Abstract
Objective: To assess the efficacy, safety, and tolerability of brexpiprazole as adjunctive treatment in adults with major depressive disorder (MDD) and an inadequate response to prior antidepressant treatment (ADT).
Methods: Patients with a current major depressive episode after prior treatment with 1−3 ADTs entered an 8- or 10-week prospective treatment phase in which they received double-blind placebo adjunct to open-label ADT. Inadequate responders were randomized (2:2:1) to brexpiprazole 2−3 mg/day, placebo, or quetiapine extended-release (XR) 150−300 mg/day, adjunct to the same ADT, for 6 weeks. The primary efficacy endpoint was the change from baseline (randomization) to week 6 in Montgomery−Åsberg Depression Rating Scale (MADRS) total score. The key secondary efficacy endpoint was the change in Sheehan Disability Scale (SDS) mean score.
Results: Adjunctive brexpiprazole showed a greater improvement in MADRS total score than adjunctive placebo (least squares mean difference [95% confidence interval] = −1.48 [−2.56, −0.39]; p = .0078), whereas adjunctive quetiapine XR did not separate from placebo (−0.30 [−1.63, 1.04]; p = .66). Adjunctive brexpiprazole failed to separate from placebo on the SDS mean score (−0.23 [−0.52, 0.07]; p = .13), but did improve functioning on two of the three SDS items (family life and social life). The most frequent treatment-emergent adverse events in patients receiving brexpiprazole were akathisia (6.1%), somnolence (5.6%), and headache (5.6%).
Conclusions: Adjunctive brexpiprazole 2−3 mg/day improved symptoms of depression compared with adjunctive placebo in patients with MDD and an inadequate response to ADTs, and was well tolerated with no unexpected side effects.
Introduction
Major depressive disorder (MDD) is a recurrent, chronic, and seriously impairing illness associated with substantial symptom severity. Despite the availability of numerous different types of antidepressant, most patients with MDD do not achieve an adequate response to such treatment. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, for example, response rates to initial antidepressant monotherapy were only ∼50%Citation1. Subsequent treatment courses resulted in even lower response rates, with over 80% of patients failing to achieve response during their third and fourth treatment attemptsCitation1. Incomplete response to treatment for MDD is associated with a prolonged loss of quality-of-life, functional status, and well-beingCitation2. Furthermore, general medical healthcare costs among patients with depression tend to increase with increasing lines of therapyCitation3.
For patients with inadequate response to antidepressant treatment (ADT), treatment options include switching to another ADT, adding a second antidepressant in combination, or adding another medication as adjunctive treatment (e.g. lithium, thyroid hormone, an atypical antipsychotic, or a stimulant)Citation4. Of these strategies, augmentation with an atypical antipsychotic is the most systematically and rigorously studied, is supported by the strongest evidence baseCitation4,Citation5, and is the only option with agents approved by the United States Food and Drug Administration.
Brexpiprazole is a serotonin−dopamine activity modulator that acts as a partial agonist at serotonin 5-HT1A and dopamine D2 receptors, and as an antagonist at serotonin 5-HT2A and noradrenaline α1B/2C receptors, all with sub-nanomolar potencyCitation6. The efficacy and safety of brexpiprazole as an adjunct to ADT over 6 weeks have been demonstrated in two fixed-dose studies in MDDCitation7,Citation8. Brexpiprazole is approved in the US as an adjunctive therapy to antidepressants for the treatment of adults with MDD, and in the US, Canada, and Australia as monotherapy for the treatment of adults with schizophrenia.
The objective of this study was to assess the efficacy, safety, and tolerability of flexibly dosed brexpiprazole as an adjunct to ADT in adults with MDD and an inadequate response to prior ADT. This study incorporated comprehensive blinding for investigators and patients to avoid the bias in rating and symptom reporting, respectively, that might occur due to knowledge of key study time points.
Methods
Patients
The study (Delphinus; ClinicalTrials.gov identifier NCT01727726; EudraCT number 2012-003948-67) was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice Guideline and local regulatory requirements. The study protocol was approved by the relevant institutional review boards (IRBs) or independent ethics committees (IECs). All patients provided written informed consent prior to the start of the study.
Patients were enrolled by investigators at 75 sites in the US (55.0% of patients), Russia (17.0%), Poland (13.0%), France (6.0%), Serbia (4.5%), Germany (4.4%), and Canada (0.0%; one patient). The study started on December 4, 2012 and was completed on November 10, 2016.
Eligible patients were male or female outpatients aged 18−65 years with a diagnosis of MDD, as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR)Citation9, and who were currently experiencing a major depressive episode of ≥8 weeks’ duration. During the current episode, patients must have reported an inadequate response, defined as <50% improved on the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire (ATRQ)Citation10, to between one and three ADTs at a therapeutic dose and for an adequate duration (≥6 weeks). In addition, eligible patients had a Montgomery−Åsberg Depression Rating Scale (MADRS)Citation11 total score of ≥26 at screening and on the first day of prospective treatment. Key exclusion criteria were treatment with adjunctive antipsychotic medication for ≥3 weeks during the current major depressive episode; a specified DSM-IV-TR Axis I diagnosis other than MDD; presenting with suicidal ideation or behavior; and substance abuse or dependence within the past 180 days.
Study design
This was a multi-center, randomized, double-blind, active-referenced, placebo-controlled study of the efficacy and safety of flexible-dose brexpiprazole (2−3 mg/day) as an adjunctive treatment for MDD. The study comprised a prospective treatment phase, a randomized treatment phase for patients who did not fully respond to prospective treatment, and a follow-up treatment phase, for a total of 18 weeks. However, to reduce potential bias, all study site personnel and patients were blinded to the phases of the study, the allocation of adjunctive treatments, the randomization criteria, the timing of randomization, and the timing of the efficacy endpoints. The unblinded study design was known only to the relevant regulatory authorities, IRBs, IECs, and sponsor.
Blinded study design
Following screening, eligible patients received an investigator-determined, open-label ADT from the following list: escitalopram, fluoxetine, paroxetine controlled-release (CR), sertraline, duloxetine, and venlafaxine extended-release (XR). Patients received the same ADT for the duration of the study. The maximum protocol-defined dose of ADT was used () unless prohibited by tolerability issues; dose changes were permitted for the first 4 weeks of treatment only.
Figure 1. Study design: (A) Blinded study design (as provided in the protocol for the investigators). (B) Unblinded study design (as per IVRS/IWRS design). From the start of prospective treatment, patients visited the study center at weekly intervals for the first 4 weeks and then every 2 weeks for the remainder of the 18-week treatment period. Abbreviations. ADT, antidepressant treatment; CR, controlled-release; IVRS/IWRS, interactive voice/web response system; XR, extended-release.
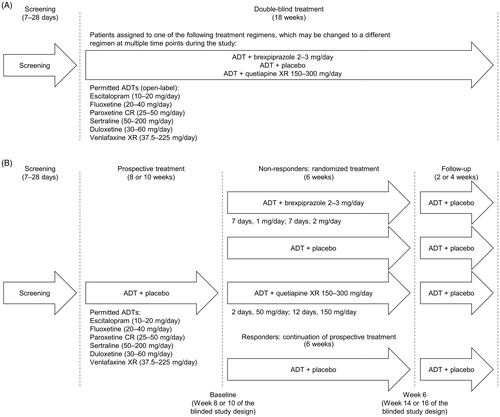
In addition to the open-label ADT, patients were assigned to double-blind adjunctive treatment. Study site personnel and patients were informed that each patient would receive either brexpiprazole, placebo, or quetiapine XR, and that this adjunctive treatment regimen might be changed at multiple time points during the study. Thus, to all study site personnel and patients, the study appeared as 18 weeks of uninterrupted treatment ().
Unblinded study design
Following screening, eligible patients entered a prospective treatment phase, during which they received placebo adjunct to ADT (). After 8 or 10 weeks,a patients were assessed for inadequate response, defined (following a protocol amendment to be more consistent with the other Phase 3 brexpiprazole studies in MDD) as meeting all of the following criteria: <50% reduction in MADRS total score between the start of prospective treatment and each 2-weekly visit; Clinical Global Impressions−Improvement (CGI-I) scaleCitation12 score ≥3 (minimally improved) at each 2-weekly visit; and MADRS total score ≥18 at the end of prospective treatment. To maintain the blinding, scores were entered into an interactive voice/web response system (IVRS/IWRS), which determined if the criteria for inadequate response were met and performed the randomization accordingly.
Patients who did not meet the criteria for inadequate response (i.e. responders to prospective ADT) continued to receive the same open-label ADT and double-blind placebo until the end of the study; these patients were not randomized. Only the randomized patients were included in the analyses.
Patients who met the criteria for inadequate response entered the randomized treatment phase, in which they were randomized in a 2:2:1 ratio to adjunctive brexpiprazole 2−3 mg/day (target dose 2 mg/day), placebo, or quetiapine XR 150−300 mg/day (target dose 150 mg/day), for 6 weeks. Quetiapine XR was included as an active reference to test assay sensitivity. Treatment assignments were based on a central, computer-generated randomization code provided by the study sponsor. Double-blind study medications were provided by the sponsor in numbered, weekly blister cards, and were assigned by the IVRS/IWRS. At any visit, an investigator could request an increase/decrease in the dose of double-blind medication, for reasons of efficacy or tolerability. If the patient was receiving an active adjunct, and the suggested dose adjustment was within the pre-defined dose range (2−3 mg/day for brexpiprazole, and 150−300 mg/day for quetiapine XR), the adjustment was implemented. Adjunctive treatments were administered orally, once-daily, at the same time as recommended for the investigator-assigned ADT, where possible. To maintain blinding, patients randomized to brexpiprazole tablets also took quetiapine XR placebo capsules, patients randomized to quetiapine XR capsules also took brexpiprazole placebo tablets, and patients randomized to placebo took both brexpiprazole and quetiapine XR placebos.
After 6 weeks of randomized treatment, all patients were switched back to double-blind placebo adjunct to the same ADT until they had completed a total of 18 weeks of treatment. Thus, the safety follow-up treatment phase was 2−4 weeks in duration, depending on the time of transition from the prospective phase into the randomized treatment phase.
Patients who completed 18 weeks of treatment may have been offered entry into an open-label rollover study. Patients who did not enter the open-label study, and any patients who withdrew early, were prescribed appropriate ADT, and had an additional safety follow-up via telephone or clinic visit 30 days after the last dose of double-blind medication.
Outcome measures
Efficacy assessments were made at screening, at the start of prospective treatment, and at each subsequent visit during the 18-week study. Depressive symptoms were measured using the clinician-rated MADRS, and overall illness severity was judged using the clinician-rated Clinical Global Impressions−Severity of illness (CGI-S) scaleCitation12. Patient functioning was assessed using the self-rated Sheehan Disability Scale (SDS)Citation13,Citation14, comprising the items (1) work/studies (including paid and unpaid volunteer work and training); (2) social life or leisure activities; and (3) family life or home responsibilities; each of which is scored from 0 (not at all disrupted) to 10 (extremely disrupted). Patients could skip the work/studies item if they had not worked/studied in the previous week for reasons unrelated to their disorder.
Safety and tolerability were assessed by the reporting of adverse events (AEs), extrapyramidal symptom (EPS) rating scales (the Simpson−Angus Scale [SAS]Citation15, the Abnormal Involuntary Movement Scale [AIMS]Citation12, and the Barnes Akathisia Rating Scale [BARS]Citation16), the Columbia Suicide Severity Rating Scale (C-SSRS)Citation17, and standard safety assessments including body weight, electrocardiograms, vital signs, and laboratory measurements.
Data analysis
Sample size was calculated based on an expected between-group difference of 3.0 points (standard deviation [SD] = 8.5) in the mean change in MADRS total score from baseline to week 6 of randomized treatment. A sample size of 200 in the ADT + brexpiprazole group, and 200 in the ADT + placebo group, was projected to yield at least 90% power to detect the treatment effects at a two-sided significance level of 0.05. Based on the 2:2:1 randomization ratio, 100 patients would be allocated to the ADT + quetiapine XR arm, which could yield 82% power to detect the same treatment effect. In order to randomize ∼500 patients, it was anticipated that ∼1,785 patients would need to be enrolled into the prospective treatment phase.
The safety population was defined as all randomized patients who took at least one dose of double-blind medication in the randomized treatment phase. The efficacy population was defined as all patients in the safety population who had a baseline and at least one post-baseline MADRS total score evaluation. Baseline was defined as the last available measurement prior to receiving the first dose of randomized treatment (provided that the measurement took place during the 2 weeks prior to randomization, for efficacy analyses).
The primary efficacy endpoint was the change from baseline to week 6 in MADRS total score, analyzed using a mixed model for repeated measures (MMRM), with fixed class effect terms for treatment, study center, and visit week, and interaction terms of treatment by visit week, and visit week by baseline MADRS total score (as covariate). The within-patient variation was modeled using an unstructured covariance matrix. The primary treatment comparison (ADT + brexpiprazole vs ADT + placebo) was tested at a significance level of 0.05 (two-sided), using least squares mean differences (LSMDs).
Sensitivity analyses were performed for the primary endpoint to address the effect of missing data being “missing not at random” (MNAR) on the estimated treatment difference. These sensitivity analyses were performed using pattern mixture models based on multiple imputation with mixed missing data mechanisms to investigate the response profile of patients who dropped out, by reason for dropout (AE or lack of efficacy; AE, lack of efficacy or withdrawal of consent; or all dropouts), under the MNAR mechanism. Delta adjustment imputation methods and placebo-based imputation methods were used.
The key secondary efficacy endpoint was the change from baseline to week 6 in SDS mean score, using the same MMRM analysis as for the primary endpoint. SDS mean score was calculated as the mean of the three individual item scores (or the mean of two scores if the work/studies item was not rated). A hierarchical testing procedure was employed in order to maintain the overall experiment-wise type I error rate at a level of 0.05. Only after statistical significance was demonstrated for the primary comparison was the key secondary endpoint tested at the same significance level of 0.05. A hierarchical testing procedure was also applied to the three SDS individual item scores, which were only tested if the SDS mean score yielded a statistically significant result, and which were tested in the following order: (1) family life; (2) social life; and (3) work/studies.
Other secondary efficacy endpoints were evaluated at a nominal 0.05 level (two-sided). Change from baseline to week 6 in CGI-S score was analyzed using MMRM. MADRS response, defined as a ≥50% reduction from baseline in MADRS total score, and MADRS remission, defined as a MADRS total score ≤10 and ≥50% reduction from baseline, were evaluated using the Cochran−Mantel−Haenszel general association test, with a last observation carried forward approach to address missing data at week 6.
To test assay sensitivity, a comparison of ADT + quetiapine XR vs ADT + placebo was performed for the primary and secondary efficacy endpoints.
Descriptive statistics are presented for safety and tolerability outcomes. In addition, the change from baseline in SAS, AIMS, and BARS scores was evaluated using analysis of covariance, adjusting for the baseline value and controlling for treatment and study center.
Results
Patients
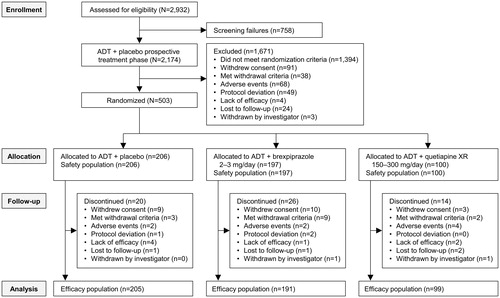
A total of 2,174 patients entered the prospective treatment phase, of whom 277 (12.7%) discontinued before the end of the phase, 1,394 (64.1%) responded to ADT + placebo at some point in the prospective treatment phase and were, therefore, excluded from randomized treatment, and 503 (23.1%) had an inadequate response to ADT + placebo and were, therefore, randomized.
Inadequate responders continued on the same ADT and were randomized to adjunctive brexpiprazole 2−3 mg/day (n = 197), adjunctive placebo (n = 206), or adjunctive quetiapine XR 150−300 mg/day (n = 100) (). All of these patients received at least one dose in the randomized phase and formed the safety population. After excluding eight patients without a post-baseline MADRS total score evaluation, the efficacy population comprised 191 patients for ADT + brexpiprazole, 205 patients for ADT + placebo, and 99 patients for ADT + quetiapine XR.
Baseline demographic and clinical characteristics of the randomized population were similar between treatment groups (). At baseline, the mean MADRS total score in each group was ∼25, and the mean CGI-S score was ∼4, indicating that the study population, on average, was moderately ill, despite 8 or 10 weeks of prospective ADT.
Table 1. Baseline demographic and clinical characteristics, and assigned antidepressant treatment (randomized population).
The randomized treatment phase was completed by 171 (86.8%) patients receiving ADT + brexpiprazole, 186 (90.3%) patients receiving ADT + placebo, and 86 (86.0%) patients receiving ADT + quetiapine XR. For patients in the ADT + brexpiprazole group, the mean dose of brexpiprazole at last visit was 2.2 mg. For patients in the ADT + quetiapine XR group, the mean dose of quetiapine XR at last visit was 198.5 mg.
Efficacy
On the primary efficacy endpoint of change from baseline to week 6 in MADRS total score (; ), the ADT + brexpiprazole group changed by a least squares mean (standard error) of −6.0 (0.4) points, and the ADT + placebo group changed by −4.6 (0.4) points. The difference between groups at week 6 was statistically significant in favor of ADT + brexpiprazole (LSMD [95% confidence interval] = −1.48 [−2.56, −0.39]; p = .0078). The ADT + quetiapine XR group did not separate from ADT + placebo at week 6, changing by −4.9 (0.6) points (LSMD = −0.30 [−1.63, 1.04]; p = .66). ADT + quetiapine XR did, however, show a benefit over ADT + placebo at week 2 (p = .010).
Figure 3. Effects of brexpiprazole, placebo, and quetiapine XR as adjunct to antidepressant treatment on Montgomery–Åsberg Depression Rating Scale (MADRS) total score (efficacy population). *p < .05, **p < .01, ***p < .001 vs placebo. Baseline MADRS total scores: ADT + placebo = 25.4; ADT + brexpiprazole = 25.3; ADT + quetiapine XR = 25.6. Abbreviations. ADT, antidepressant treatment; LS, least squares; SE, standard error; XR, extended-release.
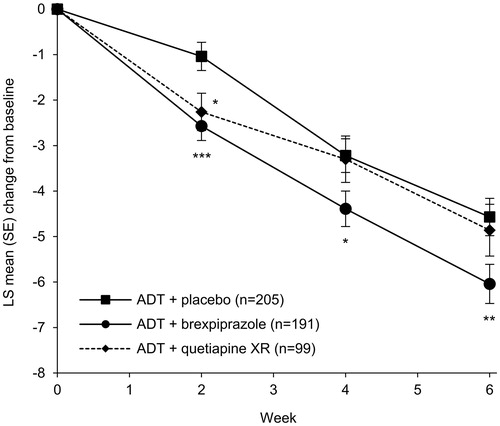
Table 2. Effects of brexpiprazole, placebo, and quetiapine XR as adjunct to antidepressant treatment on efficacy endpoints at week 6 (efficacy population).
In sensitivity analyses, the statistically significant treatment effect favoring ADT + brexpiprazole over ADT + placebo for change from baseline to week 6 in MADRS total score was maintained even when missing data were simulated with severe penalties put on ADT + brexpiprazole, using either delta adjustment or placebo-based imputation.
On the key secondary efficacy endpoint of change from baseline to week 6 in SDS mean score (; ), ADT + brexpiprazole showed improvement from baseline with a numerical benefit over ADT + placebo; however, this benefit was not statistically significant and the formal testing procedure was halted (LSMD = −0.23 [−0.52, 0.07]; p = .13). In contrast, the ADT + quetiapine XR group showed less improvement from baseline than ADT + placebo (LSMD = 0.42 [0.06, 0.78]; p = .024).
Figure 4. Effects of brexpiprazole, placebo, and quetiapine XR as adjunct to antidepressant treatment on Sheehan Disability Scale (SDS) scores at week 6 (efficacy population). *p < .05 vs placebo, in favor of active treatment (considered nominal within the formal testing hierarchy). Baseline SDS mean scores: ADT + placebo = 5.7; ADT + brexpiprazole = 5.6; ADT + quetiapine XR = 5.6. Patient numbers for the work/studies item: ADT + placebo, n = 136; ADT + brexpiprazole, n = 125; ADT + quetiapine XR, n = 67. Abbreviations. ADT, antidepressant treatment; LS, least squares; SE, standard error; XR, extended-release.
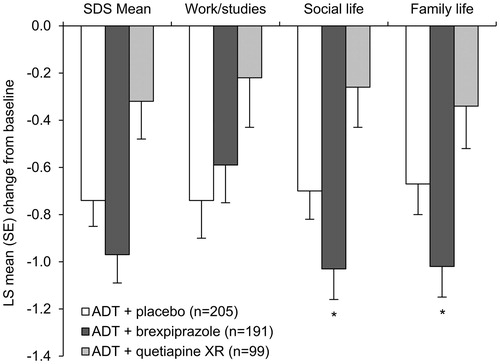
Considering the change from baseline to week 6 in individual SDS item scores (), ADT + brexpiprazole showed a benefit over ADT + placebo on the family life item (LSMD = −0.35 [−0.69, −0.01]; p = .042) and the social life item (LSMD = −0.34 [−0.66, −0.01]; p = .044), but not on the work/studies item (LSMD = 0.16 [−0.25, 0.56]; p = .45). In contrast, ADT + quetiapine XR showed no benefit over ADT + placebo on each of the three item scores: family life (LSMD = 0.33 [−0.09, 0.75]; p = .12), social life (LSMD = 0.43 [0.03, 0.84]; p = .035), and work/studies (LSMD = 0.52 [0.03, 1.02]; p = .038).
Other secondary efficacy endpoints are presented in . The ADT + brexpiprazole group showed a greater improvement in CGI-S score from baseline to week 6 compared with ADT + placebo (p = .035), whereas the ADT + quetiapine XR group did not differ from ADT + placebo (p = .56). Response rate on ADT + brexpiprazole (10.5%) was numerically higher than on ADT + placebo (6.8%), but did not separate between treatments (p = .22).
Safety and tolerability
From baseline of the randomized treatment phase, the most frequent treatment-emergent AEs (TEAEs) (≥5%) in patients receiving ADT + brexpiprazole were akathisia (6.1%), somnolence (5.6%), and headache (5.6%) (), which were all mild or moderate in severity. Aside from akathisia, other activating side effects were reported by only a few patients receiving ADT + brexpiprazole (restlessness = 5/197 [2.5%]; insomnia = 5/197 [2.5%]; agitation = 1/197 [0.5%]; anxiety = 1/197 [0.5%]). Sedating side effects other than somnolence were also uncommon with ADT + brexpiprazole (fatigue = 3/197 [1.5%]; sedation = 0/197 [0.0%]).
Table 3. Treatment-emergent adverse events (TEAEs) from baseline of the randomized treatment phase (safety population).
No TEAEs had an incidence of ≥5% in the ADT + placebo group. The most frequent TEAEs (≥5%) in patients receiving ADT + quetiapine XR were somnolence (18.0%), dry mouth (6.0%), and increased appetite (5.0%) (). Sedation was reported by 3/100 patients (3.0%).
The most frequently reported EPS-related TEAE was akathisia (6.1% in the ADT + brexpiprazole group; 1.9% in the ADT + placebo group; 3.0% in the ADT + quetiapine XR group). In the analysis of change from baseline in EPS scale scores, two of the three scales showed small treatment differences between ADT + brexpiprazole and ADT + placebo at last visit, in favor of ADT + placebo (BARS global, LSMD = 0.13, p = .0048; and SAS, LSMD = 0.11, p = .0097). ADT + quetiapine XR did not differ from ADT + placebo on any scale at last visit.
The mean change in body weight from baseline to week 6 was 1.0 kg for ADT + brexpiprazole (n = 171), 0.2 kg for ADT + placebo (n = 188), and 1.3 kg for ADT + quetiapine XR (n = 88). Body weight increase (≥7%) at any post-baseline visit was reported by 11/193 (5.7%) patients in the ADT + brexpiprazole group, 5/205 (2.4%) patients in the ADT + placebo group, and 5/99 (5.1%) patients in the ADT + quetiapine XR group.
The assessment of electrocardiograms, vital signs, and laboratory measurements (including glucose, cholesterol, and triglycerides; ) did not show any consistent differences between the ADT + brexpiprazole, ADT + quetiapine XR, and ADT + placebo groups. Changes in serum prolactin concentration in the ADT + brexpiprazole and ADT + quetiapine XR groups were comparable with those in the ADT + placebo group (). One patient in the ADT + placebo group had a mild TEAE of blood prolactin increased. During the randomized treatment phase, metabolic syndrome emerged in 2/141 (1.4%) patients in the ADT + brexpiprazole group, 1/158 (0.6%) patients in the ADT + placebo group, and 2/69 (2.9%) patients in the ADT + quetiapine XR group.
Table 4. Laboratory assessments (safety population).
Treatment with ADT + brexpiprazole did not appear to increase suicidal behavior or ideation. Only one patient exhibited treatment-emergent suicidal behavior while taking ADT + brexpiprazole. Treatment-emergent suicidal ideation was exhibited by 6/197 (3.0%) patients in the ADT + brexpiprazole group, 10/206 (4.9%) patients in the ADT + placebo group, and 3/100 (3.0%) patients in the ADT + quetiapine XR group. One patient died, during the prospective treatment phase (receiving sertraline + placebo), as a result of an accidental overdose of benzodiazepines.
Discussion
This flexible-dose study (Delphinus) confirms the results of two fixed-dose studies with adjunctive brexpiprazole 2 and 3 mg/day in MDDCitation7,Citation8. Thus, Delphinus expands the evidence base in support of a clinical role for brexpiprazole as adjunctive treatment to ADT in patients with MDD and inadequate response to ADTs. In contrast, ADT + quetiapine XR did not separate from ADT + placebo on the primary measure at week 6. Quetiapine XR was selected as an active reference since it is indicated for use as adjunctive therapy to antidepressants for the treatment of MDD in the US. Quetiapine XR 150 mg/day has shown inconsistent results in other clinical studiesCitation18,Citation19, and a definitive explanation for the lack of separation at week 6 in this trial could not be found.
As in previous studies, adjunctive brexpiprazole 2−3 mg/day improved functioning on two SDS items (family life and social life)Citation7,Citation8. Whereas previous studies showed a numerical benefit for brexpiprazole over placebo on the work/studies itemCitation7,Citation8, Delphinus did not. This lack of effect on the work/studies item may have contributed to the SDS mean score not separating—a common issue among studies that use the SDS in patients with inadequate response to ADTCitation20. Deficits in work performance can persist for 18 months among patients whose depressive symptoms have improvedCitation21. Thus, acute MDD studies may not be able to detect a benefit in work functioning, particularly among patients with inadequate response to ADT, who are at risk of persisting impairmentCitation22.
The majority of patients, in all three treatment arms, remained in treatment for the duration of the study, indicating that the outcomes are robust, and that the adjunctive treatments were well tolerated. Safety and tolerability outcomes for adjunctive brexpiprazole were generally consistent with observations from other short-term brexpiprazole MDD studiesCitation23. For adjunctive quetiapine XR, the incidences of somnolence (18.0%) and dry mouth (6.0%) were lower than those reported in previous randomized, controlled studies in MDD (somnolence including sedation, 37−43%; dry mouth, 27−40%; depending on dose)Citation24.
Putting these results into context, a meta-analysis of 14 randomized trials of adjunctive atypical antipsychotics for the treatment of MDD (conducted prior to the availability of brexpiprazole data) found that all agents showed small-to-moderate-sized benefits in terms of reducing depressive symptoms, while differentiating on the risk of specific adverse effectsCitation25. For example, aripiprazole was associated with the greatest risk of akathisia, quetiapine with the greatest risk of sedation, and olanzapine−fluoxetine combination with the greatest risk of abnormal metabolic laboratory results and weight gainCitation25. In the present brexpiprazole study, akathisia and sedating adverse events had a relatively low incidence, the mean weight gain was small, and there was no indication of an adverse effect on metabolic parameters. Thus, the introduction of brexpiprazole may provide an additional, well-tolerated treatment option in MDD.
The innovative design of Delphinus evolved during the brexpiprazole MDD clinical program as an attempt to minimize the placebo effectb—a common criticism of short-term studies of adjunctive treatment in MDD—and to more accurately identify patients with inadequate response to ADT. In Phase 2 brexpiprazole studies, as well as in studies of adjunctive aripiprazole, inadequate response to ADT was defined based on rating scale scores at the end of the 8-week prospective treatment phaseCitation26−28. However, in the Phase 2 brexpiprazole studies, a proportion of patients who were classed as inadequate responders at randomization were in fact responders at other times during the prospective treatment period, and, thus, were not “true” inadequate responders. Consequently, in the first Phase 3 brexpiprazole studies, the protocols were amended to specify that patients had to meet inadequate response criteria throughout the 8-week prospective treatment phaseCitation7,Citation8. In Delphinus, the additional blinding of response criteria and the timing of study phases (i.e. an 8- or 10-week prospective treatment phase) allowed the unbiased selection of a population of patients with inadequate response to ADT. Interestingly, the proportion of responders to ADT during the prospective treatment phase was substantially higher in Delphinus (64.1%) than in previous studies (40.2%, 39.2%)Citation7,Citation8. This may, in part, be a consequence of the comprehensive blinding in Delphinus, which lessened anticipation of randomization by investigators in the prospective treatment phase, and may have led to the expectation of getting active adjunctive treatment from the start of the study. Furthermore, for some patients, the prospective treatment phase was 2 weeks longer in this study than in previous studies, giving an additional visit at which prospective ADT response criteria could be met. The study design also had an impact in the randomized treatment phase, resulting in slightly smaller improvements from baseline in MADRS total score than in the previous brexpiprazole studies, and lower MADRS response and remission rates. However, the separation from placebo in terms of MADRS total score was maintained, further supporting the efficacy of brexpiprazole in these patients. Overall, studies such as this one with rigorous blinding are infrequently conducted (three studies of adjunctive edivoxetine in MDD used a similar methodologyCitation29), and serve as a valuable contribution to the field of clinical research in MDD with inadequate response to ADT.
The principle limitation of Delphinus was the relatively short duration of double-blind treatment. In addition, due to its blinded design and prospective treatment phase, the outcomes of this study are not directly comparable with trials that did not incorporate a prospective phase, such as other published studies of adjunctive quetiapineCitation18,Citation19. Finally, the protocol did not exclude patients who had previously received adjunctive antipsychotics, unless these agents were taken for >3 weeks.
Conclusions
Adjunctive brexpiprazole (flexible dose 2−3 mg/day) improved symptoms of depression, and showed benefits on functioning in two domains (family life and social life), compared with adjunctive placebo among patients with MDD and an inadequate response to ADTs. Brexpiprazole was well-tolerated in patients with MDD when administered as adjunctive treatment, with no unexpected side effects.
Transparency
Declaration of funding
This study was supported by Otsuka Pharmaceutical Development & Commercialization Inc. (Princeton, US) and H. Lundbeck A/S (Valby, Denmark). The sponsors were responsible for the study design and conduct; the collection, management, analysis, and interpretation of the data; and the writing and reviewing of this article.
Declaration of financial/other relationships
M.H., A.S., P.Z., C.A., C.B., R.S., and R.D.M. have disclosed that they are full-time employees of Otsuka Pharmaceutical Development & Commercialization Inc. M.K.J. and N.H. have disclosed that they are full-time employees of H. Lundbeck A/S. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work, but have no other relevant financial relationships to disclose.
Acknowledgments
Writing support was provided by Chris Watling, PhD, assisted by his colleagues at Cambridge Medical Communication Ltd (Cambridge, UK), and funded by Otsuka Pharmaceutical Development & Commercialization Inc. and H. Lundbeck A/S.
All authors contributed towards data analysis/interpretation, drafting and revising the paper, and agree to be accountable for all aspects of the work.
Notes
Notes
aPatients were randomly assigned to an 8- or 10-week prospective treatment phase (compared with an 8-week prospective treatment phase in previous adjunctive brexpiprazole studies) to further blind the timing of randomization.
References
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163:1905-17
- Mauskopf JA, Simon GE, Kalsekar A, et al. Nonresponse, partial response, and failure to achieve remission: humanistic and cost burden in major depressive disorder. Depress Anxiety 2009;26:83-97
- Russell JM, Hawkins K, Ozminkowski RJ, et al. The cost consequences of treatment-resistant depression. J Clin Psychiatry 2004;65:341-7
- Connolly KR, Thase ME. If at first you don’t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs 2011;71:43-64
- Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry 2009;166:980-91
- Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin−dopamine activity modulator. J Pharmacol Exp Ther 2014;350:589-604
- Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a Phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry 2015;76:1224-31
- Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a Phase 3, randomized, double-blind study. J Clin Psychiatry 2015;76:1232-40
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed text revision. Washington, DC: American Psychiatric Association; 2000
- Chandler GM, Iosifescu DV, Pollack MH, et al. Validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther 2010;16:322-5
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382-9
- Guy W. ECDEU assessment manual for psychopharmacology, revised. Rockville, MD: National Institute of Mental Health; 1976
- Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol 1996;11(Suppl3):89-95
- Sheehan KH, Sheehan DV. Assessing treatment effects in clinical trials with the discan metric of the Sheehan Disability Scale. Int Clin Psychopharmacol 2008;23:70-83
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970;212:11-9
- Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry 1989;154:672-6
- Posner K, Brown GK, Stanley B, et al. The Columbia−Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011;168:1266-77
- Bauer M, Pretorius HW, Constant EL, et al. Extended-release quetiapine as adjunct to an antidepressant in patients with major depressive disorder: results of a randomized, placebo-controlled, double-blind study. J Clin Psychiatry 2009;70:540-9
- El-Khalili N, Joyce M, Atkinson S, et al. Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in major depressive disorder (MDD) in patients with an inadequate response to ongoing antidepressant treatment: a multicentre, randomized, double-blind, placebo-controlled study. Int J Neuropsychopharmacol 2010;13:917-32
- Weiller E, Weiss C, Watling CP, et al. Functioning outcomes with adjunctive treatments for major depressive disorder: a systematic review of randomized placebo-controlled studies. Neuropsychiatr Dis Treat 2018;14:103-15
- Adler DA, McLaughlin TJ, Rogers WH, et al. Job performance deficits due to depression. Am J Psychiatry 2006;163:1569-76
- Trivedi MH, Morris DW, Wisniewski SR, et al. Increase in work productivity of depressed individuals with improvement in depressive symptom severity. Am J Psychiatry 2013;170:633-41
- Nelson JC, Zhang P, Skuban A, et al. Overview of short-term and long-term safety of brexpiprazole in patients with major depressive disorder and inadequate response to antidepressant treatment. Curr Psychiatry Rev 2016;12:278-90
- Seroquel XR® (quetiapine fumarate) extended-release tablets: US prescribing information [Internet]; 2017. Wilmington, DE 19850, US. AstraZeneca Pharmaceuticals LP. Available at: http://www.azpicentral.com/seroquel-xr/seroquelxr.pdf. [Last accessed 30 November 2017]
- Spielmans GI, Berman MI, Linardatos E, et al. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med 2013;10:e1001403
- Thase ME, Zhang P, Skuban A, et al. Efficacy of adjunctive brexpiprazole in patients with major depressive disorder: a clinical overview. Curr Psychiatry Rev 2016;12:291-301
- Thase M, Fava M, Hobart M, et al. Efficacy of adjunctive OPC-34712 across multiple outcome measures in major depressive disorder: a Phase II, randomized, placebo-controlled study. Neuropsychopharmacology 2011;36:S302-S304
- Pae CU, Forbes A, Patkar AA. Aripiprazole as adjunctive therapy for patients with major depressive disorder. Overview and implications of clinical trial data. CNS Drugs 2011;25:109-27
- Ball SG, Ferguson MB, Martinez JM, et al. Efficacy outcomes from 3 clinical trials of edivoxetine as adjunctive treatment for patients with major depressive disorder who are partial responders to selective serotonin reuptake inhibitor treatment. J Clin Psychiatry 2016;77:635-42