Abstract
Background
Pentasa (prolonged-release mesalazine [5-ASA]) has been available for >30 years as an effective treatment for mild-to-moderate ulcerative colitis (UC). A systematic literature review and meta-analysis was undertaken to provide an up-to-date evaluation of oral Pentasa efficacy and safety for induction and maintenance of remission.
Methods
Literature searches were conducted in PubMed, Embase and Cochrane databases, from inception to 02 December 2020. Unpublished studies were also sourced. Meta-analyses using a random-effects model and Bayesian inference compared Pentasa (tablets, granules, capsules) against placebo and other 5-ASAs.
Results
Twelve studies involving 3674 patients treated with Pentasa were identified. Pentasa 2–4 g/day was superior to placebo at inducing (absolute risk difference [ARD] at 8 weeks 0.14, 95% CI 0.07‒0.21; p < .001) and maintaining (ARD 6-12 months 0.18, 95% CI 0.04‒0.33; p < .05) remission (clinical/endoscopic). Against other 5-ASAs, Pentasa had similar efficacy for induction (ARD <0.001, 95% CI −0.05‒0.05) and maintenance (ARD 0.01, 95% CI −0.07‒0.08) treatment using randomized controlled trial data. Upon inclusion of real-world study data, Pentasa was significantly better at maintaining remission compared both to Eudragit-S mesalazine and sulfasalazine (ARD 0.04, 95% CI 0.02‒0.06; p < .001). Pentasa (1–4 g/day) had similar treatment-related adverse event rates to placebo (ARD 0.02, 95% CI −0.03‒0.06) and Eudragit-L/S mesalazines (2.25-3 vs 2.4-3 g/day, respectively; ARD −0.03, 95% CI −0.12‒0.05), but was better tolerated than sulfasalazine (3 g/day) (ARD 0.07, 95% CI 0.003‒0.14; p < .05).
Conclusion
This study confirms oral Pentasa is efficacious and well-tolerated in treating active UC and maintaining remission. The availability of multiple forms of Pentasa supports physicians’ ability to individualize treatment and optimize dosing to improve outcomes.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disorder of the colon and rectum, characterized by periods of remission and relapse, with increasing incidence and prevalence worldwideCitation1,Citation2. It can cause significant impairment of quality of life and is considered a progressive disease owing to the risks of proximal extension, colectomy, and colorectal cancerCitation1. Over the past decade, the therapeutic focus for UC has evolved from treating symptoms to mucosal healing, with the aim of modifying the natural history of the disease in order to improve long-term outcomesCitation3,Citation4. Mesalazine (5-aminosalicylate; 5-ASA) is a long established, well-tolerated oral or topical treatment for UC, that is the current standard of care for both induction and maintenance of remission in patients with mild-to-moderate diseaseCitation5,Citation6. Over 90% of patients receive a 5-ASA within the first year of diagnosis, with most continuing use for up to 15 yearsCitation7.
Several oral mesalazine preparations have been formulated with different drug delivery methods (e.g. time dependent, pH dependent, azo-bonded prodrugs) to minimize systemic absorption and maximize drug availability at the inflamed colonic epitheliumCitation8. Among these, Pentasa (prolonged-release mesalazine, Ferring Pharmaceuticals) has been shown to be effective in mild-to-moderate active UC for achieving and maintaining remissionCitation9–11. Its oral formulation consists of ethylcellulose coated microgranules that provide a consistent and reliable delivery of mesalazine throughout the gastrointestinal tract (from the duodenum to the rectum), independently of luminal pH and gut transit timeCitation8. Pentasa is available as 1 g, 500 mg and 250 mg (marketed in Japan only) tablets, 500 mg and 250 mg capsules (both marketed in USA only), and 4 g, 2 g and 1 g granules, allowing flexibility of dosing; with simplified once daily regimens proven to be an efficacious strategy to optimize outcomes in mild-to-moderate UCCitation12–15.
Recent meta-analyses of 5-ASAs in UC have focused on them more as a class, in particular, explored dosing regimens, and did not assess the mesalazines individually against placeboCitation16–18. Pentasa has been available for over 30 years with an on-going clinical development program throughout that time. Our aim was to provide an up-to-date overview of the totality of evidence amassed for the comparative safety and efficacy of oral Pentasa in UC to support physicians in treatment decisions. We conducted a systematic literature review (SLR) and meta-analysis that evaluated the efficacy and safety of Pentasa compared to placebo and active 5-ASA comparators in both induction and maintenance of remission.
Methods
Search strategy
A comprehensive search of the literature was conducted using PubMed (Medline), Embase, and Cochrane Library databases, from inception to 2 December 2020, to identify studies of oral Pentasa in adult UC. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed, including development of a protocol (Supplementary Document 1). Search terms (combined with “Medical Subject Headings” [MeSH] in PubMed and “Subject Headings” in Embase) related to drug (Pentasa, quintasa, mesalazin*, mesalasin*, mesalamin*, aminosalicylate, 5-asa, 5asa, 5-aminosalicylic*, 5aminosalicylic*, 5amino salicylic*, 5 asa, 5 aminosalicy*), formulation (tablet*, capsule*, “drug capsule”, pill, sachet*, granule*, “drug granule”), and disease (“ulcerative colitis”, UC) were used (Supplementary Document 2). Searches were filtered to include only “human” studies and no language limits were set, although a minimum of an English abstract was required. The search results were supplemented by inclusion of unpublished randomized controlled trials (RCTs) provided by Ferring; hand-searching of conference proceedings from 2006 to 2020 (Digestive Diseases Week, American College of Gastroenterology, United European Gastroenterology Week, and the Asian Pacific Digestive Week); and review of the grey literatureCitation19 (including bibliographies of key articles; targeted web searches for non-indexed articles, meeting abstracts, theses, letters etc, including using Google Scholar; and searches on clinicaltrials.gov).
Study selection
Two experienced reviewers (KP and JF) independently assessed all studies against pre-defined PICO (Population, Intervention, Comparison, Outcome) criteria. Inclusion criteria included: adults (≥18 years) with UC; use of oral Pentasa; placebo/untreated or other 5-ASA as comparators; and reporting efficacy and tolerability outcomes. All oral formulations of Pentasa (tablets, granules and capsules) were included as they can be considered therapeutically comparableCitation9, which is supported by pharmacokinetic and bioavailability studies and in vitro dissolution characteristicsCitation20,Citation21. Meta-analyses and systematic reviews, RCTs, non-RCTs, and observational studies were included. Exclusion criteria included: studies reporting use of oral prolonged-release mesalazine, but where this could not be confirmed as oral Pentasa; healthy populations or mixed populations of UC and other forms of inflammatory bowel disease (IBD); comparisons against other (not 5-ASA) IBD medications; and studies reporting only pharmacokinetic and pharmacodynamic measures. Any discrepancy in selecting studies for inclusion was resolved by a co-author (ST).
Data abstraction and quality assessment
Data relating to efficacy and safety outcomes as well as study and patient characteristics were extracted for the full study populations of the included studies. The risk of bias and methodological quality of the included RCTs were assessed using the Cochrane Collaboration’s Risk of Bias toolCitation22 and the Jadad scoreCitation23, respectively. A RCT was considered at low risk of bias when all five domains assessed (randomization method; treatment concealment; blinding; incomplete outcomes; selective reporting) were scored as such (rather than “unclear” or “high”). A moderate risk of bias was assigned when one or more domains was scored as “unclear” and the rest as “low”; and a high risk of bias was assigned when at least one domain was scored as such, or all domains were scored as “unclear”. A RCT was considered high quality when the overall score was 3-5. For observational studies, risk of bias and confounding was assessed using the RTI Item BankCitation24, with a score of 0 indicating a very high risk of bias and a score of 12 a very low risk of bias.
Outcomes of interest
The primary outcomes assessed were the induction and maintenance of remission either expressed clinically or as a composite (clinical and endoscopic) measure, as defined in the included studies, and treatment-related adverse event (AE) rates. Secondary outcomes included other efficacy (Physician Global Assessment [PGA], treatment failure, sigmoidoscopic index, biopsy score), and safety (overall AE rates, serious treatment-related AE rates, withdrawals due to AEs) measures.
For treatment of active UC, comparisons were made between oral Pentasa at low (defined as ≤3 g/day) and high (4 g/day) doses versus placebo and active comparators (no studies were identified using a dose of Pentasa >3–3.9 g/day). However, no head-to-head studies of Pentasa versus other 5-ASAs at a high-dose (≥4 g/day) were identified. From the 2020 Cochrane review of 5-ASAs for induction of remission in UCCitation17, RCTs of 4.8 g/day MMX mesalazineCitation25,Citation26 and Eudragit-S-coated mesalazineCitation27 were identified to enable indirect comparisons to 4 g/day Pentasa using placebo as a common comparator.
All analyses were first conducted using data from RCTs, with data from other study designs included only for supplementary/expanded analyses.
Data synthesis and statistical analysis
Meta-analyses to calculate the absolute effect size difference between Pentasa and comparator (placebo or active) were undertaken using random effects modelling for direct comparisons and Bayesian networking for indirect comparisonsCitation28. Random-effects models were created in Microsoft Excel 365 and results expressed as a weighted absolute risk difference (ARD) or weighted effect size (ES; for mean improvement in sigmoidoscopic index only), both with 95% confidence intervals (CIs). A p-value of <.05 was considered statistically significant. The Bayesian meta-analyses were carried out using WinBUGS 1.4.3 and used source-specific binomial dispersion and linear combinations before being converted to command line analytical code. The code for each data source combination was subjected to 10,000 sampling iterations and subsequently analyzed for mean, standard deviation (SD), median, and percentiles. A 95% credible interval (CrI) not spanning 0 (or other equivalence point) was considered to correspond to a statistical significance of p < .05. Heterogeneity between study-specific estimates was assessed using the chi-squared test (for categorical data) and I2 statistic (for continuous data).
Results
Search results and included Pentasa studies
The literature search identified 436 records from database searching and 15 from other sources (). After removal of 113 duplicates and a further 246 on review of the title and abstract, 92 records were assessed in their entirety. From these, 14 records were determined covering 12 individual studies of Pentasa that fulfilled the eligibility criteria (Supplementary Table 1). Five of the studies evaluated Pentasa for treatment of active UC, three of which have been publishedCitation29–31 and two currently unpublished (PEN2A-23_UC II [Ferring 1990] and 000174 [Ferring 2019]). Of the seven maintenance studies, six have been publishedCitation11,Citation32–36 and one was unpublished (000175 [Ferring 2019]). All of the studies were RCTs except the OPTIMUM studyCitation36, which reported 5-ASA usage as maintenance therapy in routine clinical practice in Japan. A total of 3674 (1154 excluding OPTIMUMCitation36) patients treated with oral Pentasa were included in the studies and analyzed (induction: 755; maintenance: 2919, or 399 excluding OPTIMUMCitation36).
Figure 1. PRISMA flow diagram of Pentasa studies identified for inclusion in meta-analyses. *This included two Cochrane reviews of 5-ASAs in ulcerative colitisCitation17,Citation18, which met the inclusion criteria, but did not contain information on any further Pentasa studies not already identified in the systematic review.
Efficacy of Pentasa for induction of remission
Pentasa versus placebo
Pentasa at 2 g/day (ARD 0.14, 95% CI 0.06, 0.23; p < .001), combined 2–4 g/day (ARD 0.14, 95% CI 0.07, 0.21; p < .001), and 4 g/day (ARD 0.15, 95% CI 0.03, 0.28; p < .05) was found to be significantly superior to placebo at inducing remission (defined as a composite measure) at 8 weeks (). Pentasa 4 g/day was also associated with significantly increased rates of endoscopic (ARD 0.18, 95% CI 0.08, 0.28; p < .001) and histological (ARD 0.12, 95% CI 0.03, 0.20; p < .05) remission versus placebo (). Further analysis of Pentasa 4 g/day revealed significant advantages over placebo using clinical remission assessments (ARD 0.14, 95% CI 0.09, 0.18; p < .001; Supplementary Figure 1) and using all remission definitions used in the trials (ARD 0.13, 95% CI 0.10, 0.17; p < .001; Supplementary Figure 2).
Figure 2. Pentasa versus placebo for induction of remission* at 8 weeks. Forest plot shows absolute risk difference and 95% confidence interval (CI) for Pentasa 2 g/day (A); 2–4 g/day (B); and 4 g/day (C) vs placebo. Size of blue squares represents weighting/relative size of individual studies and black diamond represents overall risk difference. *Outcomes used for remission definitions: Hanauer et al. Citation29 and PEN2A-23_UC II (Ferring 1990) = composite analysis (one or more of physician global assessment, sigmoidoscopy and biopsy); Ito et al. 2010Citation30 = Ulcerative Colitis Disease Activity Index (UCDAI); Trial 000174 (Ferring 2019) = Clinical and Endoscopic Response Score (CERS).
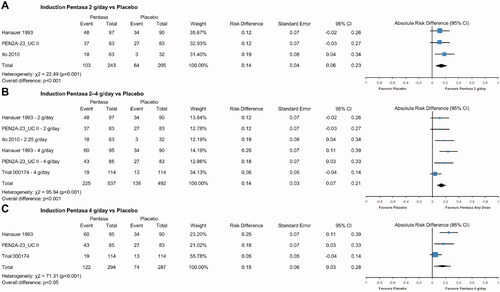
Figure 3. Pentasa versus placebo for induction of endoscopic and histologic remission* at 8 weeks. Forest plot shows absolute risk difference and 95% confidence interval (CI) for Pentasa 4 g/day vs placebo for remission assessed using Sigmoidoscopic Index (15-point scale consisting of a grade of 0–3 given for the presence of erythema, granularity/ulceration, friability, mucopus, and the appearance of the mucosal vascular pattern) (A); and biopsy score (0 = normal colonic mucosa; 1 = inactive inflammatory bowel disease [IBD]; 2 = low grade, active IBD; 3 = high grade, active IBD) (B). Size of blue squares represents weighting/relative size of individual studies and black diamond represents overall risk difference. *Outcomes used for remission definitions: (A) Hanauer et al. Citation29 and PEN2A-23_UC II (Ferring 1990) = Sigmoidoscopic Index score of 0–4 was indicative of inactive disease; (B) Hanauer et al. Citation29 and PEN2A-23_UC II (Ferring 1990) = Biopsy score of 0 or 1 with improvement of at least one category from baseline.
![Figure 3. Pentasa versus placebo for induction of endoscopic and histologic remission* at 8 weeks. Forest plot shows absolute risk difference and 95% confidence interval (CI) for Pentasa 4 g/day vs placebo for remission assessed using Sigmoidoscopic Index (15-point scale consisting of a grade of 0–3 given for the presence of erythema, granularity/ulceration, friability, mucopus, and the appearance of the mucosal vascular pattern) (A); and biopsy score (0 = normal colonic mucosa; 1 = inactive inflammatory bowel disease [IBD]; 2 = low grade, active IBD; 3 = high grade, active IBD) (B). Size of blue squares represents weighting/relative size of individual studies and black diamond represents overall risk difference. *Outcomes used for remission definitions: (A) Hanauer et al. Citation29 and PEN2A-23_UC II (Ferring 1990) = Sigmoidoscopic Index score of 0–4 was indicative of inactive disease; (B) Hanauer et al. Citation29 and PEN2A-23_UC II (Ferring 1990) = Biopsy score of 0 or 1 with improvement of at least one category from baseline.](/cms/asset/23c4ba7e-a540-4cf6-bb6e-2ffa854527ad/icmo_a_1968813_f0003_c.jpg)
Pentasa 4 g/day was also shown to be significantly better than placebo with regard to: complete or marked improvement in PGA (ARD 0.20, 95% CI 0.12, 0.28; p < .001; Supplementary Figure 3); treatment failure (ARD 0.17, 95% CI 0.09, 0.25; p < .001; Supplementary Figure 4); mean improvement in sigmoidoscopic index (ES 0.53, 95% CI 0.32, 0.74; p < .001; Supplementary Figure 5); and improvement in biopsy score (ARD 0.13, 95% CI 0.03, 0.23; p < .05; Supplementary Figure 6).
Pentasa versus other 5-ASAs
Pentasa 2.25-3 g/day was found to be not significantly different at inducing (clinical/composite) remission at 8 weeks compared to 2.4–3 g/day Eudragit-S/Eudragit-L formulations of mesalazine (ARD <0.001, 95% CI −0.05, 0.05; p > .05; ). Similarly, in Bayesian analyses, Pentasa 4 g/day induced similar remission rates to Eudragit-S/MMX mesalazine 4.8 g/day at 6/8 weeks (mean differential advantage against placebo of Pentasa over Eudragit-S/MMX mesalazine 0.47 [95% CrI −8.33, 9.33] events per 100 patients). Comparable efficacy was also seen when Pentasa 4 g/day was tested individually against Eudragit-S 4.8 g/day (Supplementary Figure 7) and MMX mesalazine 4.8 g/day (Supplementary Figure 8).
Figure 4. Pentasa versus other 5-ASAs for induction of remission* at 6/8 weeks. Forest plot shows absolute risk difference and 95% confidence interval (CI) for Pentasa 2.5–3 g/day vs Eudragit-S/Eudragit-L mesalazines 2.4–3 g/day (A). Size of blue squares represents weighting/relative size of individual studies and black diamond represents overall risk difference. Bayesian meta-analysis shows Pentasa 4 g/day vs Eudragit-S/MMX mesalazine 4.8 g/day (B). †The 5% and 95% credible intervals (CrI) cross 0, indicating no significant difference at the 5% level between Pentasa 4 g/day and Eudragit-S/MMX mesalazine 4.8 g/day. MC, Monte Carlo error. *Outcomes used for remission definitions (all at 8 weeks except where noted): Gibson et al. Citation31 = Clinical Activity Index; Ito et al.Citation30, Kamm et al.Citation25 and Lichtenstein et al.Citation26 = Ulcerative Colitis Disease Activity Index (UCDAI); Hanauer et al.Citation29 and PEN2A-23_UC II (Ferring 1990) = composite analysis (one or more of Physician Global Assessment [PGA], sigmoidoscopy and biopsy); Trial 000174 (Ferring 2019) and Feagan et al.Citation27 = clinical remission (score of 0 for stool frequency and rectal bleeding, and absence of faecal urgency) at week 6.
![Figure 4. Pentasa versus other 5-ASAs for induction of remission* at 6/8 weeks. Forest plot shows absolute risk difference and 95% confidence interval (CI) for Pentasa 2.5–3 g/day vs Eudragit-S/Eudragit-L mesalazines 2.4–3 g/day (A). Size of blue squares represents weighting/relative size of individual studies and black diamond represents overall risk difference. Bayesian meta-analysis shows Pentasa 4 g/day vs Eudragit-S/MMX mesalazine 4.8 g/day (B). †The 5% and 95% credible intervals (CrI) cross 0, indicating no significant difference at the 5% level between Pentasa 4 g/day and Eudragit-S/MMX mesalazine 4.8 g/day. MC, Monte Carlo error. *Outcomes used for remission definitions (all at 8 weeks except where noted): Gibson et al. Citation31 = Clinical Activity Index; Ito et al.Citation30, Kamm et al.Citation25 and Lichtenstein et al.Citation26 = Ulcerative Colitis Disease Activity Index (UCDAI); Hanauer et al.Citation29 and PEN2A-23_UC II (Ferring 1990) = composite analysis (one or more of Physician Global Assessment [PGA], sigmoidoscopy and biopsy); Trial 000174 (Ferring 2019) and Feagan et al.Citation27 = clinical remission (score of 0 for stool frequency and rectal bleeding, and absence of faecal urgency) at week 6.](/cms/asset/dd7adb4d-b20f-43d7-b288-ee3e3becf0e7/icmo_a_1968813_f0004_c.jpg)
Efficacy of Pentasa for maintenance of remission
Pentasa versus placebo
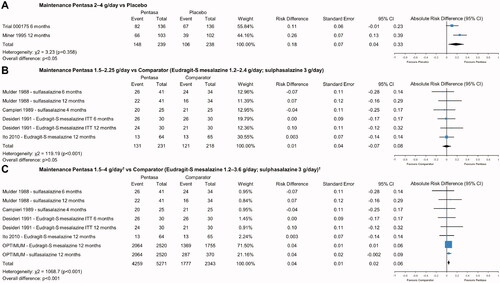
Pentasa 2–4 g/day was found to be significantly more effective than placebo at maintaining (composite) remission over 6-12 months (ARD 0.18, 95% CI 0.04, 0.33; p < .05; ). Similar results in favor of Pentasa 2 g/day over placebo were seen using data from the total and de novo (∼80% that did not follow-on from Trial 000174) patient populations and all remission definitions from Trial 000175 (Ferring 2019) (ARD 0.12, 95% CI 0.08, 0.16; p < .001; Supplementary Figure 9).
Figure 5. Pentasa 1.5–4 g/day† versus placebo and other 5-ASAs for maintenance of remission* at up to 12 months. Forest plot shows absolute risk difference and 95% confidence interval (CI) for Pentasa 2–4 g/day vs placebo (A); Pentasa 1.5–2.25 g/day vs Eudragit-S mesalazine 1.2–2.4 g/day and sulfasalazine 3 g/day (B); and Pentasa 1.5–4 g/day† g/day vs Eudragit-S mesalazine 1.2–3.6 g/day† and sulfasalazine 3 g/day† (C). Size of blue squares represents weighting/relative size of individual studies and black diamond represents overall risk difference. *Outcomes used for remission definitions: Trial 000175 (Ferring 2019) = Clinical and Endoscopic Response Score (CERS); Miner et al.Citation11 and Desideri et al.Citation34 = clinical and endoscopic measures; Mulder et al.Citation32 and Campieri et al.Citation33 = clinical, endoscopic and histological measures; Ito et al.Citation35 = Ulcerative Colitis Disease Activity Index (UCDAI); OPTIMUMCitation36 = partial UCDAI (stool frequency, bloody stool, and Physician Global Assessment). †OPTIMUMCitation36 was a real-life study where the most common doses reported for maintenance treatment were Pentasa 4 g/day (received by 20% of patients on Pentasa), Eudragit-S mesalazine 3.6 g/day (43%), and sulfasalazine 3 g/day (90%).
Pentasa versus other 5-ASAs
Pentasa 1.5–2.25 g/day was not significantly different to Eudragit-S mesalazine 1.2–2.4 g/day and sulfasalazine 3 g/day at maintaining remission for up to 12 months (ARD 0.01, 95% CI −0.07, 0.08; p > .05; ). A similar, non-significant result was seen when Pentasa 1.5–2.25 g/day was analyzed individually against Eudragit-S mesalazine 1.2–2.4 g/day (ARD 0.02, 95% CI −0.08, 0.12; p > .05; Supplementary Figure 10). Repeating the same analyses including data from the OPTIMUM studyCitation36 showed Pentasa (dose typically up to 4 g/day) to be significantly better at maintaining remission compared to both Eudragit-S mesalazine (typically up to 3.6 g/day) and sulfasalazine (typically 3 g/day) combined (ARD 0.04, 95% CI 0.02, 0.06; p < .001; ), and Eudragit-S mesalazine alone (ARD 0.04, 95% CI 0.01, 0.06; p < .05; Supplementary Figure 11).
Safety of Pentasa
There was no significant difference in overall AE rates (ARD 0.05, 95% CI −0.02, 0.11; p > .05; Supplementary Figure 12), treatment-related AE rates (ARD 0.02, 95% CI −0.03, 0.06; p > .05; ), or serious treatment-related AE rates (ARD 0.004, 95% CI −0.04, 0.04; p > .05; Supplementary Figure 13) for Pentasa (1–4 g/day) versus placebo when used as induction therapy. Similarly, treatment-related AE rates were not significantly different for Pentasa 2–4 g/day and placebo during maintenance treatment (ARD 0.04, 95% CI −0.01, 0.09; p > .05; ).
Figure 6. Safety of Pentasa vs placebo and other 5-ASAs. Forest plot shows absolute risk difference and 95% confidence interval (CI) for number of patients that experienced treatment-related adverse events (AEs) for Pentasa (1–4 g/day) vs placebo for induction treatment (A); number of patients that experienced treatment-related AEs for Pentasa (2.25–3 g/day) vs Eudragit-L mesalazine (3 g/day) and Eudragit-S mesalazine (2.4 g/day) for induction treatment; number of patients that experienced treatment-related AEs for Pentasa (2–4 g/day) vs placebo for maintenance treatment (C); and discontinuations due to AEs for Pentasa (1.5 g/day) vs sulfasalazine (3 g/day) for maintenance treatment (D). Size of blue squares represents weighting/relative size of individual studies and black diamond represents overall risk difference. Hanauer et al.Citation29 = Pentasa 1 g/day, 2 g/day or 4 g/day; PEN2A-23_UC II (Ferring 1990) = Pentasa 2 g/day or 4 g/day; Ito et al.Citation30 = Pentasa 2.25 g/day and Eudragit-S mesalazine 2.4 g/day; Trial 000174 (Ferring 2019) = Pentasa 4 g/day; Gibson et al.Citation31 = Pentasa 3 g/day and Eudragit-L mesalazine 3 g/day; Trial 000175 (Ferring 2019) = Pentasa 2 g/day; Miner et al.Citation11 Pentasa 4 g/day; Mulder et al.Citation32 and Campieri et al.Citation33 = Pentasa 1.5 g/day and sulfasalazine 3 g/day.
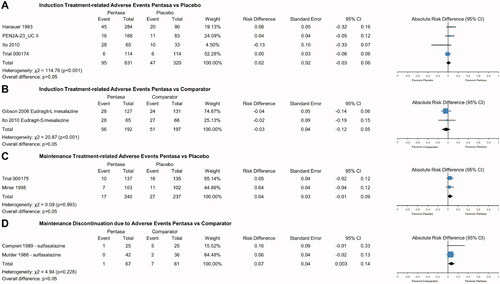
When Pentasa (2.25–3 g/day) was compared to Eudragit-L mesalazine (3 g/day) and Eudragit-S mesalazine (2.4 g/day) as induction treatment, overall AE rates (ARD 0.02, 95% CI −0.06, 0.11; p > .05; Supplementary Figure 14) and treatment-related AE rates (ARD −0.03, 95% CI −0.12, 0.05; p > .05; ) did not differ significantly. However, maintenance treatment with Pentasa (1.5 g/day) was found to result in significantly fewer discontinuations due to AEs compared with sulfasalazine (3 g/day) (ARD 0.07, 95% CI 0.003, 0.14; p < .05; ).
Study quality and heterogeneity
Two of the five induction RCTs and one of the six maintenance RCTs of Pentasa can be considered at a low risk of bias (Supplementary Table 2). Of the remaining studies, three were scored as having a moderate risk of bias (2/5 induction; 1/6 maintenance) and five were classified as high risk (1/5 induction; 4/6 maintenance), including Campieri et al.Citation33 where only the abstract could be assessed. All induction and maintenance RCTs, except Campieri et al.Citation33, were scored as having high methodological quality (Supplementary Table 3). The one observational study included, OPTIMUMCitation36, scored 8 out of 12, with scores closer to 12 indicating a lower risk of bias. Based on chi squared tests and the I2 statistic there was significant heterogeneity within the majority of individual analyses (21 of 28).
Discussion
For more than 30 years, mesalazine has been shown to be therapeutically effective and well-tolerated in the treatment of mild-to-moderate UCCitation17,Citation18 acting topically to reduce intestinal inflammation in proportion to its luminal concentrationCitation37. Of the various mesalazine formulations, Pentasa has a unique prolonged-release mechanism and is available at higher dosage strengths for simplified once daily dosingCitation8. This SLR and meta-analysis provides an up-to-date and comprehensive assessment of the clinical evidence for the effectiveness of oral Pentasa in mild-to-moderate UC, analyzing data from 12 studies, including three that are currently unpublished, and 3674 patients treated with Pentasa (1154 enrolled in RCTs). The meta-analyses undertaken confirm the consistent efficacy and safety of oral Pentasa in treating both active UC and maintaining remission.
Pentasa was found to be superior to placebo at inducing (at 2–4 g/day) and maintaining (1.5–4 g/day) remission using a range of outcomes measures. This included clinical remission (Supplementary Figure 1), composite scores of endoscopic and clinical measures () and, importantly, stricter definitions, such as biopsy score (). Pentasa was also found to have a comparable safety profile to placebo, both in terms of treatment-related AE rates () and serious treatment-related AE rates (Supplementary Figure 13). Studies comparing differing doses of Pentasa have reported AEs were not dose relatedCitation38,Citation39. In maintenance treatment, for example, a study by Fockens et al.Citation39 found Pentasa 3 g/day to be well-tolerated and not associated with more treatment-related AEs when compared to Pentasa 1.5 g/day (13% vs 11%, respectively; (p = .88).
In comparison to other 5-ASAs, Pentasa was found to be similarly effective as Eudragit-S, Eudragit-L, and MMX mesalazines for the induction of (clinical/composite) remission at lower (2.25–3 vs 2.4–3 g/day, respectively) and higher doses (4 vs 4.8 g/day) (). Similarly, Pentasa (1.5–2.25 g/day) was found to be comparable to Eudragit-S mesalazine (1.2–2.4 g/day) and sulfasalazine (3 g/day) as maintenance treatment using data only from RCTs (). Treatment-related AE rates for Pentasa and Eudragit-L/Eudragit-S mesalazines were similar, whilst significantly (p < .05) better tolerability was seen for Pentasa against sulfasalazine (). These results are consistent with previous observations, such as those from the Cochrane CollaborationCitation17,Citation18, and reflect major international guidelines on the management of UC, which recommend 5-ASAs as first-line therapy for mild-to-moderate UC and do not distinguish between the formulations in terms of efficacy, but where treatment with sulfasalazine (and olsalazine) are not considered preferable due to the high frequency of AEsCitation5,Citation6,Citation40,Citation41. Sulfasalazine is a prodrug consisting of azo-bonded mesalazine and sulfapyridine molecules, with systemic absorption of the latter contributing to the higher rate of AEs reported versus coated mesalazine formulations, such as PentasaCitation8.
Interestingly, the inclusion of real-world evidence from the OPTIMUM studyCitation36 in the meta-analysis resulted in Pentasa being significantly better in maintaining remission compared to both sulfasalazine and Eudragit-S mesalazine combined (p < .001; ) and Eudragit-S mesalazine alone (p < .05; Supplementary Figure 11). These results should be interpreted with caution due to the biases inherent in real-world data (e.g. in patient selection), but does lead to the intriguing possibility that there might be some differences in effectiveness among the 5-ASAs when used in clinical practice.
There are a number of potential limitations with the meta-analyses that should be recognized. In particular, the Pentasa studies spanned approximately 30 years where the epidemiology of UC, management strategies, definitions of disease endpoints and other factors have evolved over time. This is perhaps best illustrated in the more recent studies – Trial 000174 (Ferring 2019) and Trial 000175 (Ferring 2019) – which included a more rigorous endpoint (the proportion of subjects in remission at Week 8, defined by the Clinical and Endoscopic Response Score [CERS] as a score of: 0 for rectal bleeding, 0 or 1 with at least 1-point decrease from baseline for stool frequency and 0 or 1 for endoscopic score) than used in earlier mesalazine trials. Trial 000174 (Ferring 2019) also used central endoscopy reading for both the read-out of the primary endpoint and for determining subject eligibility. Any differences between studies, such as length of treatment period, were reflected in the significant heterogeneity found (in 21 of 28 analyses), which justified the use of a random effects approach to the meta-analyses (this assumes the data were not different samples from a uniform source).
A strength of this review is that it included only studies with confirmed use of oral Pentasa; however, it may be that some studies of Pentasa were excluded if the brand was not stated. This may have particularly affected observational studies as opposed to RCTs, with the latter perhaps more likely to report the brand. Such exclusions were not documented, but may explain why only one observational study (OPTIMUMCitation36) met the inclusion criteria. Funding sources and author conflicts were also not formally assessed, with most included studies being industry led.
In general, the RCTs included were of good methodological quality (10 of 11 studies rated “high” quality; 6 of 11 studies rated “low” or “moderate” bias), supporting the robustness of the analyses. The inclusion of three unpublished studies – Trial 000174 (Ferring 2019), PEN2A-23_UC II (Ferring 1990) and Trial 000175 (Ferring 2019) – was a potential limitation as none has been peer-reviewed. However, this is balanced by the availability of these additional data strengthening the statistical power of the analyses.
High rates of mucosal healing have been reported when oral Pentasa is combined with topical mesalazine therapy. In the MOTUS trialCitation14, mucosal healing rates of 71.1–87.5% at 8 weeks were reported in patients receiving oral Pentasa 4 g/day plus 4 weeks of enema 1 g/day. Moreover, higher rates of mucosal healing were observed in patients receiving combined oral (4 g/day) and topical (1 g/day enema for 4 weeks) Pentasa than oral treatment alone in the PINCE trial (mean change from baseline in disease activity index endoscopic mucosal appearance score −1.09 vs − 0.66, respectively; p = .024 at week 8).Citation42
Pentasa has also been reported to improve quality of life for patients with UC. In the study by Hanauer et al.Citation29 oral Pentasa (2 or 4 g/day) was shown to be significantly superior to placebo in improving each of the 12 function-related quality of life parameters scored (p < .05)Citation43. Other benefits of Pentasa include the fact that it is available in a number of different doses and formulations, including 2 g and 4 g granules, and that it can be dosed once daily (OD) to enhance patient adherenceCitation44. The MOTUS trialCitation14 found that a 4 g OD dose of Pentasa granules was non inferior to a 4 g twice daily (BID) dose, in induction of remission in mild-to-moderate UC. Similarly, the PODIUM trialCitation12 found a OD regimen of Pentasa 2 g granules non-inferior, and even superior, to Pentasa 1 g BID regimen in maintaining clinical remission in patients with quiescent UC (p = .024). Also in maintenance of UC remission, Watanabe et al.Citation45 demonstrated the non-inferiority of OD administration of Pentasa tablets to three times daily administration.
This comprehensive systematic review and meta-analysis has demonstrated the efficacy and safety of oral Pentasa for the treatment of active mild-to-moderate UC. With therapeutic aims in UC becoming more ambitious, targeting endoscopic and histological remission as well as symptomatic improvement, there is increasing emphasis on optimizing currently available treatments and identifying patients who could benefit from dose escalationCitation46. The multiple formulations of Pentasa provide physicians’ with the scope for optimizing patients’ 5-ASA dosing and thus impact not only the likelihood of symptomatic improvement and remission, but also treatment adherence, quality of life, and long-term outcomes.
Transparency
Declaration of funding
This work was supported by Ferring Pharmaceuticals.
Declaration of financial/other relationships
KP is an employee of Ferring Pharmaceuticals. JF is an employee of Violicom Medical Limited that has received funding from Ferring for work on various projects. ST has received Grants/Research Support from: AbbVie, Buhlmann, Celgene, IOIBD, Janssen, Lilly, Pfizer, Takeda, UCB, Vifor, and Norman Collisson Foundation; Consulting Fees from: Abacus; AbbVie; Actial; ai4gi; Alcimed; Allergan; Amgen; Aptel; Arena; Asahi; Aspen; Astellas; Atlantic; AstraZeneca; Barco; Biocare; Biogen; BLPharma; Boehringer Ingelheim; BMS; Buhlmann; Calcico; Celgene; Cellerix; Cerimon; ChemoCentryx; Chiesi; CisBio; ComCast; Coronado; Cosmo; Ducentis; Dynavax; Elan; Enterome; Falk; Ferring; FPRT Bio; Galapagos; Genentech/Roche; Genzyme; Gilead; Glenmark; Grunenthal; GSK; GW Pharmaceuticals; Immunocore; Immunometabolism; Indigo; Janssen; Lexicon; Lilly; Medarex; Medtrix; Merck; Merrimack; Millenium; Neovacs; Novartis; Novo Nordisk; NPS-Nycomed; Ocera; Optima; Origin; Otsuka; Palau; Pentax; Pfizer; Pharmaventure; Phillips; P&G; Pronota; Proximagen; Resolute; Robarts; Sandoz; Santarus; Satisfai; Sensyne; Shire; SigmoidPharma; Souffinez; Syndermix; Synthon; Takeda; Theravance; Tigenix; Tillotts; Topivert; Trino Therapeutics with Wellcome Trust; TxCell; UCB Pharma; Vertex; VHsquared; Vifor; Warner Chilcott and Zeria; Speaker Fees from: AbbVie, Amgen, Biogen, Falk; Ferring, Janssen, Pfizer, Shire, Takeda, UCB; ST holds no stocks or share options.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
KP planned and designed the study. KP and JF undertook the systematic review supported by ST. JF analyzed the data and performed the statistical calculations. KP, JF and ST edited and approved the manuscript. JF is the overall guarantor of the content in the article.
Supplemental Material
Download PDF (1 MB)Supplemental Material Table 4
Download MS Word (43 KB)Supplemental Material Table 3
Download MS Word (43.5 KB)Supplemental Material Table 2
Download MS Word (50.5 KB)Supplemental Material Table 1
Download MS Word (45.4 KB)Supplementary_Document_2_Search_Strings_1.0_210809.docx
Download MS Word (32.7 KB)Supplemental Material Document 1 SLR Protocol 2.0 200810
Download MS Word (51.7 KB)Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
References
- Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Prim. 2020;6:74.
- Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN study). Scand J Gastroenterol. 2009;44(4):431–440.
- Ungaro R, Colombel J-F, Lissoos T, et al. A treat-to-target update in ulcerative colitis: a systematic review. Am J Gastroenterol. 2019;114(6):874–883.
- Colombel J-F, D’haens G, Lee W-J, et al. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020;14(2):254–266.
- Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769–784.
- Ko CW, Singh S, Feuerstein JD, et al. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology. 2019;156(3):748–764.
- Fumery M, Singh S, Dulai PS, et al. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol. 2018;16(3):343–356.e3.
- Ye B. Mesalazine preparations for the treatment of ulcerative colitis: are all created equal? World J Gastrointest Pharmacol Ther. 2015;6(4):137.
- Farup PG, Hinterleitner TA, Lukáš M, et al. Mesalazine 4 g daily given as prolonged-release granules twice daily and four times daily is at least as effective as prolonged-release tablets four times daily in patients with ulcerative colitis. Inflamm Bowel Dis. 2001;7(3):237–242.
- Marteau P, Probert CS, Lindgren S, et al. Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active ulcerative colitis: a randomised, double blind, placebo controlled study. Gut. 2005;54(7):960–965.
- Miner P, Hanauer S, Robinson M, et al. Safety and efficacy of controlled-release mesalamine for maintenance of remission in ulcerative colitis. Digest Dis Sci. 1995;40(2):296–304.
- Dignass AU, Bokemeyer B, Adamek H, et al. Mesalamine once daily is more effective than twice daily in patients with quiescent ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7(7):762–769.
- Feagan BG, MacDonald JK. Once daily oral mesalamine compared to conventional dosing for induction and maintenance of remission in ulcerative colitis: a systematic review and Meta-analysis. Inflamm Bowel Dis. 2012;18(9):1785–1794.
- Flourié B, Hagège H, Tucat G, et al. Randomised clinical trial: once- vs. twice-daily prolonged-release mesalazine for active ulcerative colitis. Aliment Pharmacol Ther. 2013;37(8):767–775.
- D'Haens GR, Sandborn WJ, Zou G, et al. Randomised non-inferiority trial: 1600 mg versus 400 mg tablets of mesalazine for the treatment of mild-to-moderate ulcerative colitis. Aliment Pharmacol Ther. 2017;46(3):292–302.
- Barberio B, Segal JP, Quraishi MN, et al. Efficacy of oral, topical, or combined oral and topical 5-aminosalicylates, in ulcerative colitis: systematic review and network meta-analysis. J Crohns Colitis. 2021;15:1184–1196.
- Murray A, Nguyen TM, Parker CE, et al. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2020;8:CD000543.
- Murray A, Nguyen TM, Parker CE, et al. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2020;8:CD000544.
- Paez A. Gray literature: an important resource in systematic reviews. J Evid Based Med. 2017;10(3):233–240.
- Wilding IR, Kenyon CJ, Hooper G. Gastrointestinal spread of oral prolonged-release mesalazine microgranules (PENTASA) dosed as either tablets or sachet. Aliment Pharmacol Ther. 2000;14(2):163–169.
- Ferring Pharmaceuticals. 3.2.P.2 Pharmaceutical Development (Version 6). 2013.
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, editor. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration. 2011. Available from: www.handbook.cochrane.org
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
- Viswanathan M, Berkman ND, Dryden DM, et al. Assessing risk of bias and confounding in observational studies of interventions or exposures: further development of the RTI item bank. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013.
- Kamm MA, Sandborn WJ, Gassull M, et al. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology. 2007;132(1):66–75.
- Lichtenstein GR, Kamm MA, Boddu P, et al. Effect of once- or twice-daily MMX mesalamine (SPD476) for the induction of remission of mild to moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2007;5(1):95–102.
- Feagan BG, Sandborn WJ, D’Haens G, et al. The role of centralized reading of endoscopy in a randomized controlled trial of mesalamine for ulcerative colitis. Gastroenterology. 2013;145(1):149–157.e2.
- Deeks JJ, Higgins JPT, Altman DG, editors. Chapter 10: analysing data and undertaking Meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane; 2021. Available from: www.training.cochrane.org/handbook
- Hanauer S, Schwartz J, Robinson M, et al. Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. Am J Gastroenterol. 1993;88:1188–1197.
- Ito H, Iida M, Matsumoto T, et al. Direct comparison of two different mesalamine formulations for the induction of remission in patients with ulcerative colitis: a double-blind, randomized study. Inflamm Bowel Dis. 2010;16(9):1567–1574.
- Gibson PR, Fixa B, Pekarkova B, et al. Comparison of the efficacy and safety of Eudragit-L-coated mesalazine tablets with ethylcellulose-coated mesalazine tablets in patients with mild to moderately active ulcerative colitis. Aliment Pharmacol Ther. 2006;23(7):1017–1026.
- Mulder CJJ, Tytgat GNJ, Weterman IT, et al. Double-blind comparison of slow-release 5-aminosalicylateand sulfasalazine in remission maintenance in ulcerative colitis. Gastroenterology. 1988;95(6):1449–1453.
- Campieri M, Gionchetti PB, et al. Slow-release 5-aminosalicylic acid (PENTASA) versus sulphasalazine (SASP) in the maintenance treatment of ulcerative colitise. Scand J Gastroenterol. 1989;24:130.
- Desideri S, Ardizzone S, Petrillo M. Two different oral formulations of 5-Aminosalicylic acid (5-ASA) in maintenance treatment of ulcerative colitis. Ital J Gastroenterol. 1991;23:643.
- Ito H, Iida M, Matsumoto T, et al. Direct comparison of two different mesalamine formulations for the maintenance of remission in patients with ulcerative colitis: a double-blind, randomized study. Inflamm Bowel Dis. 2010;16(9):1575–1582.
- Nagahori M, Kochi S, Hanai H, et al. Real life results in using 5-ASA for maintaining mild to moderate UC patients in Japan, a multi-center study, OPTIMUM Study. BMC Gastroenterol. 2017;17(1):47.
- Ham M, Moss AC. Mesalamine in the treatment and maintenance of remission of ulcerative colitis. Expert Rev Clin Pharmacol. 2012;5(2):113–123.
- Safdi M, Schwartz J, Kaplan M, et al. Controlled-release mesalamine capsules for the treatment of ulcerative colitis: final results of a multicenter open-label trial. Am J Gastroenterol. 1991;16:A284.
- Fockens P, Mulder CJJ, Tytgat GNJ, et al. Comparison of the efficacy and safety of 1.5 compared with 3.0 g oral slow-release mesalazine (Pentasa) in the maintenance treatment of ulcerative colitis. Dutch Pentasa Study Group. Eur J Gastroenterol Hepatol. 1995;7(11):1025–1030.
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106.
- Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384–413.
- Probert CS, Dignass AU, Lindgren S, et al. Combined oral and rectal mesalazine for the treatment of mild-to-moderately active ulcerative colitis: rapid symptom resolution and improvements in quality of life. J Crohns Colitis. 2014;8(3):200–207.
- Robinson M, Hanauer S, Hoop R, et al. Mesalamine capsules enhance the quality of life for patients with ulcerative colitis. Aliment Pharmacol Ther. 2007;8(1):27–34.
- Kane SV. Systematic review: adherence issues in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2006;23(5):577–585.
- Watanabe M, Hanai H, Nishino H, et al. Comparison of QD and TID oral mesalazine for maintenance of remission in quiescent ulcerative colitis: a double-blind, double-dummy, randomized multicenter study. Inflamm Bowel Dis. 2013;19(8):1681–1690.
- Solitano V, D’Amico F, Fiorino G, et al. Key strategies to optimize outcomes in mild-to-moderate ulcerative colitis. J Clin Med. 2020;9:2905.

