–2009 to reduce waiting times for diagnostics and treatment [Citation16]. Similar national cancer strategies have been implemented in the other Nordic countries following Denmark (Supplementary Table 10).
In addition to these strategic efforts, large advancements in breast cancer treatment during the last decades are likely an important contributor to the increasing survival trends. The Breast Cancer Groups in each Nordic country have facilitated rapid translation of clinical trial results to clinical practice via national care guidelines based on national and international recommendations. These advancements include both surgical techniques, such as breast conservation and axillary lymph node dissection for broader groups of patients, and systemic treatments, such as endocrine therapy, anti-HER2 therapy, and new chemotherapy agents [Citation17]. In older patients, we found increasing yet lower survival compared to younger patients, which may be due to a lower treatment intensity, partly due to comorbidity, or lower access to new treatments [Citation18]. Treatment guidelines in Denmark previously excluded patients 70 years and above from chemotherapy, but since year 2007 these patients should receive standard treatment also after consideration for comorbidity according to revised guidelines [Citation19]. Older patients may also be diagnosed with more advanced tumors as they are outside of screening age. The country variation in survival was larger among older women, possibly indicating differences in access to and practices of treatment in the elderly across countries.
Screening programs for breast cancer have been implemented in all Nordic countries since the 1990s, except for Denmark where national screening was fully implemented 2007–2009 (Supplementary Table 10). Current screening ages are 50–69 years in Denmark, Norway, and Finland, while Iceland invite women aged 40–69 years and Sweden 40–74 years. Screening attendance has been high and stable over time, with participation around 80% among invited, except in Iceland where attendance is lower (around 60%) [Citation20–24]. Screening leads to a shift in the stage-distribution of detected cases, and can lead to overdiagnosis, and might therefore have an effect on age-specific incidence rates. However, it will likely not have a large effect on the age-standardised incidence rate over the time frame presented in this study. We observed increasing breast cancer incidence in all countries during the study period, in particular in ages 60–69 years but also ages 70 years and above. Possible reasons for the increasing incidence in women above screening age include decreased physical activity, obesity and alcohol use, and changed childbearing patterns [Citation25–27]. Although the use of hormone replacement therapy has decreased over the past 20 years, previous use may also influence incidence in the oldest age group [Citation28]. Mortality, however, decreased in most but not all countries and age groups. Anderson et al. [Citation29] have previously reported increasing incidence trends of estrogen receptor positive breast cancer, and declining trends of estrogen receptor negative breast cancer. Thus, a shift toward the more favorable biology of disease may in part explain the improved survival.
Our period estimates suggest a continued improvement in survival for all countries after 2008, with >80% 10-year relative survival in Finland, Norway, and Sweden. In the latest period, Finland had the highest 10-year relative survival at 85%. Despite the large improvements over time, a slightly lower 5- and 10-year survival was observed in Denmark and Iceland. The remaining differences translate into the fewest number of life-years lost in Finland, on average 3.3 years, while women in Denmark and Iceland lose 4.7 and 4.9 years, respectively. Important to note is that such comparisons are usually hampered by country-specific differences in other cause mortality, whereas our results utilized the new method of reference-adjusted background mortality and the same average Nordic expected mortality.
In comparison to Europe and the rest of the world, the Nordic countries have the highest survival for breast cancer patients [Citation30,Citation31]. The EUROCARE-5 study covering a study period of 1999–2007 found similar results as we did, with poorer survival among older patients and a lower survival in Denmark [Citation31]. Our study extends on these findings by including longer follow-up and providing more insights into trends across age.
The strengths of the study included the population-based approach covering a population of more than 27 million inhabitants, and the additional 13 years of diagnosis data compared to the previous Nordic trend analysis [Citation3]. The Nordic countries have similar tax-funded healthcare systems, including cancer screening programs, available to all residents. Trends in cancer survival are important for assessing how well healthcare systems meet the needs for diagnosis and treatment of cancer in the population. For this purpose a powerful database such as NORDCAN plays a vital role. We reported a wide range of survival measures, including new statistical techniques to improve comparability across countries. With improved survival and more high-quality data available both in the Nordic countries and beyond, there is an increasing need for new methods and a variety of measures to quantify the survival experience of different patients.
An important limitation of our study was the lack of adjustment for TNM stage at diagnosis. Due to differences in coding practices across time and countries, it was not possible to make a fair comparison of stage-specific survival in the Nordic countries. Other international benchmark projects have also had difficulties making stage-specific survival comparisons [Citation32]. Hence, there is an urgent need to harmonize the stage information and improve the reporting to the NORDCAN database. For example, the Swedish stage information was mainly based on clinical examination where non-palpable tumors were coded as T0 causing a high proportion of stage 0, whereas in the other countries pathological information was used to a higher extent. TNM stage information from clinical and pathological staging should be reported separately and coherently across regions and time. We urge decision-makers to invest in such harmonization to enable important future comparisons, in particular as the data are already being collected in each country.
Another limitation was the lack of information on tumor biology, including estrogen receptor status and grade, which is not available in the NORDCAN database. We were therefore not able to explore age differences in survival across subtypes of breast cancer.
In conclusion, since 1990 the survival improvements seen for women diagnosed with breast cancer has continued in all Nordic countries and benefited patients across age. However, a larger country variation was observed in older women, where survival was also lower overall. The previously observed survival disadvantage in Denmark had declined, with similar 1-year relative survival in 2015 to the other Nordic countries, while the gap for 5- and 10-year relative survival had narrowed. Today, women diagnosed with breast cancer in Finland do have the most favorable prognosis of all the Nordic countries with 85% relative survival 10 years after diagnosis. The most likely explanations for these improvements are several important hallmarks of cancer treatment, as well as the implementation of national cancer care strategies and screening programs.
Author Contributions
FEL: Conceptualization, Formal analysis, Methodology, Visualization, Writing-Original draft preparation (LEAD), Writing-Reviewing and Editing (LEAD). NK: Writing-Original draft preparation, Writing-Reviewing and Editing. ML: Writing-Reviewing and Editing. TMLA: Writing-Reviewing and Editing. GE: Data curation, Writing-Reviewing and Editing. TBJ: Writing-Reviewing and Editing. AV: Writing-Reviewing and Editing. DP: Writing-Reviewing and Editing. EJÓ: Writing-Reviewing and Editing. HB: Writing-Reviewing and Editing. PCL: Conceptualization, Funding acquisition, Writing-Reviewing and Editing. LSM: Writing-Original draft preparation, Writing-Reviewing and Editing. ALVJ: Conceptualization, Methodology, Writing-Original draft preparation (LEAD), Writing-Reviewing and Editing (LEAD).
Supplemental Material
Download MS Word (82.9 KB)Disclosure Statement
PCL received support from the Swedish Cancer Society and the Swedish Research Council for the submitted work; ML owns stock in Pfizer and Astra Zeneca; the authors have no other relationships or activities that could appear to have influenced the submitted work.
Data Availability Statement
The data underlying this article were provided by NORDCAN by permission. Data will be shared on request to the corresponding author with permission of the NORDCAN secretariat.
Additional information
Funding
References
- Larønningen S, Ferlay J, Bray F, et al. NORDCAN: cancer incidence, mortality, prevalence and survival in the Nordic countries, version 9.1 (27.09.2021) [Internet]. Association of the Nordic Cancer Registries. Oslo, Norway: Cancer Registry of Norway. 2021; [cited 2022 Feb 3]. https://nordcan.iarc.fr/
- Lundberg FE, Andersson TM-L, Lambe M, et al. Trends in cancer survival in the Nordic countries 1990–2016: the NORDCAN survival studies. Acta Oncol. 2020;59(11):1266–1274.
- Tryggvadóttir L, Gislum M, Bray F, et al. Trends in the survival of patients diagnosed with breast cancer in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010;49(5):624–631.
- Land LH, Dalton SO, Jensen M-B, et al. Impact of comorbidity on mortality: a cohort study of 62,591 Danish women diagnosed with early breast cancer, 1990–2008. Breast Cancer Res Treat. 2012;131(3):1013–1020.
- Engholm G, Ferlay J, Christensen N, et al. NORDCAN – a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49(5):725–736.
- Pukkala E, Engholm G, Højsgaard Schmidt LK, et al. Nordic cancer registries – an overview of their procedures and data comparability. Acta Oncol. 2018;57(4):440–455.
- Laugesen K, Ludvigsson JF, Schmidt M, et al. Nordic health registry-based research: a review of health care systems and key registries. Clin Epidemiol. 2021;13:533–554.
- Lambert PC, Dickman PW, Rutherford MJ. Comparison of different approaches to estimating age standardized net survival. BMC Med Res Methodol. 2015;15(1):64.
- Royston P, Lambert PC. Flexible parametric survival analysis using Stata: beyond the Cox model. College Station (TX): Stata Press; 2011. [Database]
- Syriopoulou E, Mozumder SI, Rutherford MJ, et al. Robustness of individual and marginal model-based estimates: a sensitivity analysis of flexible parametric models. Cancer Epidemiol. 2019;58:17–24.
- Andersson TM-L, Rutherford MJ, Møller B, et al. Reference adjusted loss in life expectancy for population-based cancer patient survival comparisons – with an application to colon cancer in Sweden. Cancer Epidemiol Biomarkers Prev. 2022;31(9):1726.
- Andersson TM-L, Dickman PW, Eloranta S, et al. Estimating the loss in expectation of life due to cancer using flexible parametric survival models. Stat Med. 2013;32(30):5286–5300.
- Lambert PC, Andersson TM-L, Rutherford MJ, et al. Reference-adjusted and standardized all-cause and crude probabilities as an alternative to net survival in population-based cancer studies. Int J Epidemiol. 2020;49(5):1614–1623.
- Stata Corp LLC. Stata statistical software: release 17. College Station (TX): Stata Corp LLC; 2021.
- Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9(2):265–290.
- Probst HB, Hussain ZB, Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians—a National Danish project. Health Policy. 2012;105(1):65–70.
- Ejlertsen B, Offersen BV, Overgaard J, et al. Forty years of landmark trials undertaken by the Danish Breast Cancer Cooperative Group (DBCG) nationwide or in international collaboration. Acta Oncol. 2018;57(1):3–12.
- Baban CK, Devane L, Geraghty J. Change of paradigm in treating elderly with breast cancer: are we undertreating elderly patients? Ir J Med Sci. 2019;188(2):379–388.
- Møller S, Jensen M-B, Ejlertsen B, et al.; Danish Breast Cancer Cooperative Group. The clinical database and the treatment guidelines of the Danish Breast Cancer Cooperative Group (DBCG); its 30-years experience and future promise. Acta Oncol. 2008;47(4):506–524.
- Lagerlund M, Åkesson A, Zackrisson S. Population-based mammography screening attendance in Sweden 2017–2018: a cross-sectional register study to assess the impact of sociodemographic factors. Breast. 2021;59:16–26.
- Jacobsen KK, von Euler Chelpin M, Vejborg I, et al. Impact of invitation schemes on breast cancer screening coverage: a cohort study from Copenhagen, Denmark. J Med Screen. 2017;24(1):20–26.
- Sebuødegård S, Sagstad S, Hofvind S. [Attendance in the Norwegian Breast Cancer Screening Programme]. Tidsskr Nor Laegeforen. 2016;136(17):1448–1451.
- Anttila A, Lehtinen M, Mäki S, et al. Breast Cancer Screening Programme – annual review 2021 [Internet]. Finnish Cancer Registry. [cited 2022 Feb 18]; https://cancerregistry.fi/screening/breast-cancer-screening/.
- Sigurdsson K, Ólafsdóttir EJ. Population-based service mammography screening: the Icelandic experience. Breast Cancer. 2013;5:25.
- Andersson TM-L, Engholm G, Pukkala E, et al. Avoidable cancers in the Nordic countries – the impact of alcohol consumption. Eur J Cancer. 2018;103:299–307.
- Andersson TM-L, Engholm G, Lund ASQ, et al. Avoidable cancers in the Nordic countries—the potential impact of increased physical activity on postmenopausal breast, Colon and endometrial cancer. Eur J Cancer. 2019;110:42–48.
- Andersson TM-L, Weiderpass E, Engholm G, et al. Avoidable cancer cases in the Nordic countries – the impact of overweight and obesity. Eur J Cancer. 2017;79:106–118.
- Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394(10204):1159–1168.
- Anderson WF, Rosenberg PS, Petito L, et al. Divergent estrogen receptor-positive and -negative breast cancer trends and etiologic heterogeneity in Denmark. Int J Cancer. 2013;133(9):2201–2206.
- Allemani C, Matsuda T, Di Carlo V, et al.; CONCORD Working Group. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075.
- Sant M, Chirlaque Lopez MD, Agresti R, et al.; EUROCARE-5 Working Group. Survival of women with cancers of breast and genital organs in Europe 1999–2007: results of the EUROCARE-5 study. Eur J Cancer. 2015;51(15):2191–2205.
- Minicozzi P, Innos K, Sánchez M-J, et al.; EUROCARE-5 Working Group. Quality analysis of population-based information on cancer stage at diagnosis across Europe, with presentation of stage-specific cancer survival estimates: a EUROCARE-5 study. Eur J Cancer. 2017;84:335–353.