Abstract
Objectives
Cold agglutinin disease (CAD) is a rare form of autoimmune hemolytic anemia that may manifest in complement-mediated chronic hemolytic anemia, profound fatigue, and transient agglutination-mediated circulatory symptoms. This study compared the healthcare resource utilization (HRU) of patients with CAD with a matched non-CAD comparison cohort using national Danish health registry data.
Methods
All cases of CAD were identified from 1 January 1999 to 30 June 2016, in the Danish National Patient Registry using the International Classification of Diseases, Tenth Revision, discharge diagnosis codes. A subcohort of patients with primary CAD was identified based on the absence of secondary predisposing concomitant diseases. CAD cases were matched to individuals without CAD from the general population based on birth year, sex, and 19 disease categories of the Charlson Comorbidity Index. Comparative analyses assessed inpatient hospitalizations, outpatient clinic visits, emergency room visits, transfusion use, and expensive drug use between cohorts 6 months before and 12 months after the admission date of the first hospital visit with CAD diagnosis (index date).
Results
A total of 104 patients with CAD were matched to 1003 comparison cohort members. Throughout the 12 months after the index date, patients with CAD were more likely to have at least one inpatient hospitalization (odds ratio [OR], 3.9; 95% confidence interval [CI], 2.5–6.0), outpatient clinic visit (OR, 17.2; 95% CI, 6.8–43.1), and blood transfusion (OR, 93.0; 95% CI, 33.3–259.8) than matched comparisons. HRU was similarly higher among patients with CAD than matched comparisons during the 6 months before the index date. Findings were similar among patients with primary CAD.
Conclusions
Characterization of HRU among European patients with CAD has not previously been conducted. This study shows that patients with CAD utilize significant resources in Denmark. Increased HRU uses among patients with CAD before diagnosis presents opportunities for earlier diagnosis and management.
Introduction
Cold agglutinin disease (CAD), a rare subtype of autoimmune hemolytic anemia, affects an estimated 16 per 1 million individuals, with a median survival of 8.5–12.5 years after disease onset in European cohortsCitation1,Citation2. CAD is mediated by monoclonal immunoglobulin M autoantibodies, called cold agglutinins, which bind to the I antigen on the surface of red blood cellsCitation3. Cold agglutinins are most pathogenic when their thermal amplitude (the highest temperature at which they bind to red blood cell antigens) overlaps with vascular temperatures at the lower limit of normalCitation4. When bound to red blood cells, cold agglutinins activate complement component 1 (C1 complex) and trigger the classical complement pathway, resulting in extravascular hemolysis and, to a lesser extent, intravascular hemolysisCitation3,Citation5,Citation6.
CAD can occur at any age, but the average age at diagnosis is between 60 and 70 years, and it occurs more frequently among femalesCitation2,Citation5. Clinical manifestations include anemia and profound fatigue as well as cold-induced circulatory symptoms such as Raynaud’s phenomenon and acrocyanosisCitation5,Citation7. Primary CAD is considered a clonal lymphoproliferative disorder, distinguishable from CAD, which is secondary to underlying infections (Epstein-Barr virus infection, Mycoplasma pneumoniae) or aggressive lymphomaCitation6,Citation8.
Currently, there is no approved treatment for CAD. However, the burden of CAD is substantial and includes an increased risk of thromboembolic eventsCitation8,Citation9 and early mortalityCitation2. Previous studies have evaluated healthcare resource utilization (HRU) among patients with CAD in the United States (US) and found high HRU in both a tertiary medical center and large insurance planCitation10,Citation11. To date, no study has evaluated HRU among patients with CAD in Europe. The objective of this study is to characterize HRU for patients with CAD and their matched comparisons in Denmark using national health registries.
Methods
Data source
This population-based cohort study was conducted using Danish medical and administrative registries covering the study period from 1 January 1999 to 31 December 2016. In Denmark, the National Health Service provides universal, tax-supported healthcare for all Danish residents, guaranteeing access to general practitioners and hospitals free of chargeCitation12. The Danish registries are linkable at individual levels through the civil personal registration number, which is a unique 10-digit identifier assigned to all residents at birth or upon immigration.
The Danish National Patient Registry (DNPR) was used to identify patients with a CAD diagnosis and to define comorbidities and HRU. The DNPR contains nonpsychiatric hospitalizations since 1977 and outpatient and emergency room visits since 1995Citation13. The outpatient clinic visits included contacts with hospital-based (ambulatory) specialty clinics but not with general practitioners or private practice specialistsCitation13. Diagnoses were coded using the eighth revision of the International Classification of Diseases (ICD) until the end of 1993 and the tenth revision (ICD-10) thereafter. Information is recorded immediately after discharge and includes admission and discharge dates, one primary diagnosis, and up to 20 secondary diagnosesCitation13. Drugs administered in hospitals are also coded in the DNPR using the SKS Health Care Classification System of the Danish Health and Medicines Authority. The non-CAD comparison cohort was sampled using the DNPR and the Danish Civil Registration System (DCRS)Citation14. The DCRS tracks vital status and migration nationwide since 1968 with daily electronic updatesCitation14.
Study population
Patients with CAD were identified by any inpatient or outpatient hospital visit with ICD-10 code D591A either as a primary or secondary diagnosis. The index date of patients with CAD was defined as the admission date of the first hospital or outpatient clinic visit with CAD diagnosis. To ensure at least 6 months of follow-up, patients were included from 1 January 1999, through 30 June 2016.
We randomly sampled with replacement up to 10 individuals from the general population of Denmark to each member of the CAD cohort and matched on birth year (±3 years), sex, and 19 disease categories of the Charlson Comorbidity Index (CCI). The comparator’s index date was set to the corresponding patient with CAD for their admission date, and they were required to be alive and CAD-free at this date.
To evaluate HRU specifically in patients presumed to have primary CAD, a sensitivity analysis was conducted by excluding patients who had coexisting malignant conditions (i.e. B-cell lymphomas and Waldenström macroglobulinemia), as well as specific infections (i.e. the Epstein-Barr virus, cytomegalovirus, and Mycoplasma pneumoniae). The ICD codes used to identify these patients are presented in Supplemental Table 1. Comparison cohort members who were matched to the excluded CAD cases or had conditions associated with secondary CAD were also removed from the analyses of primary CAD.
Outcomes
Inpatient hospitalizations, outpatient clinic visits, and emergency room visits were evaluated for patients with CAD and their matched comparisons 6 months prior to and 12 months after the index date. Those hospital visits that cover the index date (i.e. admission date at or before index and discharge date at or before index) were excluded from the analyses. Admission date was used to define the timing of the visits (i.e. 6 months prior to and 12 months after index date). The proportion of patients having HRU and the number of HRUs per patient were evaluated separately for each visit type and period. For inpatient hospitalizations, the total hospital days per person were also reported and compared.
Hospital visits with ICD-10 codes D591, D649, D594, and D599 either as a primary or secondary diagnosis were identified as CAD-related visits. Inpatient and outpatient visits for CAD-related events were evaluated in a separate analysis. The procedure codes used to identify treatments with expensive drugs (bortezomib, antithymocyte globulin, high-dose immunoglobulin, bendamustine, daunorubicin, and other biological treatments) are in Supplemental Table 2. Emergency room visits, transfusion, and expensive drug use could not be evaluated for CAD-specific events because the number of events was too small in either the CAD cohort or comparison cohort for meaningful comparisons to be made.
Red blood cell transfusions were identified with treatment code BOQ. The exact quantity of transfusions and drugs was not addressed in this analysis; a small proportion of patients received multiple transfusions per day, and the dosage of the selected drugs is not registered in the DNPR. The proportion of patients having transfusions/expensive drugs was reported separately for each period. The number of transfusion/drug days for those who received the treatment was also evaluated.
If a patient had less than 1 year of follow-up because of the end of the study period (31 December 2016) or emigration/loss of follow-up, the number of HRU, hospital days, and days with a transfusion or expensive drug were annualized. During annualization, the outcome utilizations were divided by the actual follow-up time (in days) and multiplied by 365. No annualization was applied if the follow-up ended with death.
Statistical analyses
Baseline demographic information, including gender, age at CAD diagnosis, calendar year, and CCI score, were summarized with descriptive statistics. The proportions of patients with hospital visits, transfusion, and drug use in the CAD and comparison cohorts were compared using conditional logistic regression models. The odds ratios (ORs) of HRU in the CAD cohort compared with the comparison cohort and their 95% confidence interval (CI) were estimated. The rate of utilizations in the CAD and comparison cohorts were compared with negative binomial mixed models. The rate ratios (RRs) and 95% CIs of HRU in the CAD cohort compared with the comparison cohort were estimated. The ORs and RRs were controlled for matching variables by study design.
The number of hospital days per patient in the CAD and comparison cohorts were compared using a generalized linear mixed model with the zero-inflated negative binomial distribution. The zero-inflated negative binomial model consists of the following 2 parts: 1) a logit part modeling the proportion of patients with zero hospital days and 2) a negative binomial part modeling the number of hospital days. The reported P values belong to the negative binomial part.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
This is the first study to examine HRU of newly diagnosed CAD patients in a European population. A total of 104 patients with CAD were identified in the DNPR and matched to 1003 comparison individuals without CAD from the general Danish population, with a mean ratio of 9.6:1 (). Demographics and baseline characteristics were similar between the CAD cohort and the matched non-CAD cohort (). The median age of patients at the index date was 72.3 years (interquartile range [IQR], 62.7–78.2) in the CAD cohort and 71.5 years in the comparison cohort (IQR, 63.0–78.6). The overall CAD cohort comprised slightly more women than men (52.9% vs. 47.1%, respectively). The majority of patients had one or more comorbidities; 63.5% of patients with CAD and 60.0% of patients with primary CAD had a CCI score of 1 or higher (, Supplementary Table 3).
Figure 1. Patient flow diagram. Danish health registries (1999–2016). Abbreviations. CAD, Cold agglutinin disease; DNPR, Danish National Patient Registry.
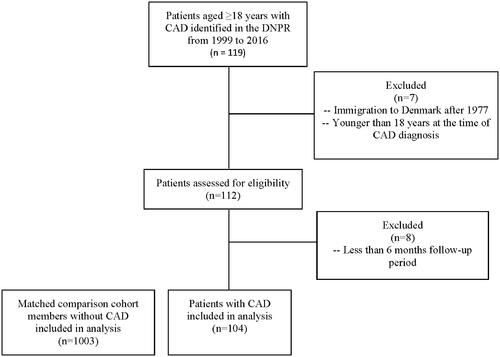
Table 1. Demographic and clinical baseline characteristics of the Danish CAD cohort and matched comparison cohort: Danish health registries (1999–2016).
During the 12 months after the index date, 52.8% (55/104) of the CAD cohort compared with 22.6% (227/1003) of matched comparisons required inpatient hospitalizations, with the odds of having at least 1 inpatient admission being 3.9 times (95% CI, 2.5–6.0) higher for patients with CAD than for the comparisons (). Patients with CAD had 5.1 times (95% CI, 3.6–7.3) increased rate of inpatient hospitalization admissions (p < .0001) and more inpatient hospital days than matched comparisons (median 1.5 days for patients with CAD vs median 0 d for comparisons; p = .001) ().
Table 2. Total and CAD-related healthcare resource utilization of the Danish CAD and matched comparison cohorts within 12 months months after the index date.
During the 12 months after the index date, 95.2% (99/104) of patients with CAD compared with 58.8% (590/1003) of matched comparisons had at least 1 outpatient visit; the odds of having at least 1 outpatient visit were 17.2 times (95% CI, 6.8–43.1) higher for patients with CAD than for the comparisons (). The median number of outpatient clinic visits was 10 and 1 in the CAD and comparison cohorts, respectively. Patients with CAD had a 5.3 times (95% CI, 4.1–6.8) higher rate of outpatient visits than comparison cohort members (). The use of emergency care was also greater in the CAD cohort than in the comparison cohort; however, the increase was less substantial than for the other visit settings. Specifically, 19.2% (20/104) of the patients with CAD visited the emergency room at least once, compared with 13.7% (137/1003) in the comparison cohort. Transfusion use was also significantly greater for patients with CAD than for matched comparisons (43.3% vs 1.3%; p < .0001) (). The median number of transfusion days during the 12 months after the CAD diagnosis was 4.0 for the 43.3% of patients with CAD who received the treatment. The expensive drug use during the 12 months after index was also significantly greater for patients with CAD than for matched comparisons (10.6% vs. 1.2%; p < .0001). High-dose immunoglobulin was the most frequently applied drug; accounting for 31.7% and 58.8% of the treatments for patients with CAD and the matched comparisons, respectively. However, one should note that these results were collected for a study period from 1999 to 2016 and treatment practices changed substantially during these years; e.g. eculizumab was not even approved before 2007.
Analyses limited to inpatient hospitalizations or outpatient clinic visits likely to be associated with CAD diagnosis showed similar but stronger results for patients with CAD and CAD-related HRU during the 12-month post-diagnosis period. Regression models showed increased odds of inpatient stay (OR, 15.3; 95% CI, 8.3–28.2) and outpatient visit (OR, 36.4; 95% CI, 18.2–72.7) compared with matched comparison cohort members ().
HRU in the 6 months prior to the index date was also significantly higher for patients with CAD. Patients with CAD had more inpatient admissions (p < .0001), outpatient visits (p < .0001), and emergency room visits (p < .0001) when compared with the members of the comparison cohort. The percentage of patients with transfusion was 20.2% in the CAD cohort and 1.3% in the comparison cohort. The percentage of patients with expensive drug use was slightly higher for patients with CAD than for the comparison cohort; the difference was around the limit of significance (p = .017). (). These trends persisted for CAD-related utilization ().
Table 3. Total and CAD-related healthcare resource utilization of the Danish CAD and matched comparison cohorts within 6 months months prior to the index date.
Sensitivity analysis: primary CAD
To evaluate HRU among patients with likely primary CAD only, sensitivity analyses were conducted excluding patients with diseases known to be associated with cold agglutinin syndrome (i.e. secondary CAD) (Supplementary Table 1). Within the CAD cohort, 85 patients (81.7% of the total CAD cohort) lacked any conditions known to be associated with secondary CAD and were presumed to have primary CAD. These patients were matched to 826 non-CAD comparison cohort members. While the overall cohort had slightly more men than women, this was reversed among patients with primary CAD (44.7% vs. 55.3%, respectively) (Supplementary Table 3). Patients with primary CAD experienced increased HRU for inpatient and outpatient visits, emergency room visits, and transfusion use versus matched general population comparisons (Supplementary Table 4). These trends in inpatient hospitalizations and outpatient clinic visits persisted for patients with CAD-related utilization (Supplementary Table 4), for HRU within 6 months of initial diagnosis overall (Supplementary Table 5), and specific to CAD-related HRU (Supplementary Table 5). Expensive drug use was not evaluated for the 6 months before the diagnosis because the number of events was too small in either the CAD cohort or comparison cohort for meaningful comparisons to be made.
Discussion
This study evaluated HRU among patients with CAD and primary CAD in Denmark. Versus their matched comparisons, patients with CAD and primary CAD experienced significantly higher HRU, including inpatient hospitalizations, emergency room visits, outpatient clinic visits, and transfusions, in the 12 months post-diagnosis. Interestingly, HRU was also increased for patients with CAD in the 6 months prior to diagnosis, which may be a reflection of the presence of anemia at disease onset. In the literature, Barcellini et al.Citation15, Prabhu et al.Citation16, Mullins et al.Citation10, Schöllkopf et al.Citation17, and Berentsen et al. (in 1997Citation18 and 2000Citation19) found that a significant proportion of patients had anemia at CAD diagnosis. In fact, 84% of patients in 1 study (Prabhu et alCitation16) and more than 40% in 2 other studiesCitation10,Citation15 reported severe anemia at diagnosis.
Two US studies have also examined HRU in this population. One of the studies conducted at Stanford University Medical Center in the USCitation10 assessed the patient journey for 29 patients with CAD diagnosed between 2000 and 2016. The second US study used the Optum–Humedica databaseCitation11. The proportion of patients with CAD who had at least 1 inpatient visit was different in the 2 US studies; 67% at Stanford and 36% in Optum, while the proportion of patients with an inpatient visit in Denmark (53%) was between these 2 US estimates. The percentage of patients with CAD who had at least 1 outpatient visit was similar in each study (100% at Stanford, 95% in Optum, and 95% in Denmark). The proportion of patients seen in the emergency room was smaller in Denmark than in the 2 US studies (19% in Denmark vs. 53% at Stanford and 26% in Optum). The observed differences may reflect differences in healthcare systems between the US and Denmark, clinical practice guidelines, patient behavior, or a combination of factors. Firstly, the Danish health system is universal, comprehensive, and free. By contrast, the US health system relies on public and private health insurance coverage, and many uninsured rely on hospital emergency rooms for primary care. This may provide reasoning for the decreased emergency room visits seen in the Danish cohort. Another reason could be the 2014 Denmark hospital restructure, wherein emergency department access changed from a walk-in basis to referral-based, requiring a doctor referral or activation of the emergency medical services. This may have inadvertently resulted in fewer emergency room visits or diverted these patients to other treatment settingsCitation20. Further, there may be differences in clinical practice guidelines between countries, such that the phase of care may differ on a case-by-case basis, ultimately resulting in Denmark having an increased inpatient hospitalization rate compared with the US.
A recent study in 8 Italian hematological centers followed 378 patients with primary autoimmune hemolytic anemia (AIHA), including CAD, to capture both clinical and HRU parametersCitation15. Similar to our study, the authors found high HRU among patients with AIHA. A total of 64% of patients with AIHA required at least 1 hospital admission, and this percentage was lower for the Danish patients with CAD (53%). The median number of outpatient visits per year was twice as large in the CAD cohort as among the patients with AIHA (10 vs. 5 visits per year). Subgroup analyses for patients with CAD (within the AIHA cohort) revealed that about 50% of patients required at least 1 transfusion, which is similar to the 43% that we found in the Danish CAD cohort.
In the current study, the high HRU observed after the index date may reflect limitations associated with current CAD therapeutic approaches, given the dearth of approved treatments. To verify that the increase in HRU was not caused by comorbid conditions such as diabetes or heart disease, patients with and without CAD were matched based on CCI disease categories. Furthermore, we conducted sensitivity analyses that excluded patients with underlying conditions that can be associated with CAD, such as specific infections and B-cell lymphoma. The persistence of increased HRU across all parameters (inpatient, outpatient, emergency room, transfusion days, and expensive drug use) in the CAD cohort after making these adjustments indicates that our findings cannot be solely owing to the presence of underlying comorbidities or related disorders.
This analysis is strengthened with the use of the Danish health registries with complete patient follow-up. Denmark also utilizes an ICD-10 code specific to CAD [D591A] in healthcare practice, where in the US, determination of CAD diagnosis must be considered through procedural codes and department-level utilizationCitation10,Citation11. However, as no ICD-10 code for primary CAD currently exists, in a sensitivity analysis we developed a primary CAD group based on concomitant diseases likely to be secondary to CAD based on prior studies in CADCitation2,Citation11. Similarly, CAD-related utilization was based on the identification of ICD-10 codes likely to be associated with the disease. The primary CAD subcohort and the CAD-related utilization measure may therefore be subject to misclassification.
Conclusions
This population-level analysis of HRU in patients with CAD is the first to date in a European cohort. The results of this study demonstrate that compared with a matched non-CAD cohort from the general population, patients with CAD utilize significantly more healthcare resources both prior to diagnosis and 1 year post-diagnosis. The burden of disease among these patients is suggestive of unmet needs in earlier management of the condition.
Transparency
Declaration of funding
EpidStrategies received a grant from Bioverativ, a Sanofi company, for this research.
Declaration of financial/other relationships
EKV and EHP have nothing to declare. JS was an employee of Bioverativ, a Sanofi company, at the time this research was conducted. NH, GN, and JPF are employees of EpidStrategies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors’ contributions
JS, JPF, GN, EKV, and EHP contributed to the study concept and design. EV and EHP performed data acquisition. All authors contributed to data analysis and interpretation, and manuscript preparation and review. All authors approved the final version of this manuscript and agree to be accountable for all aspects of this work.
Supplemental Material
Download PDF (161.4 KB)Acknowledgements
Editorial assistance for the development of this paper was provided by Fishawack Communications Ltd., part of Fishawack Health, and was funded by Sanofi.
Data availability statement
Data used in this analysis is protected by the Danish government and is not available.
References
- Berentsen S, Ulvestad E, Langholm R, et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica. 2006;91(4):460–466.
- Bylsma LC, Ording AG, Rosenthal A, et al. Occurrence, thromboembolic risk, and mortality in danish patients with cold agglutinin disease. Blood Adv. 2019;3(20):2980–2985.
- Berentsen S. Complement activation and inhibition in autoimmune hemolytic anemia: focus on cold agglutinin disease. Semin Hematol. 2018;55(3):141–149.
- Rosse WF, Adams JP. The variability of hemolysis in the cold agglutinin syndrome. Blood. 1980;56(3):409–416.
- Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood. 2013;122(7):1114–1121.
- Berentsen S. Cold agglutinin disease. Hematology Am Soc Hematol Educ Program. 2016;2016(1):226–231.
- Berentsen S. How I manage patients with cold agglutinin disease. Br J Haematol. 2018;181(3):320–330.
- Kamesaki T, Nishimura J, Wada H, et al. Demographic characteristics, thromboembolism risk, and treatment patterns for patients with cold agglutinin disease in Japan. Int J Hematol. 2020;112(3):307–315.
- Broome C, Cunningham JM, Mullins M, et al. Incidence of thromboembolic events is increased in a retrospective analysis of a large cold agglutinin disease (CAD) cohort. Blood. 2017;130(Suppl_1):928–928.
- Mullins M, Jiang X, Bylsma LC, et al. Cold agglutinin disease burden: a longitudinal analysis of anemia, medications, transfusions, and health care utilization. Blood Adv. 2017;1(13):839–848.
- Su J, Bylsma LC, Jiang X, et al. Healthcare resource utilization among commercially insured patients with cold agglutinin disease in the United States. J Med Econ. 2020;23(8):902–907.
- Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591.
- Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490.
- Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549.
- Barcellini W, Zaninoni A, Fattizzo B, et al. Predictors of refractoriness to therapy and healthcare resource utilization in 378 patients with primary autoimmune hemolytic anemia from eight Italian reference centers. Am J Hematol. 2018;93(9):E243–E246.
- Prabhu R, Bhaskaran R, Shenoy V, et al. Clinical characteristics and treatment outcomes of primary autoimmune hemolytic anemia: a single center study from South India. Blood Res. 2016;51(2):88–94.
- Schöllkopf C, Kjeldsen L, Bjerrum OW, et al. Rituximab in chronic cold agglutinin disease: a prospective study of 20 patients. Leuk Lymphoma. 2006;47(2):253–260.
- Berentsen S, Bø K, Shammas FV, et al. Chronic cold agglutinin disease of the “idiopathic” type is a premalignant or low-grade malignant lymphoproliferative disease. APMIS. 1997;105(5):354–362.
- Berentsen S, Tjønnfjord GE, Shammas FV, et al. No response to cladribine in five patients with chronic cold agglutinin disease. Eur J Haematol. 2000;65(1):88–90.
- Fløjstrup M, Bogh SB, Henriksen DP, et al. Increasing emergency hospital activity in Denmark, 2005-2016: a nationwide descriptive study. BMJ Open. 2020;10(2):e031409.

