Abstract
Objective: Benralizumab, an anti-eosinophilic monoclonal antibody, in combination with high-dosage inhaled corticosteroids and long-acting β2-agonists (ICS/LABA), significantly reduced asthma exacerbations, improved lung function, and reduced symptoms for patients with severe, uncontrolled asthma with blood eosinophil counts ≥300 cells/μL in the Phase III SIROCCO and CALIMA studies. To understand the efficacy and safety of benralizumab for patients with eosinophil-driven disease with blood eosinophil counts lower than 300 cells/μL, we evaluated the effect of applying an eosinophil cutoff of ≥150 cells/μL.
Methods: Adult patients with uncontrolled asthma despite high-dosage ICS/LABA ± additional asthma controller(s) received subcutaneous benralizumab 30 mg every 8 weeks (Q8W; first three doses every 4 weeks) or placebo for 48 (SIROCCO) or 56 (CALIMA) weeks. Efficacy measures including annual exacerbation rate, prebronchodilator FEV1, and total asthma symptom score were analyzed by baseline blood eosinophil counts ≥150 vs. <150 cells/μL.
Results: Benralizumab reduced asthma exacerbation rates by 42% in SIROCCO (rate ratio = 0.58; 95% CI = 0.46–0.74; p < 0.001; n = 325) and 36% in CALIMA (rate ratio = 0.64; 95% CI = 0.50–0.81; p < 0.001; n = 300) vs. placebo (n = 306 for SIROCCO, n = 315 for CALIMA) for patients with blood eosinophil counts ≥150 cells/μL. Benralizumab increased prebronchodilator FEV1 (both studies, p ≤ 0.002) and improved total asthma symptom score in SIROCCO (p = 0.009) at end of treatment vs. placebo for patients with blood eosinophil counts ≥150 cells/μL. The overall adverse events frequency was similar between treatment groups and eosinophil count cohorts.
Conclusion: These results support the efficacy and safety of benralizumab for patients with severe asthma and blood eosinophil counts ≥150 cells/μL.
Introduction
Severe, uncontrolled asthma affects approximately 5?10% of an estimated 315 million patients with asthma worldwideCitation1,Citation2. Severe asthma requires medium-/high-dosage inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) with add-on therapies (e.g., oral corticosteroids, anti-immunoglobulin E [omalizumab] or anti–interleukin-5 [IL-5] agents) to control the disease. For many patients, the disease remains uncontrolled, despite such extensive treatmentCitation3, placing them at risk of severe asthma exacerbationsCitation4. Thus, a significant unmet medical need remains. Patients with severe, uncontrolled asthma have increased morbidity, reduced health-related quality of life and increased health care resource use, and, therefore, need additional therapeutic optionsCitation4–7.
Eosinophilic inflammation is evident in approximately half of the patients with asthma, and is associated with increased disease severity, exacerbation frequency, and symptom burden, as well as with decreased lung functionCitation8–10. IL-5, a critical cytokine for eosinophil development, activation, and survival, is present in increased concentrations in patients with asthmaCitation11,Citation12. Current asthma treatment guidelines recommend add-on anti–IL-5 therapy for patients with severe, uncontrolled, eosinophilic asthmaCitation3. Blood eosinophil count is one of the predictive biomarkers of eosinophilic asthma. However, blood eosinophil count cutoff criteria have varied from study to study, and a clear definition of blood eosinophilia has not been established. Eosinophil counts can fluctuate with circadian rhythm and exposure to systemic steroidsCitation13. Furthermore, patients with low blood eosinophil counts may still have eosinophil-driven disease, and would benefit from therapies that target eosinophilic inflammation.
Benralizumab is a humanized, afucosylated, anti-eosinophilic monoclonal antibody against IL-5 receptor α, which is expressed on the surface of eosinophils and basophilsCitation14,Citation15. Benralizumab is under development for patients with severe, uncontrolled, eosinophilic asthma. Benralizumab induces direct, rapid, and nearly complete depletion of eosinophils through enhanced antibody-dependent cell-mediated cytotoxicity, an apoptotic process of eosinophil elimination involving natural killer cellsCitation16. In this respect, benralizumab differs from the two marketed anti–IL-5 antibodies (mepolizumab and reslizumab) that bind IL-5 and result in passive, indirect eosinophil reductionCitation17–19. In two Phase III studies, SIROCCO and CALIMA, adding subcutaneous benralizumab 30 mg every 4 weeks (Q4W) or every 8 weeks (Q8W; Q4W for the first three doses) to ICS/LABA significantly reduced asthma exacerbations, increased lung function (forced expiratory volume in 1 s [FEV1]), improved symptom control, and depleted blood eosinophils for patients with severe, uncontrolled asthma and a screening blood eosinophil count of ≥300 cells/μLCitation20,Citation21. Both benralizumab dosing regimens reduced the frequency of exacerbations relative to that with placebo for patients with blood eosinophil counts <300 cells/μL in CALIMA and with the benralizumab Q4W regimen in SIROCCOCitation20,Citation21.
Mepolizumab and reslizumab, the two marketed anti–IL-5 antibodies indicated for severe asthma, have been studied in patients with blood eosinophil counts ≥150 cells/μL at screening or ≥300 cells/μL in the prior year and with ≥400 cells/μL, respectivelyCitation17–19. In contrast, the Phase III benralizumab trials included patients irrespective of blood eosinophil count, so it was possible to assess efficacy across a range of blood eosinophils. Of note, benralizumab demonstrated efficacy for patients with a screening blood eosinophil count <300 cells/μLCitation20,Citation21. The aim of the current study was to further characterize the patient population most likely to benefit from benralizumab treatment. To this end, our analysis examined the efficacy and safety results from the SIROCCO and CALIMA studies for patients aged ≥18 years with severe, uncontrolled asthma with blood eosinophil counts ≥150 cells/μL vs. <150 cells/μL.
Methods
Study design and participants
The SIROCCO (NCT01928771) and CALIMA (NCT01914757) studies were randomized, double-blind, parallel-group, placebo-controlled, Phase III studies that enrolled patients at 303 and 374 clinical research centers, respectively, in Africa, Asia, Europe, North America, and South AmericaCitation20,Citation21. The study design comprised an enrollment visit (Week –4), a 4-week screening/run-in phase, randomization (Week 0), a treatment period from Weeks 0 to 48 (SIROCCO) or 56 (CALIMA), and a final follow-up visit 4 weeks following the end of the treatment period.
Enrollment criteria for the studies have been publishedCitation20,Citation21. The study included male and female patients aged 12–75 years, with a weight of ≥40 kg, and with physician-diagnosed asthma that required treatment with medium- to high-dosage ICS/LABA (defined as fluticasone ≥500 μg/day or equivalent total daily dosage) for ≥12 months prior to enrollment. In this current analysis, only patients aged ≥18 years treated with high-dosage ICS/LABA (defined as fluticasone >500 μg/day or equivalent total daily dosage) were evaluated. Patients must have had at least two asthma exacerbations requiring systemic corticosteroid therapy or a temporary increase in their usual maintenance dosages of oral corticosteroids within 12 months prior to enrollment. Patients must have had documented treatment of ICS/LABA with or without oral corticosteroids or additional asthma controllers for ≥3 months prior to enrollment. Studies were conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use/Good Clinical Practice guidelines, and the ethics committee at each participating site.
Patients from all regions were randomized 1:1:1 to receive either subcutaneous benralizumab 30 mg Q4W, benralizumab 30 mg Q8W, or placebo Q4W. Treatment was double blind and lasted for 48 (SIROCCO) or 56 weeks (CALIMA). Patients with blood eosinophil counts ≥300 cells/μL and <300 cells/μL were recruited at screening in a ratio of approximately 2:1, respectively.
Because there was no apparent safety or efficacy benefit to more frequent benralizumab dosing in either the SIROCCO or CALIMA studies, only benralizumab 30 mg Q8W was explored further in the current analysisCitation20,Citation21. The Q8W regimen has indicated greater numerical trends (statistical tests not evaluated) toward efficacy benefits, and a smaller drug burden compared with the Q4W regimenCitation20,Citation21.
Outcomes
The primary efficacy endpoint was annual asthma exacerbation rate. Annual asthma exacerbation rate was calculated as the total number of exacerbations × 365.25/total duration of follow-up within the treatment group (days). An exacerbation was defined as a worsening of asthma that led to one of the following: (1) use of systemic corticosteroids (or a temporary increase in a stable oral corticosteroids background dosage) for at least 3 days or a single, injected dose of corticosteroids, (2) emergency department (ED)/urgent care visit (<24 hours) related to asthma that required systemic corticosteroids, or (3) inpatient hospitalization (≥24 hours) related to asthma. Worsening of asthma was defined as any new or increased symptoms or signs that were concerning to the patient or related to an Asthma Daily Diary alertCitation20,Citation21.
Key secondary endpoints (i.e., multiplicity [type-1 error]–protected endpoints) included prebronchodilator FEV1 and total asthma symptom score. FEV1 was measured by spirometry at the study center, with equipment provided by a central vendor. Spirometry was performed according to American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines by the investigator or authorized delegateCitation22. Asthma symptom scores were derived from night-time and daytime symptom recordings by patients each morning and evening, respectively, with electronic patient-reported outcome devices. Symptoms were reported on a 4-point response scale (0–3), with 0 indicating no symptoms. In addition, symptom severity was rated from 1 (low) to 3 (high). Total asthma symptom score was a sum of night-time and morning scores, with a maximum score of 6.
Additional secondary endpoints were Standardized Asthma Quality of Life Questionnaire for 12 years and older (AQLQ[S] + 12) score and Asthma Control Questionnaire 6 (ACQ-6) score. The AQLQ(S) + 12 questionnaire measured the health-related quality of life experienced by patients aged ≥12 years with asthmaCitation23. Four separate domains were part of the questionnaire: symptoms, activity limitations, emotional function, and environmental stimuli. Patients were asked to recall their experiences during the previous 2 weeks and to score each of the questions on a 7-point scale ranging from 7 (no impairment) to 1 (severe impairment). The overall score was calculated as the mean response to all questions. The ACQ-6 assessed asthma symptoms (night-time waking, symptoms on waking, activity limitation, shortness of breath, and wheezing) and short-acting bronchodilator useCitation24. Patients were asked to recall their experiences during the previous week, and to score each of the questions on a 7-point scale ranging from 0 (totally controlled) to 6 (severely uncontrolled). The overall score was calculated as the mean response to all questions. For the AQLQ(S) + 12 and ACQ-6, total score changes of ≥0.5 were considered clinically meaningful. Electronic patient-reported outcome devices were used to record AQLQ(S) + 12 and ACQ-6 assessments. Safety outcomes included adverse events and serious adverse events.
Statistical analysis
To account for the 2:1 stratification for baseline blood eosinophil counts (≥300 cells/μL and <300 cells/μL), we reweighted patients with baseline blood eosinophil counts <300 cells/μL using the ratio of the number of patients who had baseline blood eosinophil counts ≥300 cells/μL to the number of those who had <300 cells/μL. The analysis of treatment outcomes by blood eosinophil counts ≥150 cells/μL and <150 cells/μL was not part of the formal testing strategy; therefore, all p-values were nominal. Exacerbation rates were calculated using a negative binomial model, with adjustment for treatment, region, oral corticosteroid use at time of randomization, and prior exacerbations. The estimated treatment effect (rate ratio of benralizumab vs. placebo at end of treatment), corresponding 95% CI, and two-sided p-value for the rate ratio were determined. Secondary endpoints were determined using a mixed-effects model for repeated measures analysis, with adjustment for treatment, baseline value, region, oral corticosteroid use at time of randomization, visit, and visit × treatment. Based on these analyses, covariate adjusted or least squares means, treatment differences in least squares means, 95% confidence intervals (CIs), and p-values were calculated.
The efficacy of subcutaneous benralizumab 30 mg was analyzed by blood eosinophil count <150 cells/μL and ≥150 cells/μL for the Q8W dosing arm in adult patients on high-dosage ICS/LABA for both SIROCCO and CALIMA studies. The full analysis set, including all patients randomized and receiving either benralizumab or placebo, irrespective of protocol adherence or continued study participation, was used for the analyses. Statistical calculations were performed using SAS Version 9.2.
Results
These analyses evaluated adult patients with severe, uncontrolled asthma and screening blood eosinophil counts ≥150 cells/μL and <150 cells/μL who had received benralizumab Q8W or placebo plus high-dosage ICS/LABA in the SIROCCO (≥ 150 cells/μL, n = 631; < 150 cells/μL, n = 122; total n = 753) and CALIMA (≥ 150 cells/μL, n = 615; < 150 cells/μL, n = 88; total n = 703) studies. Demographics and baseline clinical characteristics for the adult patients in the SIROCCO and CALIMA studies for the respective eosinophil count groups were similar, except for nasal polyposis, which was present at a greater frequency in the ≥150 cells/μL subgroup than in the <150 cells/μL subgroup ().
Table 1. Demographics and baseline clinical characteristics (adults).
Reductions in annual exacerbation rate with benralizumab vs. placebo were seen for adult patients with baseline blood eosinophil counts ≥150 cells/μL in the SIROCCO (42%; rate ratio [95% CI] = 0.58 [0.46–0.74]; p < 0.001) and CALIMA (36%; rate ratio [95% CI] = 0.64 [0.50–0.81]; p < 0.001) studies (). Numerical differences in annual exacerbation rates favoring treatment were observed between the benralizumab and placebo groups for patients with baseline blood eosinophil counts <150 cells/μL in both studies. In the SIROCCO study, a decrease was observed in the annual exacerbation rate for exacerbations leading to emergency room visits or hospitalizations with benralizumab vs. placebo for patients with baseline blood eosinophil counts ≥150 cells/μL (rate ratio vs. placebo = 0.54 [95% CI = 0.32–0.90; p = 0.018]).
Table 2. Annual exacerbation rates for patients with baseline blood eosinophil counts ≥150 and <150 cells/μL treated with benralizumab plus high-dosage ICS/LABA (adults).
Benralizumab treatment resulted in increases in prebronchodilator FEV1 at the end of treatment for patients with baseline blood eosinophils ≥150 cells/μL in both studies (). The least squares mean treatment differences vs. placebo were 0.163 L (95% CI = 0.087–0.239; p < 0.001) for the SIROCCO study and 0.116 L (95% CI = 0.041–0.191; p = 0.002) for the CALIMA study. Improvements in FEV1 for patients with baseline blood eosinophils ≥150 cells/μL who received benralizumab were observed after the first dose by the first 4-week assessment in both studies (p < 0.05; ). A greater reduction vs. placebo in total asthma symptom score was achieved in the SIROCCO study (–0.23 points, 95% CI = –0.41 to –0.06; p = 0.009) and in the CALIMA study (–0.16 points, 95% CI = –0.33 to 0.01; p = .0.073), representing 13–19% greater improvement, for patients with baseline blood eosinophil counts ≥150 cells μL (). Numerical improvements in FEV1 and total asthma symptom score between the benralizumab and placebo groups were observed only in the SIROCCO study for patients with baseline blood eosinophil counts <150 cells/μL.
Figure 1. Change from baseline in prebronchodilator FEV1 for patients receiving high-dosage ICS/LABA with baseline eosinophil counts (A, B) ≥ 150 cells/μL and (C, D) < 150 cells/μL for the SIROCCO (A, C) and CALIMA (B, D) studies (adults). FEV1, forced expiratory volume in 1 s; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; Q8W, every 8 weeks (first three doses every 4 weeks). * p < 0.05 for benralizumab Q8W vs. placebo treatment comparison.
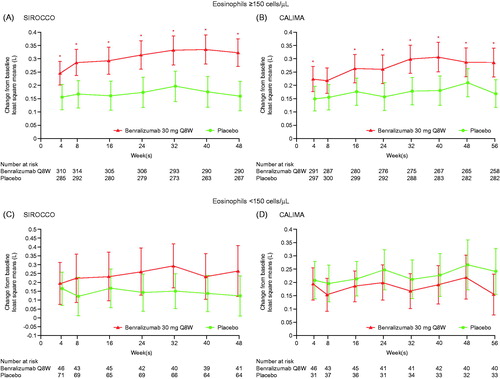
Table 3. Change in key secondary endpoints with benralizumab plus high-dosage ICS/LABA treatment for patients with blood eosinophil counts ≥150 or <150 cells/μL (adults).
For adult patients with blood eosinophil counts ≥150 cells/μL, benralizumab treatment increased AQLQ(S) + 12 scores relative to placebo in the SIROCCO (0.19; 95% CI = 0.01–0.37; p = 0.036) and CALIMA studies (0.20; 95% CI = 0.02–0.38; p = 0.029; ). Decreases in ACQ-6 scores in comparison with placebo were observed in both the SIROCCO (–0.15; 95% CI = –0.31 to 0.02; p = 0.084) and CALIMA studies (–0.22; 95% CI = –0.39 to –0.06; p = 0.008; ) for patients with blood eosinophil counts ≥150 cells/μL. Improvements in AQLQ(S) + 12 and ACQ-6 scores with benralizumab treatment relative to placebo were observed only in the SIROCCO study (p ≤ 0.056) for patients with blood eosinophil counts <150 cells/μL.
Table 4. Change in AQLQ(S) + 12 and ACQ-6 with benralizumab plus high-dosage ICS/LABA treatment for patients with blood eosinophil counts ≥150 or <150 cells/μL (adults).
Adverse events during the on-study period were reported by similar percentages of patients who received benralizumab or placebo for either blood eosinophil subgroup for both SIROCCO and CALIMA studies (72–79% for benralizumab cohorts, ). For patients with blood eosinophil counts ≥150 cells/μL who received benralizumab, the most common adverse events were asthma worsening (SIROCCO and CALIMA, 12% each) and nasopharyngitis (SIROCCO, 11%; CALIMA, 20%). For patients with blood eosinophil counts <150 cells/μL who received benralizumab, the most common adverse events were asthma worsening (15%), nasopharyngitis (10%), and upper respiratory tract infection (10%) for the SIROCCO study, and nasopharyngitis (29%) and upper respiratory tract infection (15%) for the CALIMA study. Serious adverse events were reported by similar or lesser percentages of patients who received benralizumab vs. placebo for either blood eosinophil subgroup. For both blood eosinophil count subgroups, percentages of patients with injection-site reactions were similar between the benralizumab and placebo cohorts (2–4% for the benralizumab cohorts). Percentages of patients experiencing hypersensitivity adverse events were similar or lesser for the benralizumab cohorts vs. placebo cohorts.
Table 5. Adverse events, injection-site reactions, and hypersensitivity during on study period (adults).
Discussion
In the SIROCCO and CALIMA studies, benralizumab 30 mg administered Q8W in combination with high-dosage ICS/LABA significantly improved annual exacerbation rates, prebronchodilator FEV1, and total asthma symptom scores compared with placebo for patients with severe, uncontrolled asthma with baseline blood eosinophil counts ≥300 cells/μLCitation20,Citation21. The results of the current analyses extend the efficacy profile for benralizumab to patients with severe, uncontrolled asthma with baseline blood eosinophil counts ≥150 cells/μL. With benralizumab treatment, these patients had consistent improvements in exacerbation reduction, in lung function based on FEV1, and in disease-specific, health-related quality of life based on AQLQ score. Recently, the ZONDA study demonstrated that benralizumab treatment substantially reduced oral glucocorticoid use for patients with blood eosinophil counts ≥150 cells/μL, further supporting potential use of this agent in this patient populationCitation25.
Numerical differences in these measures were observed for the subset of patients with blood eosinophil counts <150 cells/μL. However, the number of patients in this subgroup was small, and the results were less consistent than those for patients with baseline blood eosinophil counts ≥150 cells/μL. For patients with severe, uncontrolled asthma with blood eosinophil counts <150 cells/μL for whom the possibility of eosinophil-mediated airway inflammation remains high, health care providers should consider the use of sputum or other airway assessmentsCitation26.
Mepolizumab (administered Q4W), an anti–IL-5 monoclonal antibody, has been studied for patients with severe, eosinophilic asthma defined by an eosinophil inclusion criterion of ≥150 cells/μL at screening or ≥300 cells/μL in the previous 12 months. In the combined mepolizumab Phase III DREAMCitation18 and MENSACitation17 studies, the asthma exacerbation rate ratio of mepolizumab vs. placebo was 0.48 (95% CI = 0.39–0.58) for patients with blood eosinophil counts ≥150 cells/μL. For prebronchodilator FEV1, improvement with mepolizumab for the combined studies vs. placebo was 0.064 L (95% CI = 0.001–0.127) for patients with blood eosinophil counts of ≥150 cells/μL. Our ability to compare results from the mepolizumab studies and the current study directly is limited because of differences in baseline characteristics. However, both studies show that their respective agents reduce exacerbation frequency and improve lung function for patients with severe, uncontrolled asthma with blood eosinophil counts ≥150 cells/μL.
The gold-standard diagnosis of eosinophilic asthma relies on the demonstration of eosinophilic inflammation in the airways, based on bronchial biopsies or induced sputumCitation27. These methods are invasive and require specialized training, and are not feasible for routine clinical practice. Of a range of different biomarkers, blood eosinophil counts offer the greatest diagnostic accuracy for sputum eosinophilia, with increasing specificity as blood eosinophil counts increaseCitation28,Citation29. However, given the natural variability of blood eosinophil counts (even within 24 hours) and their sensitivity to corticosteroids, low blood eosinophil counts may belie clinically significant eosinophilic airway inflammationCitation13,Citation30,Citation31. The results of the current analyses underscore the potential limitations of defining probable responders to eosinophil depletion therapy, based on a blood eosinophil count of ≥300 cells/μL alone. More detailed characterization of the eosinophilic phenotype beyond blood eosinophil counts is needed that uses a combination of clinical characteristics (e.g., nasal polyposis), along with blood eosinophil countsCitation27. Blood eosinophil counts should be measured at several time points to address variability issues that could cause missed diagnoses for patients with eosinophilic inflammationCitation32. The data from our current analysis demonstrate that benralizumab has clinical efficacy at blood eosinophil counts ≥150 cells/μL for patients with severe uncontrolled asthma, although greater eosinophil counts were predictive of a greater magnitude of response across asthma outcomes, including exacerbations, lung function, and symptomatology.
In the current analysis, the safety profile for benralizumab was consistent with that previously reported for the SIROCCO and CALIMA studiesCitation20,Citation21. In both the SIROCCO and CALIMA studies, the frequency of overall adverse events and serious adverse events reported by benralizumab recipients was similar to that reported by placebo recipients for both the ≥150 cells/μL and <150 cells/μL blood eosinophil cohorts. Injection-site reactions were infrequent and occurred in similarly small percentages of patients in the benralizumab and placebo cohorts.
Conclusions
Adding benralizumab to a high-dosage ICS-based treatment regimen improved several important measures of asthma control, including asthma exacerbation rate, lung function, and disease-specific, health-related quality of life, for patients with severe, uncontrolled asthma with baseline blood eosinophil counts ≥150 cells/μL. These results suggest that benralizumab reduces the burden of disease and health care costs for this difficult-to-treat population with limited treatment options.
Transparency
Declaration of funding
Funding for the SIROCCO and CALIMA studies was provided by AstraZeneca and Kyowa Hakko Kirin. Funding for the analyses was provided by AstraZeneca.
Declaration of financial and other interests
MG, IH, JGZ, PN, and XX are full-time employees of AstraZeneca/MedImmune LLC. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work, but have no other relevant financial relationships to disclose.
Author contributions
All authors conceived and designed the study, acquired the data, and accessed, analyzed, and interpreted the data. All authors participated in the development and critical review of the manuscript. All authors provided final approval for publication submission and are accountable for the accuracy and integrity of the work.
Acknowledgments
The authors would like to thank the investigators, health care providers, research staff, and patients who participated in the SIROCCO and CALIMA studies. Writing and editing support, including preparation of the draft manuscript under the direction and guidance of the authors, incorporating author feedback, and manuscript submission, was provided by Alan Saltzman, PhD, of Endpoint Medical Communications, Conshohocken, PA, USA, and Michael A. Nissen, ELS, of AstraZeneca (Gaithersburg, MD, USA). This support was funded by AstraZeneca.
References
- To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health 2012;12:204
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343-73
- Global Initiative for Asthma. Global strategy for asthma management and prevention; 2017. http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/. Accessed March 27, 2017
- Peters SP, Ferguson G, Deniz Y, et al. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med 2006;100:1139-51
- Fernandes AG, Souza-Machado C, Coelho RC, et al. Risk factors for death in patients with severe asthma. J Bras Pneumol 2014;40:364-72
- Sadatsafavi M, Lynd L, Marra C, et al. Direct health care costs associated with asthma in British Columbia. Can Respir J 2010;17:74-80
- Bahadori K, Doyle-Waters MM, Marra C, et al. Economic burden of asthma: a systematic review. BMC Pulm Med 2009;9:24
- Zhang JY, Wenzel SE. Tissue and BAL based biomarkers in asthma. Immunol Allergy Clin North Am 2007;27:623-32
- Price D, Wilson AM, Chisholm A, et al. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy 2016;9:1-12
- Talini D, Novelli F, Bacci E, et al. Sputum eosinophilia is a determinant of FEV1 decline in occupational asthma: results of an observational study. BMJ Open 2015;5:e005748
- Tan LD, Bratt JM, Godor D, et al. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy 2016;9:71-81
- Patterson MF, Borish L, Kennedy JL. The past, present, and future of monoclonal antibodies to IL-5 and eosinophilic asthma: a review. J Asthma Allergy 2015;8:125-34
- Spector SL, Tan RA. Is a single blood eosinophil count a reliable marker for “eosinophilic asthma?”. J Asthma 2012;49:807-10
- Lopez AF, Vadas MA, Woodcock JM, et al. Interleukin-5, interleukin-3, and granulocyte-macrophage colony-stimulating factor cross-compete for binding to cell surface receptors on human eosinophils. J Biol Chem 1991;266:24741-7
- Yamada T, Sun Q, Zeibecoglou K, et al. IL-3, IL-5, granulocyte-macrophage colony-stimulating factor receptor α-subunit, and common β-subunit expression by peripheral leukocytes and blood dendritic cells. J Allergy Clin Immunol 1999;101:677-82
- Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti–IL-5 receptor α mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol 2010;125:1344-53
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014;371:1198-207
- Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012;380:651-9
- Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015;3:355-66
- Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016;388:2115-27
- FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016;388:2128-41
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38
- Juniper EF, Svensson K, Mork AC, et al. Modification of the asthma quality of life questionnaire (standardised) for patients 12 years and older. Health Qual Life Outcomes 2005;3:58
- Juniper EF, Svensson K, Mork AC, et al. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med 2005;99:553-8
- Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med 2017;376:2448-58
- Mukherjee M, Nair P. Blood or sputum eosinophils to guide asthma therapy? Lancet Respir Med 2015;3:824-5
- Coumou H, Bel EH. Improving the diagnosis of eosinophilic asthma. Expert Rev Respir Med 2016;10:1093-103
- Westerhof GA, Korevaar DA, Amelink M, et al. Biomarkers to identify sputum eosinophilia in different adult asthma phenotypes. Eur Respir J 2015;46:688-96
- Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax 2015;70:115-20
- Hastie AT, Moore WC, Li H, et al. Biomarker surrogates do not accurately predict sputum eosinophils and neutrophils in asthma. J Allergy Clin Immunol 2013;132:72-80
- Korevaar DA, Westerhof GA, Wang J, et al. Diagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: a systematic review and meta-analysis. Lancet Respir Med 2015;3:290-300
- Mathur SK, Fichtinger PS, Evans MD, et al. Variability of blood eosinohil count as an asthma biomarker. Ann Allergy Asthma Immunol 2016;117:551-3
