Abstract
Objective: Topical delivery of drugs is an alternative to oral administration, often with similar efficacy but potentially a more favorable tolerability profile. However, topical formulations need to be able to penetrate the skin and permeate to the target areas in quantities sufficient to exert a therapeutic effect. Many factors can affect this process, including the physicochemical properties of the drug, the formulation used, and the site and mode of application. It is believed that measurement of drug concentrations at the sites of action may be an indicator of their likely efficacy. This review addresses these issues, with reference to topically administered diclofenac in osteoarthritis.
Methods: Articles relevant to this review were identified after a systematic search of Medline and Embase, using the key words “diclofenac”, "topical administration" and “osteoarthritis” in the search strategy.
Results: The sparse data available indicate that topical diclofenac can penetrate and permeate to deeper tissues, with a lower plasma to tissue ratio than oral diclofenac. The tissue diclofenac levels after topical delivery are sustained over time (at least several hours). However, there is not enough data to establish how diclofenac levels in the joint compare with IC50 levels (50% of the maximum inhibition of prostaglandin synthesis) established following oral administration.
Conclusions: After topical application, diclofenac can penetrate the skin and permeate to deeper tissues, where it reaches a concentration that appears to be sufficient to exert a therapeutic effect. More robust methods are required for in vivo characterization to better estimate the clinical efficacy of topically applied drugs.
Introduction
Topical administration of drugs can be a practical alternative to oral delivery, not least because they avoid first-pass metabolism, are associated with a lower rate of systemic adverse events, and allow direct application over the target areasCitation1. Topical formulations should be easy and acceptable to use, but importantly need to be able to penetrate the skin and permeate to the target areas in quantities sufficient to exert a therapeutic effect. Topical analgesics are often used in acute and chronic painful conditions, delivering non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, diclofenac, and acetylsalicylic acid directly to the site of injury to relieve pain. They can be particularly useful in the management of osteoarthritis (OA), a chronic condition where a regular intake of oral NSAIDs to control painful flares may be associated with systemic adverse events, especially in the older population that typically suffers from OACitation2 and may be more prone to adverse eventsCitation3–5. Topical NSAIDs have been proven to be as effective as and better tolerated than oral NSAIDs in the treatment of OACitation4,Citation6–8, and are recommended in certain international guidelines before the use of oral NSAIDs in OA of the knees or handsCitation9–11. In a preference study, almost three times as many OA patients chose to use a topical rather than oral NSAID, particularly those who were more concerned about toxicity such as the elderlyCitation12,Citation13. It seems that topical NSAIDs are currently underutilizedCitation14, and their efficacy in pain relief remains debatableCitation15. Clinicians still seem to be unsure of the value of topical NSAIDsCitation16, with many regarding them as little more than placeboCitation15. Indeed, a large placebo effect of around 50% (after 12 weeks) has been observed in studies of topical NSAIDs, twice as high as that in studies with oral placebo (25% in a pooled analysis)Citation15. Despite this, real-life studies in OA indicate that topical NSAIDs are as effective as oral NSAIDs over 1 year of treatmentCitation4.
To relieve pain effectively, topical NSAIDs need to work at the appropriate site of action. However, in OA there is still uncertainty regarding the target tissues and how OA-associated pain is generated. There is often a disparity between the degree of pain perception or functional impairment and the extent of damage in the OA joint, and the pain mechanisms are likely to be complexCitation17. Pain perception appears to be influenced by peripheral factors (e.g. damaged structures impinging on other local structures), as well as activation of central pain-processing pathwaysCitation17. And there is evidence that there is an inflammatory component to OA, including the activation and release of local proinflammatory mediators such as cytokines or prostaglandinsCitation17–19. Topical NSAIDs appear to work on peripheral pain receptors in OA, with relatively few central effectsCitation20. They relieve peripheral pain by inhibiting the cyclooxygenase (COX)-2 enzyme, thereby reducing the production of prostaglandins that would otherwise increase sensitivity to pain by sensitizing peripheral nociceptors to painful stimuliCitation21. This is the established mechanism of action, but there are putative mechanisms that include inhibition of leukotriene synthesis, inhibition of phospholipase A2, modulation of arachidonic acid levels, inhibition of the N-methyl-D-aspartate (NMDA) pathway and increase in plasma β-endorphin levelsCitation22,Citation23. Emerging mechanisms include inhibition of peroxisome proliferator-activated receptor gamma, reduction in plasma and synovial substance P and interleukin-6 levels, inhibition of the thromboxane-prostanoid receptor, and inhibition of acid-sensing ion channels. There are no nociceptors in articular cartilage, the main structure affected morphologically in OA. Thus, the pain that occurs when the cartilage wears away must instead originate from other structures within the joint, such as the synovial membrane or tissue, bone, or periarticular muscles and ligaments, for exampleCitation17,Citation24–27. Nociceptors are abundant in many articular tissuesCitation21 that are in contact with the intra-articular environmentCitation28. A clearer understanding of the drug concentrations that can be achieved in synovial tissue and fluid following application of a topical NSAID, particularly in relation to plasma levels, may provide a useful insight into the potential therapeutic effect in OA and the potential liability for systemic adverse events.
The current review considers the general characteristics that influence the effectiveness of topical products, with specific illustration using diclofenac in the treatment of OA-related pain. Factors addressed include issues regarding skin penetration and tissue permeation, and the concentrations that have been reached in and around the articular joint and in plasma in published studies. We chose diclofenac because analysis suggests that it is the most potent COX-2 inhibitor compared with other commonly used NSAIDsCitation22,Citation29–31. Topical diclofenac has been widely available since its first approval in 1985, and is one of the most extensively investigated NSAIDs, often used as a benchmark in clinical studies in OACitation32. Topical diclofenac is proven to be effective in relieving the pain of OACitation4,Citation15,Citation33, and is the only NSAID approved for topical treatment of OA pain in the United StatesCitation33. Specific aims of the review are to determine: (1) whether topical NSAIDs, specifically diclofenac, can penetrate through the skin and permeate to deep sites of action; (2) whether the amount of diclofenac at the site of action is known, and how the formulation can be optimized and measured; (3) whether topically administered diclofenac is pharmacologically effective. To identify relevant articles, a systematic search of Medline and Embase was performed (to October 2016), using the key words “diclofenac”, “topical administration” and “osteoarthritis” in the search strategy. Further details of the search strategy used can be found in the Supplementary Appendix.
Rationale for using topical vs. oral NSAIDs
Topical products were developed to provide well tolerated, effective targeted therapies, based on the drug’s pharmacokinetics and penetration to the site of action. There are various reasons to use a topical NSAID in preference to an oral NSAID ().
Table 1. Advantages of using topical NSAIDs in preference to oral NSAIDs in osteoarthritis.
Topical therapies are delivered to the site of action, avoiding the first-pass metabolism of oral drugsCitation34. Most importantly, topical NSAIDs were developed to reduce the risk of gastrointestinal (GI), cardiovascular (CV), and renal adverse events associated with oral NSAIDsCitation33,Citation35. Although some of the drug does enter the systemic circulation from the dermal microcirculation, systemic exposure to NSAIDs is reduced. For example, plasma levels after topical administration have been reported to fall within a range of 0.2% to 8% of those achieved after oral administrationCitation33,Citation36,Citation37. Thus, complications such as GI bleeding and gastric ulcerations associated with oral administration of NSAIDsCitation38,Citation39, as well as CV and renal toxicityCitation40, are less common following use of topical NSAIDsCitation41–43. This is important in older patients who form the OA population, who often have co-morbid conditions or an increased risk for GI, CV or renal complicationsCitation3–5. A meta-analysis of the safety data for topical diclofenac found that although the overall risk of adverse events was similar between topical diclofenac and oral comparators, the risk of systemic effects was significantly lower with topical administrationCitation44. The risk of local effects was significantly higher with topical delivery, howeverCitation44, and future development should aim to minimize local effects with better topical formulations. Use of a topical NSAID may also have a treatment-sparing effect, such that it may be possible to substantially reduce the overall dose of concomitant oral NSAIDs required to manage OA by 40%, and thereby reduce the risk of systemic adverse eventsCitation4. In older OA patients, who are likely to have high concomitant medication use, the low systemic exposure associated with topical NSAIDs will reduce the potential for clinically relevant drug–drug interactions (e.g. with warfarin, antihypertensives or low-dose aspirin used for cardioprotection) associated with oral NSAIDsCitation3,Citation45.
Although there is a sound rationale for using a topical NSAID, questions remain as to how the drug reaches the target tissues. In OA, it is important that a topically applied drug reaches the target tissues in sufficient amounts for there to be a pharmacodynamically active concentration present, that it is unequivocally effective in the disease indicated, and that it has no local toxic or allergic effects or undesirable, dose-related systemic effectsCitation46.
Pathways used by topical drugs to reach target tissues
The efficacy of a topical drug at relieving pain and inflammation is dependent on its ability to penetrate skin and permeate to the target tissues. It has been suggested that topical NSAIDs exert their action locally at structures that surround superficial joints such as the knee or hand and within the joint itselfCitation15,Citation47, and must reach a concentration in those areas that is sufficient to inhibit the COX enzymesCitation46.
Oral drugs are dependent on absorption into the circulation and subsequent distribution to the peripheral tissues. The pharmacological action of topical drugs instead relies on penetration through the stratum corneum and permeation into the lower layers of the skin, illustrated in .
Figure 1. Penetration of topical diclofenac through the skin and permeation through the deeper layers to the inflamed joint.
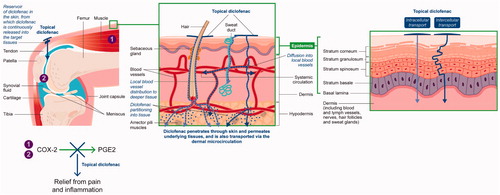
The stratum corneum is the outermost, horny layer of skin that limits penetration of substances to protect the more delicate structures underneath. Therefore, it can be very difficult to penetrate passively, and is the rate-limiting step for epidermal drug transportCitation48. Topically applied drugs may have a depot effect, such that they accumulate for a prolonged time in the stratum corneum, epidermis, dermis and subcutaneous fatty tissue to form a reservoir, from which there is a sustained release of drug into the surrounding tissuesCitation49–54. The efficiency of the reservoir is dependent on the active pharmaceutical ingredients (APIs), including lipid/water solubility, protein-binding capacity, percutaneous absorption, compound concentration, clearance, application time, and application modeCitation51. The highly bound nature of diclofenac may contribute to the formation of a reservoir, with retention occurring when diclofenac is highly bound in tissues underlying the topical absorption siteCitation53. Movement of the drug through the skin is a passive diffusional process, relying on diffusion down concentration gradients (following Fick’s LawCitation55) and partitioning into tissues and solutesCitation1,Citation56,Citation57. Active transport processes can occur, but the mechanisms have not been identifiedCitation1,Citation58. Some of the drug may travel from the surface of the skin via hair follicles or sweat ducts to reach the lower layersCitation59,Citation60.
At the dermal level (), the drug may enter the local blood vessels for distribution to deeper tissuesCitation60. Uptake of the drug from the dermal microcirculation into the systemic circulation may also occur (for example, diclofenac has been found in treated and untreated tissues after topical applicationCitation56,Citation61,Citation62) – although total systemic exposure is lowCitation33. Alternatively, the drug may diffuse deeper into inflamed tissuesCitation56,Citation63,Citation64 and/or be absorbed via lymphatic drainageCitation58. It should be noted that the exact mechanisms involved remain unclearCitation65.
Factors affecting penetration and permeation
Several factors can affect penetration of the drug through the stratum corneum and permeation to the underlying tissues (outlined in ), which must be considered when choosing a topical NSAID.
Table 2. Overview of the factors affecting the ability of a topical drug to optimally penetrate skin and permeate through the underlying tissues.
Permeation through the layers of skin, and the factors that influence this process, are commonly assessed using the Franz cellCitation78–80. This technique is an efficient in vitro method of evaluating drug movement through excised human or animal skin or synthetic skin; it can be used to identify the changing concentrations of APIs in different physiological layers of the skin, and can be combined with a variety of imaging techniques to visualize the movement of drug through the skinCitation79. Physiologically, the Franz cell assesses the penetration of APIs through the skin, the first step in the passage of the APIs to the site of action. Yet permeation through the tissue layers to the deeper sites of action still needs to be evaluated.
Properties influencing penetration through the skin
Innate drug properties
Topical drugs must be small enough to pass through the skin (molecular weight <500 g/mol)Citation1, and molecular size is probably the main determinant of flux across the skin (i.e. the amount of drug that can penetrate the skin per unit of time)Citation66. Diclofenac is a small molecule (296 g/molCitation67) (Supplementary Table 1), allowing it to pass more easily through the stratum corneum. The drug must be water solubleCitation68, yet also have adequate lipophilicity to penetrate through the lipid matrix of the stratum corneumCitation1,Citation56. Diclofenac is a weak organic acid (pKa 3.9Citation69) and thus amphiphilic, with both lipophilic and hydrophilic properties that allow it to access all tissues including the stratum corneum and other skin tissues, but also cell membranes such as in the synovial lining of jointsCitation33,Citation50,Citation63. Diclofenac has a low molecular weight, high lipophilicity, and has been shown to have the highest in vitro permeation rate constant compared to other NSAIDs that were studiedCitation81. However, it also has a modest flux (evaluated in the Franz cell) and a relatively low clearance from skin into muscleCitation64,Citation81. The physicochemical characteristics of diclofenac allow diffusion of the drug through the skin although with the need for permeation enhancers.
Topical formulation properties
The topical formulation is also important, and affects the ability of a drug to penetrate the skin. Few drugs readily penetrate skin when used alone and so topical delivery can be locally enhanced by altering a drug’s formulation, which can have a substantial impact on the rate of skin absorption and on the subsequent penetration depthCitation42,Citation82. Characteristics of the vehicle used to carry the drug need to be considered, such as the solubility, molecular mass, depth of penetration, pharmacology and toxicology of its componentsCitation70,Citation71. Gels, sprays and microemulsions may be absorbed through the skin more effectively than creamsCitation41, and an in vitro study has suggested that diclofenac gel has faster flux than a diclofenac solution or patchCitation72. An aqueous solution of diclofenac has been shown to penetrate to a depth of around 3–4 mm into the underlying dermis and subcutaneous tissueCitation56. It should be noted that different formulations of topical diclofenac may penetrate more deeply and at different velocities, reflecting the varying effects of excipientsCitation56,Citation65,Citation83.
Formulations providing faster skin penetration would seem desirable, seemingly promising faster absorption to sites of action. Penetration-enhancing factors can affect the formation of a skin reservoir, resulting in faster formation and emptying of the reservoirCitation51. However, faster penetration (assessed in vitro) can also have the unintended consequence of increasing plasma exposure (and thus the risk of systemic adverse events), while leaving tissue levels largely unchanged (cf. Brunner et al.Citation83, Nivsarkar et al.Citation84). For example, the pharmacokinetic parameters of diclofenac in skeletal muscle were variable and indistinguishable following topical administration of diclofenac diethylamine gel compared to that of a novel diclofenac formulation (whose skin transport of diclofenac was reported to be ten-fold faster than the gel)Citation83. Furthermore, the bioequivalence 90% confidence intervals of the novel formulation versus the gel included 100% in all instances, indicating that there were no significant differences in exposure. Although skin penetration speed had no effect on tissue permeation, it did lead to higher levels of diclofenac in the plasma: diclofenac exposure following topical administration of diclofenac diethylamine gel is around 6% of that observed compared to three times daily oral administration of diclofenac 50 mg; in contrast, the plasma exposure following administration of the novel formulation was 2.8–4.8 times higherCitation83. Similar outcomes were seen with a comparison of four topical ibuprofen productsCitation65,Citation85. In all of these studies with diclofenac and with ibuprofen, the speed of penetration through the skin – as measured in the Franz cell – did not translate to proportional changes in tissue permeation.
Use of penetration enhancers
A penetration enhancer may be used to encourage local absorption through the skin into the underlying tissues and could increase the depth of local direct penetrationCitation42,Citation47,Citation73. It should be noted that the vehicle and enhancers themselves can have a clinical effect, for example topically administered dimethylsulfoxide (DMSO) used to treat veterinary systemic inflammationCitation15,Citation73,Citation86. The vehicles/enhancers should ideally be inactive with no adverse effects on the skin – the high concentrations of DMSO used for enhancing penetration of diclofenac can cause erythema and wheals of the stratum corneum and may denature some proteinsCitation73.
Site and mode of application
The site of application affects how readily the drug can reach the target tissues, and a topical NSAID is more likely to reach superficial joints such as the finger or knee, rather than deeper structures such as the hip jointCitation15. In addition, the mode of application of the drug influences penetration. For example, rubbing or local heat can increase local blood flow and facilitates uptake into the blood, maintaining the concentration gradient that drives the passive diffusionCitation87. Prolonged rubbing has been shown to greatly increase the flux of diclofenac gel through the skinCitation74.
Once in the blood circulation, the drug can be redistributed into local tissues or into other body compartments. Skin surface occlusion, which hydrates the stratum corneum, often facilitates penetration through the skin and into the underlying tissuesCitation75,Citation76. Repetitive administration can greatly increase the bioavailability of the drugCitation77. Single-dose topical administration of diclofenac resulted in drug levels in the plasma and tissue indicative of direct penetration (pathways in ), while multiple-dose administration led to redistribution from the systemic circulation to the tissueCitation88. Furthermore, repeated daily applications of NSAIDs provide potentially effective concentrations of the drug in skeletal muscleCitation89. Interindividual variability is often reported in in vivo studies of topical drugsCitation90, because of differences in skin permeability due to age, disease or damage, hydration of the epidermis, local blood flow, for example, or the presence of metabolic skin enzymes that may break down the drug and reduce its potencyCitation91.
The penetration of topical products through the skin is dependent on the active drug substance and the formulation, yet optimal skin penetration may not consistently result in the optimal downstream delivery to tissue. While skin penetration assays are well understood, broadly used and robust, our evaluation of the available evidence – and that of other authorsCitation80,Citation92,Citation93 – indicates that their results may not be suitable for anticipating tissue penetration. This is in contrast to establishing that in vitro methods can reflect systemic bioavailabilityCitation94, as this is not consistently reflective of target tissue permeation. Several issues regarding the active drug need to be determined, including how fast it can penetrate to the site of action, how deep it can directly penetrate, and how its delivery to the site of action can be optimized.
Distribution to the target tissues
It is important to note, as demonstrated above, that the rate of absorption is not the only factor that is important in the efficacy of a topical NSAID. Physiology and the API characteristics already mentioned also play an important roleCitation45,Citation56,Citation64. However, these factors do not always lead to understood outcomes; data on topical ibuprofen, for example, shows that even if topical NSAIDs with the same APIs have the same rate of skin penetration, they do not necessarily reach the target tissues in the same concentrationsCitation65,Citation85,Citation95. Ultimately, it is the concentration of the drug at the joint which is of paramount importance.
Preferential distribution of diclofenac to the sites of inflammation, the "effect" compartments, is influenced by several factors. For example, like all NSAIDs diclofenac is bound to plasma proteins, primarily albumin, and concentrations of the drug should be higher and persist where there is a larger concentration of albumin. Furthermore, the partition coefficient (Ks) of a drug indicates the ratio of the mean concentration in synovial fluid and plasma over long-term administrationCitation96. Thus, drugs that have a Ks value greater than one, such as diclofenac after multiple administration (Ks 1.1Citation96), should be present in greater amounts in synovial fluid than in plasma.
The pH of topical drugs may also be a factor that could promote uptake and retention of the drug in the acidic microenvironment of inflamed tissues. In an acidic environment, protein binding is decreased and the more acidic NSAIDs (those with low pKa values, such as 3.9 for diclofenacCitation69) will be un-ionized and able to cross membrane barriers; such NSAIDs will thus reach a higher concentration at cell membranes and in neutral intracellular spaces containing COX-2 enzymes than in the relatively acidic extracellular space of inflamed tissueCitation56,Citation63,Citation97.
Preferential distribution of diclofenac to areas of inflammation rather than plasma is facilitated by its lipophilicity and higher distribution coefficient, and thus a greater likelihood of penetrating lipophilic membranes such as the synoviumCitation63. Despite the short half-life and relatively fast elimination of diclofenac from plasma, there are persisting therapeutic concentrations of the drug in areas of inflammationCitation34,Citation63. The synovial mean transit time has been estimated as 2–2.5 hCitation98 and its effect can be observed in the time course reported by Liauw and colleaguesCitation99, where the plasma to synovial fluid ratio of diclofenac levels increases for approximately 8 h (signaling a loss of drug from plasma with a slower loss from synovial fluid). A similar effect is seen for soft tissue, where muscle levels are sustained longer than those in plasmaCitation90. The duration of effect in tissue may be sustained by the reservoir of diclofenac that accumulates in the skin after repeated applicationCitation100, from which diclofenac is continuously released into the target tissues and the relatively slower clearance from tissuesCitation90.
As already indicated, the penetration of diclofenac into tissue depends on the formulation and route of administration – but the speed of skin penetration (as measured by the Franz cell) does not consistently translate into tissue concentrationsCitation83. This implies that other formulation characteristics must also be influential. Similar data collected on topical diclofenac compared with oral diclofenac tablets provides additional complexityCitation83,Citation90. Comparison of these topical products shows that they have different ratios of tissue exposure relative to plasma exposure ().
Table 3. Ratios of average plasma to tissue concentrations for topical versus oral diclofenac after administration over 3 days (data adapted from Brunner et al.Citation83,Citation90).
A higher ratio, which derives from higher diclofenac levels in the tissue relative to plasma, is indicative of better penetration. The ratios following topical application contrast greatly with tissue exposure following oral administration; plasma levels are significantly higher while tissue levels are similar, thus yielding a significantly lower partition into tissue (i.e. a much smaller ratio). This may reflect direct penetration of topical products from the site of administration and minimal systemic redistribution. Optimizing this direct penetration requires more than fast in vitro skin penetration – data with the same APIs suggests a role for excipients in influencing tissue penetration alongside skin permeation, requiring further understanding and evaluation techniques in addition to in vitro data.
Suitability of diclofenac for topical administration
The described characteristics of diclofenac are thought to be optimal for penetration and uptake into local sites ()Citation50, although methods are needed to fully evaluate its potential. Topical diclofenac has been determined to exhibit acceptable efficiency for external use, based on the ratio between skin penetration to concentration in the target tissue, called the index of topical anti-inflammatory activity (ITAA)Citation101. The ITAA takes into account both biopharmaceutic (i.e. the facility to reach the target in the skin) and pharmacodynamic aspects (i.e. demonstration of a local therapeutic effect) of a drug and may give an indication of the anti-inflammatory efficacy of a topical NSAIDCitation101. Diclofenac has been shown to have a high transdermal penetrationCitation81, and was the most potent inhibitor of COX-2 activityCitation101, and therefore had the highest ITAA at 50%, 75% and 90% of COX-2 inhibition. However, the dermis was the target tissueCitation101, and it is less clear whether the concentrations of topical diclofenac are sufficient in peri- and intra-articular tissues.
Forecasting whether a formulation and its APIs led to those APIs achieving active concentrations in target tissue may not be consistently accurate, as demonstrated in our previous examples and in the seeming dislocation of rate of skin penetration and tissue penetration/API pharmacokinetics. There are limitations with in vitro dataCitation80,Citation92, and in silico based physiologically based pharmacokinetic (PBPK) modeling is relatively recent and focuses on predicting rates of penetration and plasma exposure rather than target tissue exposureCitation58,Citation102,Citation103. Alternatives include more labor intensive in vivo approaches that can measure at the site of action, including microdialysis and (specific to knee OA) joint sampling techniques like synovial biopsies and arthroplastiesCitation80,Citation104,Citation105. Animal models can also be used to anticipate human resultsCitation106. However appropriate these methods are, they all require data to establish the bioactivity of forecast drug levels in tissue.
Concentration of topical diclofenac in target tissues compared with plasma
Data obtained after oral application
The extent to which an NSAID reaches inflamed tissues gives an indication of the likely efficacy of the drugCitation107,Citation108. The data obtained following oral administration of diclofenac provides some characterization of the drug levels required in the knee joint. This oral data provides a context for understanding diclofenac levels achieved in the knee. They also provide context for levels that need to be achieved when developing new products. Most of the recent data is collected in studies where knee bioactivity and diclofenac levels in the knee and plasma are measured exclusively, forgoing the chance to identify the relationships between systemic drug levels, site of action drug levels, and the bioactivity they provide. Prostaglandin production (specifically PGE2) is a surrogate measure of COX-2 activityCitation109. Therefore, inhibition of PGE2 may be regarded as an indicator of COX-2 inhibition. Few studies have measured the therapeutically active concentrations of diclofenac in vivo or ex vivo. Chlud and Wagener determined that 100–500 ng/ml of diclofenac reduced PGE2 in the OA synovium and was "therapeutically effective"Citation110. Liauw et al. investigated the concurrent diclofenac levels in plasma and synovial fluid and PGE2 levels in synovial fluid after oral treatment with 75 mg diclofenac tabletsCitation99. The diclofenac IC50 (i.e. the concentration that produces 50% of the maximum inhibition of prostaglandin synthesisCitation31) for PGE2 in synovial fluid was calculated to be 45 ng/mlCitation99. Using this IC50, the therapeutically active concentrations of 100–500 ng/ml predict PGE2 reductions of 85–100%, consistent with the effects noted by Chlud and WagenerCitation110. Martel-Pelletier and colleagues confirmed the effects of active diclofenac levels by spiking synovial tissue samples (ex vivo, stimulated with lipopolysaccharide stimulation [LPS]) with 125 and 250 ng/ml diclofenac, leading to >90% inhibition of PGE2 synthesisCitation111. Therefore, based on our own calculations (cf. Liauw et al.Citation99, Chlud and WagenerCitation110, Martel-Pelletier et al.Citation111), a diclofenac concentration in synovial tissues of 45 ng/ml is associated with a 50% reduction (IC50) in PGE2 (acting as a surrogate for inhibition of COX-2), while >100 ng/ml is associated with >80% reduction in PGE2. This data and the corresponding IC50 values for the synovial tissue differ significantly from those estimated in whole blood (which can be found in ); the in vivo IC50 levels are much higher than these in vitro levels, and higher diclofenac concentration levels would be therefore required to reach in vivo levels.
Table 4. Minimal effective therapeutic concentrations of diclofenac in target tissues, as reported in the literature.
The inconsistency between synovium and whole blood results may be due to differences in experimental process and/or differences in the biological environment. The in vitro whole blood evaluation of PGE2 following LPS stimulation follows a set protocolCitation109, whereas the clinical data followed an alternative methodology as the patient data from Liauw et al. did not require LPS stimulationCitation117. The data from Martel-Pelletier et al. (in synovial tissue with LPS stimulation, similar to whole blood assay) was consistent with the patient dataCitation111 and not the whole blood IC50 results. Another possible reason for the difference is the biological environment. The whole blood assay is performed using 10 μg/mL LPS in heparinized whole blood and so contains albumin, lymphocytes, etc. associated with diclofenac disposition and the immune response. Conversely, synovial fluid does not contain the same amount of lymphocytes and has a small and significant difference in plasma protein bindingCitation118. Whether due to assay or environment, the differences indicate that the in vitro estimates of COX-2 inhibition through inhibition of PGE2 production greatly overestimate the inhibition caused by diclofenac in the synovial environment. The PGE2 whole blood assay is a convenient in vitro method for assessing COX-2 potency in blood and it matches the ex vivo inhibition seen in human subject blood following administration of NSAIDs. However, the observed human in vivo/ex vivo potency is shown to be different, so evaluation of COX-2 inhibition should be done with human samples in vivo or by replicating the assay in synovial explanted tissueCitation104,Citation111,Citation117.
This potency data for diclofenac in OA knee tissue following oral administration provides context to the diclofenac levels achieved following topical administration. This would allow an initial evaluation, based on COX-2 and PGE2 pharmacology, of whether the levels achieved are active or not. Further context to expected bioactivity/efficacy would also be provided by considering the downstream therapeutic and biochemical consequences of the PGE2 synthesis inhibition, with effects on therapeutic endpoints such as the Western Ontario and McMaster Universities Arthritis (WOMAC) index and/or inflammatory cytokines in the PGE2 signal transduction pathwayCitation104.
Inflammatory cytokines are downstream of PGE2 within the signal transduction pathwayCitation119. The PGE2 levels can correlate with inflammatory cytokines, including interleukin (IL)-6 and tumor-necrosis factor (TNF)α – reduction of PGE2 synthesis coincides with reduction in these cytokinesCitation120,Citation121. Gallelli and colleagues demonstrated that IL-6 and TNFα reduced in a dose-dependent manner in OA patient synovial tissue when patients were treated orally with either 75 mg or 150 mg diclofenac per dayCitation104. This reduction in cytokines matched the reduction in PGE2 observed at the same dose, and also matched an improvement in the composite OA score, the WOMAC index. As might be expected, there was a smaller improvement in WOMAC score with oral diclofenac 75 mg/day compared with 150 mg/day, but the improvement was nonetheless clinically meaningful. A dose of 75 mg/day diclofenac represents the over-the-counter (OTC) limit in many countries. It is associated with an approximate average synovium diclofenac concentration of 50–175 ng/ml (which we estimated from Elmquist et al.Citation98, Liauw et al.Citation99 and Fowler et al.Citation122 [Supplementary Tables 2–4], assuming linear pharmacokinetics) following single and steady state dosing respectively associated with estimated peak PGE2 inhibition of >50%.
Topical versus oral application
In the studies that directly compared topical and oral administration, maximum plasma concentrations of diclofenac after topical application were generally lower than after oral administration, falling within a range of 0.4–2.4% of those achieved after oral administration (Supplementary Table 3). This observation is in agreement with the 0.2–8% reported in other reviewsCitation33,Citation36,Citation37. The mean plasma concentrations and plasma AUC values were also lower after topical versus oral administration (between 6–71% and 0.6–21% lower, respectively; Supplementary Tables 2 and 4), indicating that plasma diclofenac concentrations were lower over time with topical administration than with oral administration. Furthermore, maximum plasma diclofenac concentrations were achieved more slowly after topical administration (1.25–30 hours) compared with oral administration (20 minutes to 6.5 hours). The results from Brunner et al.Citation90 indicate a steep tissue-to-plasma gradient; the relative bioavailability of diclofenac in the target tissue (subcutaneous adipose and skeletal muscle) was substantially higher after topical dosing (324%) than oral dosing (29%), whereas relative plasma bioavailability was 50-fold lower.
Topical application
Topical NSAIDs are considered effective in treating joint painCitation15,Citation43,Citation45. This efficacy was established in clinical trials using endpoints such as the WOMAC score, for which the oral data suggests a link with joint drug levels and their effects on PGE2. Evaluating the joint concentrations of diclofenac, in the context of the oral data, may shed insight into how these products have an effect and what might be target levels of drug.
In OA, topical NSAIDs seem to work by permeating through the skin to reduce inflammation in periarticular structures, and travelling via the local blood supply to reduce inflammation within the joint itselfCitation15. Therefore, it is important to have a clear understanding of the concentrations of topical diclofenac that can be reached in synovial fluid and synovial tissue. Accordingly, we searched the literature to identify studies that measured the concentration of diclofenac in various compartments after topical administration (Supplementary Tables 2–4). It is important to note that there were many inconsistencies in methodological approaches between these topical diclofenac studies, which makes it difficult to draw firm conclusions on concentrations in the target tissues. The available data is variable due to differences in study designs (e.g. dose size, regimen, etc.), but also between similar trials and between subjects within the trials. It was not possible to differentiate the data, thus the concentration data discussed below does not reflect the dose or formulation used and only general trends can be observed. Future studies should adopt a standardized approach using consistent criteria to enable a more robust comparison.
Despite the above-mentioned shortcomings, it has been demonstrated that topically administered diclofenac penetrates through the skin and permeates to the target tissues in appreciable amounts, with different concentrations within the knee (Supplementary Tables 2–4). The mean diclofenac concentrations varied across tissues, from 90.6 ng/ml in subcutaneous tissue to 9.3–63.3 ng/g in muscle, 4.99–20.4 ng/g in synovial tissue, 1.02–25.5 ng/ml in synovial fluid, and 1.42–40.6 ng/ml in plasma (Supplementary Table 2), with a similar pattern in the maximum concentrations obtained (Supplementary Table 3). Overall, the mean concentrations of diclofenac after topical administration appear to be similar in synovial fluid and plasma. Diclofenac concentrations were generally higher in synovial tissue than in synovial fluid or plasma after topical administration. Although there is little data, it appears that after topical administration the concentration of diclofenac declined from subcutaneous tissue > muscle > synovial tissue > synovial fluid ≈ plasma. As might be expected, repeat dosing led to higher levels than following single dose administration.
A typical topical OTC dose is 160 mg of diclofenac per day. There is sparse joint concentration data, with most obtained from single time points and few at relevant doses. The scarcity of data means that it is not possible to draw any conclusions about what levels of diclofenac are reached for comparison with oral data or evaluation of prostaglandin inhibition – further studies are required.
Duration of exposure in target tissue
The tissue concentrations of diclofenac achieved after oral administration demonstrate a durable and sustained exposure compared to plasma levels (Supplementary Table 4). A similar trend in joints is expected following topical administration, where soft tissue exposure is similarly durable and consistent and independent of the dose routeCitation96. This was observed in an in vivo comparison of oral vs. topical diclofenacCitation90. Diclofenac concentrations were sustained over 48 h in subcutaneous tissue and muscle after both topical and oral administration, although higher levels were observed after topical delivery; plasma levels were significantly lower after topical administrationCitation90. Supplementary Table 4 indicates that the mean diclofenac concentration AUC values over the 12 hour period after topical application ranged 1.41–8867 ng·h/ml in the subcutaneous tissue, 1.09–18.2 ng·h/ml in muscle, and 7.30–1224.19 ng·h/ml in plasma, with a median value between 93 and 142 in synovial fluid (Supplementary Table 4). In general, the AUC declined from plasma > synovial fluid > subcutaneous tissue ≈ muscle. This order was also observed after oral administration, but the AUC was much higher in plasma while the AUC was much lower in subcutaneous tissue and muscle compared with topical application.
Effective concentrations in target tissues after topical application
To determine whether the concentrations of diclofenac reported throughout the skin and joint after topical application are sufficient to exert a therapeutic effect, they may be compared against the minimal concentrations of diclofenac (IC50) that have been reported to have a therapeutic effect. The human data, which provides a more cautious IC50 compared to in vitro data, suggests a higher IC50 of approximately 45 ng/ml. It should be noted that the mechanisms of COX inhibition are variable and complex and there should be a certain degree of caution when interpreting the IC50 valuesCitation123. The contributing data for diclofenac levels in joints following topical administration are sparse and insufficient to estimate the levels of PGE2 inhibition. We know the relationship between synovium drug levels and PGE2 from oral studies, but there is poor data on either endpoint for topical NSAIDs. Furthermore, we are unable to determine the degree of effectiveness of the diclofenac concentrations observed in Supplementary Tables 2–4. We know that topical diclofenac is effective, but we can’t associate this effectiveness with a joint diclofenac concentration or reduction in PGE2 levels. There is a high placebo effect, but nevertheless topical NSAIDs are as effective as oral diclofenac in OACitation4. There is a link to clinical outcomes such as the WOMAC scale, but it needs to be finalized. More data is required to define a minimum effective drug concentration in the synovium and see how current or future topical diclofenac concentrations compare.
Even with low systemic availability, topical diclofenac can be effective in OA, supporting the notion that plasma concentrations are not necessarily an indication of efficacyCitation124. For example, in one study plasma levels of diclofenac were undetectable after topical administration for up to 4 hours; however, the antihyperalgesic effect 1 hour after dosing was 2.2-fold greater with topical diclofenac than oral diclofenac, corresponding to a subcutaneous tissue AUC value that was 2.6-fold higherCitation20. Topical diclofenac has been used effectively for many years in the management of OACitation4,Citation15,Citation33, with a lower rate of systemic adverse events than oral diclofenacCitation44.
We noted that in some studies after topical administration, diclofenac levels were higher in plasma than in synovial fluid or tissue levels. This may reflect the fact that faster penetration can increase plasma exposure, which is an undesirable situation for a topical drug as there is a potentially greater risk of systemic adverse events. Patient variability is also an important factor that influences how quickly effective concentrations are reached, with intra- and interindividual skin properties influencing percutaneous absorptionCitation42,Citation90. Interindividual variability has been shown to result in different concentrations of diclofenac in subcutaneous tissues, with a subsequent antihyperalgesic effect that was highest in patients with the highest tissue AUC valuesCitation20. For this reason, it is difficult to accurately compare different formulations of a topical NSAID in clinical trials.
A preferred trial approach would include a design where the plasma levels of drug are collected in parallel with concentrations at the sites of action and measures of bioactivity, i.e. PGE2, inflammatory cytokines, etc., in an approach similar to that used by Liauw and colleaguesCitation99. This could be performed using a combination of synovial biopsy and arthroplasty. The patients would already be scheduled for arthroplasty, making it unnecessary to schedule a significant intervention that is not beneficial to the patient. Use of synovial biopsy prior to surgery can strengthen the study design by allowing a repeated measures approach, where the biopsy provides the baseline followed by arthroplasty to evaluate the drug effect. Additionally, the arthroplasty could provide tissue suitable for advanced imaging techniques such as MALDI mass spectrometry. This approach would provide a holistic characterization of drug exposure and effect, increase the value of data provided, and provide important context between target drug levels, systemic exposure and drug effect.
Summary
Topical products were developed to reduce the potential for systemic effects that have been reported with orally administered drugs, and to deliver the active drug locally to the site of injury to relieve pain. They can be an effective alternative to orally administered drugs, and topical NSAIDs are recommended before the use of oral NSAIDs in the treatment of knee OA. The pharmacological action of topical drugs relies on penetration and permeation through the skin into the lower layers. Many factors can affect this process and need to be considered in the topical administration of NSAIDs, including the innate properties of the drug, the formulation used, the methods of application, and patient inter- and intraindividuality.
Taking these factors into consideration, there is a sound rationale to use topical diclofenac to relieve pain and inflammation in OA. The available evidence suggests that after topical application, the drug can penetrate the skin and permeate to deeper tissues, with generally higher levels in muscle than in plasma compared with oral administration. The concentrations achieved in the target tissues appear to be sufficient to exert a therapeutic effect, although these may be minimally effective levels. Repeat dosing is beneficial. Nevertheless, there is room for improvement with future formulations.
More data is required to evaluate the penetration and permeation after topical delivery. The available data for the concentration of diclofenac within various tissues after topical administration is old, sparse and inconsistent. Use of the Franz cell alone, although useful, only evaluates penetration and possibly systemic exposure, and does not provide an insight regarding the likely site of action levels and resulting efficacy of the drug. A better screening cascade incorporating Franz cell and other assays is needed. The absence of additional in vitro or in silico methods means downstream tissue permeation requires in vivo characterization of tissue concentrations and bioactivity. The synovium IC50 (approximately 45 ng/mL) is higher than that determined using the whole blood PGE2 assay. To estimate the clinical efficacy, the synovial approach is better than the whole blood approach as the latter will overestimate the efficacy. Thus, COX-2 inhibition should be done with human samples in vivo or replicating the assay in synovial explanted tissue. The use of IC50 needs to be clarified, particularly regarding the differences between in vitro and in vivo values.
Despite uncertainty regarding the concentration of diclofenac required to inhibit COX-2, it is clear that topically administered diclofenac is pharmacologically effective, and patients report significant pain relief in mild to moderate OA that extends beyond the placebo effect and is comparable to oral diclofenac. Thus, combined with its more favorable safety profile, there is a sound basis to use diclofenac administered topically rather than orally.
Transparency
Declaration of funding
There is no sponsorship or funding to declare from GlaxoSmithKline Consumer Healthcare or otherwise.
Author contributions: M.H. and M.B. were involved in the conception of the review, analysis and interpretation of the data, drafting of the manuscript and revising it critically for intellectual content, and final approval of the version to be published. Both authors are accountable for all aspects of the work.
Declaration of financial/other relationships
M.H. and M.B. have disclosed that they are salaried employees of GlaxoSmithKline Consumer Healthcare, who also paid for the services of the medical writer, Deborah Nock.
Supplementary Tables
Download MS Word (89.7 KB)Supplementary Appendix
Download MS Word (19.3 KB)Acknowledgements
Thanks are given to Beata Coffey, Information Specialist at the Royal Society of Medicine Library, for her systematic review of the literature. The manuscript was drafted and edited by a professional medical writer, Deborah Nock (Medical WriteAway, Norwich, UK).
References
- Brown MB, Martin GP, Jones SA, et al. Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv 2006;13:175-87
- Stanos SP. Osteoarthritis guidelines: a progressive role for topical nonsteroidal anti-inflammatory drugs. J Multidiscip Healthc 2013;6:133-7
- Barkin R, Berckerman M, Blum S, et al. Should nonsteroidal anti-inflammatory drugs (NSAIDs) be prescribed to the older adult? Drugs Aging 2010;27:775-89
- Rannou F, Pelletier JP, Martel-Pelletier J. Efficacy and safety of topical NSAIDs in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum 2016;45(4 Suppl):S18-S21
- Argoff C, Gloth FM. Topical nonsteroidal anti-inflammatory drugs for management of osteoarthritis in long-term care patients. Ther Clin Risk Manag 2011;7:393-9
- Chou R, McDonagh MS, Nakamoto E, et al. Analgesics for osteoarthritis: an update of the 2006 comparative effectiveness review. Comparative Effectiveness Review, Number 38. AHRQ Publication No. 11(12)-EHC076-EF. Rockville (MD): Agency for Healthcare Research and Quality (US). 2011
- Mason L, Moore R, Edwards J, et al. Topical NSAIDs for chronic musculoskeletal pain: systematic review and meta-analysis. BMC Musculoskelet Dis 2004;5:28
- Lin J, Zhang W, Jones A, et al. Efficacy of topical NSAIDs in the treatment of osteoarthritis: a meta-analysis of randomized, controlled trials. BMJ 2004;329:324
- National Institution for Health and Care Excellence (NICE). Osteoarthritis. Care and Management. Clinical Guidelines. 2014
- Bruyère O, Cooper C, Pelletier JP, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 2014;44:253-63
- Zhang W, Doherty M, Leeb BF, et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007;66:377-88
- Underwood M, Ashby D, Cross P, et al. Advice to use topical or oral ibuprofen for chronic knee pain in older people: randomised controlled trial and patient preference study. BMJ 2008;336:138-42
- Carnes D, Anwer Y, Underwood M, et al. Influences on older people’s decision making regarding choice of topical or oral NSAIDs for knee pain: qualitative study. BMJ 2008;336:142
- Nicholas Hall’s global OTC database D. 2015
- Derry S, Conaghan P, Da Silva JAP, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev 2016;4:CD007400
- Efe T, Sagnak E, Roessler PP, et al. Penetration of topical diclofenac sodium 4% spray gel into the synovial tissue and synovial fluid of the knee: a randomised clinical trial. Knee Surg Sports Traumatol Arthrosc 2014;22:345-50
- Sofat N, Ejindu V, Kiely P. What makes osteoarthritis painful? The evidence for local and central pain processing. Rheumatology 2011;50:2157-65
- Rahmati M, Mobasheri A, Mozafari M. Inflammatory mediators in osteoarthritis: a critical review of the state-of-the-art, current prospects, and future challenges. Bone 2016;85:81-90
- Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 2013;21:16-21
- Burian M, Tegeder I, Seegel M, et al. Peripheral and central antihyperalgesic effects of diclofenac in a model of human inflammatory pain. Clin Pharmacol Ther 2003;74:113-20
- Malfait A-M, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol 2013;9:654-64
- Gan T. Diclofenac: an update on its mechanism of action and safety profile. Curr Med Res Opin 2010;26:1715-31
- Dong X-D, Svensson P, Cairns B. The analgesic action of topical diclofenac may be mediated through peripheral NMDA receptor antagonism. Pain 2009;147:36-45
- Barron M, Rubin B. Managing osteoarthritic knee pain. J Am Osteopath Assoc 2007;107(Suppl 6):ES21-7
- Felson D. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am 2004;42:1-9
- O’Neill TW, Parkes MJ, Maricar N, et al. Synovial tissue volume: a treatment target in knee osteoarthritis. Ann Rheum Dis 2016;75:84-90
- Witt KL, Vilensky JA. The anatomy of osteoarthritic joint pain. Clin Anat 2014;27:451-4
- Creamer P, Hunt M, Dieppe P. Pain mechanisms in osteoarthritis of the knee: effect of intraarticular anesthetic. J Rheumatol 1996;23:1031-6
- Giuliano F, Warner TD. Ex vivo assay to determine the cyclooxygenase selectivity of non-steroidal anti-inflammatory drugs. Br J Pharmacol 1999;126:1824-30
- FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 2001;345:433-42
- Huntjens DRH, Danhof M, Della Pasqua OE. Pharmacokinetic–pharmacodynamic correlations and biomarkers in the development of COX-2 inhibitors. Rheumatology 2005;44:846-59
- Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg 2013;21:571-6
- Altman R, Bosch B, Brune K, et al. Advances in NSAID development: evolution of diclofenac products using pharmaceutical technology. Drugs 2015;75:859-77
- Davies N, Anderson K. Clinical pharmacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin Pharmacokinet 1997;33:184-213
- Evans JMM, McMahon AD, McGilchrist MM, et al. Topical non-steroidal anti-inflammatory drugs and admission to hospital for upper gastrointestinal bleeding and perforation: record linkage case control study. BMJ 1995;311:22-6
- McPherson ML, Cimino NM. Topical NSAID formulations. Pain Med 2013;14:S35-S9
- Weaver AL. Current and emerging treatments for mild/moderate acute ambulatory pain. Am J Ther 2008;15(Suppl 10):S12-S16
- Henry D, Lim L, Garcia Rodriguez L, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ 1996;312:1563-6
- Odom DM, Mladsi DM, Saag KG, et al. Relationship between diclofenac dose and risk of gastrointestinal and cardiovascular events: meta-regression based on two systematic literature reviews. Clin Ther 2014;36:906-17
- Balmaceda CM. Evolving guidelines in the use of topical nonsteroidal anti-inflammatory drugs in the treatment of osteoarthritis. BMC Musculoskelet Dis 2014;15:27
- Zacher J, Altman R, Bellamy N, et al. Topical diclofenac and its role in pain and inflammation: an evidence-based review. Curr Med Res Opin 2008;24:925-50
- Heyneman C, Lawless-Liday C, Wall G. Oral versus topical NSAIDs in rheumatic diseases. A comparison. Drugs 2000;60:555-74
- Moore R, Tramer M, Carroll D, et al. Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs. BMJ 1998;316:333-8
- Taylor RS, Fotopoulos G, Maibach H. Safety profile of topical diclofenac: a metaanalysis of blinded, randomized, controlled trials in musculoskeletal conditions. Curr Med Res Opin 2011;27:605-22
- Barkin RL. Topical nonsteroidal anti-inflammatory drugs: the importance of drug, delivery, and therapeutic outcome. Am J Ther 2015;22:388-407
- Chlud K, Wagener H. Percutaneous non-steroidal anti-inflammatory drug (NSAID) therapy with particular reference to pharmacokinetic factors. EULAR Bull 1987;2:40-3
- Massey T, Derry S, Moore R, et al. Topical NSAIDs for acute pain in adults. Cochrane Database Syst Rev 2010;6:CD007402
- Barry B. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharmaceut Sci 2001;14:101-14
- Dreiser RL. Topical antirheumatic drug therapy: current practice and future trends. Eur J Rheumatol Inflamm 1994;14:3-8
- Rainsford K, Kean W, Ehrlich G. Review of the pharmaceutical properties and clinical effects of the topical NSAID formulation, diclofenac epolamine. Curr Med Res Opin 2008;24:2967-92
- Clijsen R, Baeyens JP, Barel AO, et al. In vivo determination of the diclofenac skin reservoir: comparison between passive, occlusive, and iontophoretic application. Drug Des Dev Ther 2015;9:835-40
- Roberts MS, Cross SE, Anissimov YG. The skin reservoir for topically applied solutes. In: Bronaugh RL, Maibach H, eds. Percutaneous Absorption: Drugs, Cosmetics, Mechanisms, Methods. CRC Press, 2005:213-34
- Roberts MS, Cross SE. A physiological pharmacokinetic model for solute disposition in tissues below a topical application site. Pharm Res 1999;16:1392-8
- Riess W, Schmid K, Bott L, et al. [The percutaneous absorption of diclofenac]. Arzneim-Forsch/Drug Res 1986;36:1092-6
- Flynn G, Yalkowsky S, Roseman T. Mass transport phenomena and models. J Pharm Sci 1974;63:479-510
- Singh P, Roberts MS. Skin permeability and local tissue concentrations of non-steroidal anti-inflammatory drugs after topical application. J Pharm Exp Ther 1994;268:144-51
- Roberts MS, Cross SE, Anissimov YG. Factors affecting the formation of a skin reservoir for topically applied solutes. Skin Pharmacol Physiol 2004;17:3-16
- Jepps OG, Dancik Y, Anissimov YG, et al. Modeling the human skin barrier – towards a better understanding of dermal absorption. Adv Drug Deliv Rev 2013;65:152-68
- Lademann J, Knorr F, Richter H, et al. Hair follicles – an efficient storage and penetration pathway for topically applied substances. Skin Pharmacol Physiol 2008;21:150-5
- Scheuplein RJ. Mechanism of percutaneous adsorption: I. Routes of penetration and the influence of solubility. J Invest Dermatol 1965;45:334-46
- Gondolph-Zink B, Gronwald U. Active substance concentration in articular and periarticular tissues of the knee joint after cutaneous application of diclofenac-diethylammonium emulgel. Aktuelle Rheumatologie 1996;21:298-304
- Radermacher J, Jentsch D, Scholl MA, et al. Diclofenac concentrations in synovial fluid and plasma after cutaneous application in inflammatory and degenerative joint disease. Br J Clin Pharmac 1991;31:537-41
- Brune K. Persistence of NSAIDs at effect sites and rapid disappearance from side-effect compartments contributes to tolerability. Curr Med Res Opin 2007;23:2985-95
- Lee CM, Maibach H. Deep percutaneous penetration into muscles and joints. J Pharm Sci 2006;95:1405-13
- Herkenne C, Naik A, Kalia YN, et al. Ibuprofen transport into and through skin from topical formulations: in vitro-in vivo comparison. J Invest Dermatol 2007;127:135-42
- Magnusson BM, Anissimov YG, Cross SE, et al. Molecular size as the main determinant of solute maximum flux across the skin. J Invest Dermatol 2004;122:993-9
- Fini A, Laus M, Orienti I, et al. Dissolution and partition thermodynamic functions of some nonsteroidal anti-inflammatory drugs. J Pharm Sci 1986;75:23-5
- Hadgraft J, Somers G. Percutaneous absorption. J Pharm Pharmacol 1956;8:625-34
- Sengupta C, Afeche P, Meyer-Brunot H, et al. Diclofenac sodium. In: Rainsford K, ed. Anti-Inflammatory and Anti-Rheumatic Drugs, Volume II: Newer Anti-Inflammatory Drugs. Boca Raton, FL: CRC Press, 1985:49-63
- Raza K, Kumar M, Kumar P, et al. Topical delivery of aceclofenac: challenges and promises of novel drug delivery systems. Biomed Res Int 2014;2014:406731
- Hadgraft J. Passive enhancement strategies in topical and transdermal drug delivery Int J Pharm 1999;184:1-6
- Folzer E, Gonzalez D, Singh R, et al. Comparison of skin permeability for three diclofenac topical formulations: an in vitro study. Pharmazie 2014;69:27-31
- Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev 2012;64(Suppl):128-37
- Hasler-Nguyen N, Fotopoulos G. Effect of rubbing on the in vitro skin permeation of diclofenac-diethylamine 1.16% gel. BMC Res Notes 2012;5:321
- Reiter S. Topical non-steroidal anti-inflammatory drugs. Bundesgesundheitsbl – Gesundheitforsch – Gesundheitsschutz 2000;12:950-9
- Zhai H, Maibach H. Effects of skin occlusion on percutaneous absorption: an overview. Skin Pharmacol Appl Skin Physiol 2001;14:1-10
- Hewitt P, Poblete N, Wester R, et al. In vitro cutaneous disposition of a topical diclofenac lotion in human skin: effect of a multi-dose regimen. Pharm Res 1998;15:988-92
- Patel P, Schmieder S, Krishnamurthy K. Research techniques made simple: drug delivery techniques, part 2: commonly used techniques to assess topical drug bioavailability. J Invest Dermatol 2016;136:e43-9
- Bolzinger M, Briançon S, Pelletier J, et al. Penetration of drugs through skin, a complex rate-controlling membrane. Curr Opin Colloid Interface Sci 2012;17:156-65
- Herkenne C, Alberti I, Naik A, et al. In vivo methods for the assessment of topical drug bioavailability. Pharm Res 2008;25:87
- Cordero JA, Alarcon L, Escribano E. A comparative study of the transdermal penetration of a series of nonsteroidal antiinflammatory drugs. Pharm Sci 1997;86:503-8
- Goh C, Lane M. Formulation of diclofenac for dermal delivery. Int J Pharmaceut 2014;473:607-16
- Brunner M, Davies D, Martin W, et al. A new topical formulation enhances relative diclofenac bioavailability in healthy male subjects BJCP 2011;71:852-9
- Nivsarkar M, Maroo SH, Patel KR, et al. Evaluation of skin penetration of diclofenac from a novel topical non aqueous solution: a comparative bioavailability study. J Clin Diagnostic Res 2015;9:FC11-13
- Stahl J, Wohlert M, Kietzmann M. The effect of formulation vehicles on the in vitro percutaneous permeation of ibuprofen. BMC Pharmacol 2011;11:12
- Roth SH. The importance of differentiating between topical NSAID. Postgrad Med 2011;123:241-52
- Akomeah F, Nazir T, Martin GP, et al. Effect of heat on the percutaneous absorption and skin retention of three model penetrants. Eur J Pharm Sci 2004;21:337-45
- Dehghanyar P, Mayer BX, Namiranian K, et al. Topical skin penetration of diclofenac after single- and multiple-dose application. Int J Clin Pharmacol Ther 2004;42:353-9
- Müller M, Rastelli C, Ferri P, et al. Transdermal penetration of diclofenac after multiple epicutaneous administration. J Rheumatol 1998;25:1833-6
- Brunner M, Dehghanyar P, Seigfried B, et al. Favourable dermal penetration of diclofenac after administration to the skin using a novel spray gel formulation. Br J Clin Pharmacol 2005;60:573-7
- Stanos SP. Topical agents for the management of musculoskeletal pain. J Pain Symptom Manage 2007;33:342-55
- Ternullo S, de Weerd L, Flaten G, et al. The isolated perfused human skin flap model: a missing link in skin penetration studies? Eur J Pharm Sci 2017;96:334-41
- Flaten G, Palac Z, Engesland A, et al. In vitro skin models as a tool in optimization of drug formulation. Eur J Pharm Sci 2015;75:10-24
- Raney S, Franz T, Lehman P, et al. Pharmacokinetics-based approaches for bioequivalence evaluation of topical dermatological drug products. Clin Pharmacokinet 2015;54:1095-106
- Chik Z, Tucker AT, Shiel JI, et al. Comparative pharmacokinetic assessments of topical drugs: evaluation by dermatopharmacokinetics, microdialysis, and systemic measurement. J Invest Dermatol 2010;130:2828-30
- Day RO, McLachlan AJ, Graham GG, et al. Pharmacokinetics of nonsteroidal anti-inflammatory drugs in synovial fluid. Clin Pharmacokinet 1999;36:191-210
- Dutta SK, Basu SK, Sen KK. Binding of diclofenac sodium with bovine serum albumin at different temperatures, pH and ionic strengths. Indian J Exp Biol 2006;44:123-127
- Elmquist WF, Chan KK, Sawchuk RJ. Transsynovial drug distribution: synovial mean transit time of diclofenac and other nonsteroidal antiinflammatory drugs. Pharm Res 1994;11:1689-97
- Liauw H, Walter S, Lee L, et al. Effects of diclofenac on synovial eicosanoid product formation in arthritic patients. J Clin Pharmacol 1985;25:455-74
- Sioufi A, Pommier F, Boschet F, et al. Percutaneous absorption of diclofenac in healthy volunteers after single and repeated topical application of diclofenac Emulgel. Biopharm Drug Dispos 1994;15:441-9
- Cordero J, Camacho M, Obach R, et al. In vitro based index of topical anti-inflammatory activity to compare a series of NSAIDs. Eur J Pharm Biopharm 2001;51:135-42
- Anissimov Y, Roberts M. Mathematical models for topical and transdermal drug products. In: Shah VP, Maibach HI, Jenner J, eds. Topical Drug Bioavailability, Bioequivalence, and Penetration. New York, USA: Springer, 2014:249-98
- Chen L, Han L, Saib O, et al. In silico prediction of percutaneous absorption and disposition kinetics of chemicals. Pharm Res 2015;32:1779-93
- Gallelli L, Galasso O, Falcone D, et al. The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthritis Cartilage 2013;21:1400-8
- Gallelli L, Galasso O, Urzino A, et al. Characteristics and clinical implications of the pharmacokinetic profile of ibuprofen in patients with knee osteoarthritis. Clin Drug Invest 2012;32:827-33
- Wible JH, Barrett T, Devarakonda K, et al. Biodistribution of diclofenac following repeated topical applications of two diclofenac sodium formulations to minipigs. Biopharm Drug Dispos 2014;35:87-96
- Escribano E, Calpena AC, Queralt J, et al. Assessment of diclofenac permeation with different formulations: anti-inflammatory study of a selected formula. Eur J Pharm Sci 2003;19:203-10
- Benson MD, Aldo-Benso M, Brandt KD. Synovial fluid concentrations of diclofenac in patients with rheumatoid arthritis or osteoarthritis. Semin Arthritis Rheum 1985;15(12 Suppl):65-7
- Patrignani P, Panara MR, Greco A, et al. Biochemical and pharmacological characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthases. J Pharmacol Exp Therapeut 1994;271:1705-12
- Chlud K, Wagener H. Percutaneous therapy with non-steroidal anti-inflammatory drugs. Pharmacokinetic criteria of effectiveness. Fortschr Med 1991;109:59-60
- Martel-Pelletier J, Cloutier J-M, Pelletier J-P. Effects of aceclofenac and diclofenac on synovial inflammatory factors in human osteoarthritis. Clin Drug Invest 1997;14:226-32
- Kato M, Nishida S, Kitasato H, et al. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of non-steroidal anti-inflammatory drugs: investigation using human peripheral monocytes. J Pharmacy Pharmacol 2001;53:1679-85
- Riendeau D, Percival MD, Brideau C, et al. Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharm Exp Ther 2001;296:558-66
- Warner TD, Giuliano F, Vojnovic I, et al. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA 1999;96:7563-8
- Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med 1998;104:413-21
- Pairet M, van Ryn J, Schierok H, et al. Differential inhibition of cyclooxygenases-1 and -2 by meloxicam and its 4’-isomer. Inflammation Res 1998;47:270-6
- Liauw HL, Ku E, Brandt KD, et al. Effects of Voltaren on arachidonic acid metabolism in arthritis patients. Agents Actions Suppl 1985;17:195-9
- Chan KK, Vyas KH, Brandt KD. In vitro protein binding of diclofenac sodium in plasma and synovial fluid. J Pharm Sci 1987;76:105-8
- van Erk M, Wopereis S, Rubingh C, et al. Insight in modulation of inflammation in response to diclofenac intervention: a human intervention study. BMC Med Genomics 2010;3:5
- Haseeb A, Haqqi TM. Immunopathogenesis of osteoarthritis. Clin Immunol 2013;146:185-96
- Inoue H, Takamori M, Shimoyama Y, et al. Regulation by PGE2 of the production of interleukin-6, macrophage colony stimulating factor, and vascular endothelial growth factor in human synovial fibroblasts. Br J Pharmacol 2002;136:287-95
- Fowler PD, Dawes PT, John VA, et al. Plasma and synovial fluid concentrations of diclofenac sodium and its hyroxylated metabolites during once-daily administration of a 100mg slow release formulation. Eur J Clin Pharmacol 1986;31:469-72
- Gierse JK, Koboldt CM, Walker MC, et al. Kinetic basis for selective inhibition of cyclo-oxygenases. Biochem J 1999;339(Pt 3):607-14
- Muller PY, Milton MN. The determination and interpretation of the therapeutic index in drug development. Nat Rev Drug Discovery 2012;11:751-61

