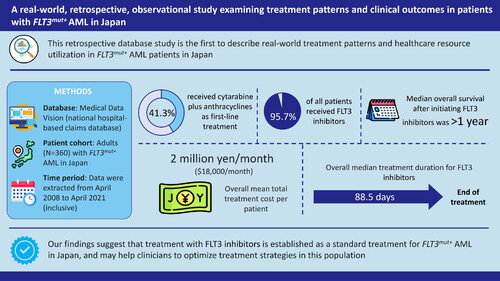
Abstract
Objective
Acute myeloid leukemia (AML) is the most common form of leukemia among adults in Japan. This study aimed to understand the treatment patterns, health care resource utilization, and costs of FMS-like tyrosine kinase 3 mutation-positive (FLT3m+) AML patients in Japan.
Methods
A retrospective cohort study of Japanese FLT3m + AML patients was conducted using data extracted from a national hospital-based claims database provided by Medical Data Vision Co. Ltd. (MDV; Tokyo, Japan). Patients were identified from the MDV database between April 2008 and April 2021 inclusive.
Results
A total of 360 patients were included in this study. The study results suggest that cytarabine + anthracyclines was the most common first-line (1 L) treatment, accounting for 41.3% of the patients. FLT3 inhibitors (FLT3i) was the most common treatment across the study period (95.7%). The mean age of patients was 62.4 years, and most were 65 years or older. The median overall survival (OS) after initiating FLT3i treatment was 394 days. The median treatment duration of FLT3i was 88.5 days, while it was 66.0 days for patients treated with FLT3i within 60 days after hematopoietic stem cell transplantation (HSCT). The overall mean monthly total treatment cost was JPY 2,009,531.7/per patient per month (PPPM) (USD 17,967.9/PPPM).
Conclusions
The study found specific treatment patterns, trends and features in patients with FLT3m + AML. FLT3i was the most prescribed treatment across the study period and the overall median OS after initiating FLT3i treatment was over 1 year. The findings of this study could be helpful for clinicians to optimize treatment strategies for FLT3m + AML in Japan.
Graphical Abstract
Introduction
Acute myeloid leukemia (AML) is a heterogeneous disorder of myeloblasts in the bone marrow and peripheral blood [Citation1]. In 2019, the annual global prevalence and incidence of AML ranged from 2.2–2.8 and 1.4–1.7 per 100,000, respectively [Citation2]; and, AML is the most common form of leukemia among adults [Citation3], with an annual incidence of 5.6 per 100,000 in Japan [Citation4]. The annual incidence of AML in Japan has increased with the advancing population age to 10–17 per 100,000 in patients over 69 years old, compared with 0.6–6.0 per 100,000 in patients under 70 years old [Citation4].
According to the Japanese Practical Guidelines for Hematological Malignancies published by the Japanese Society of Hematology, induction therapy for AML consists of high-intensity chemotherapy, which includes an anthracycline such as daunorubicin or idarubicin, along with cytarabine [Citation5]. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an option for patients who cannot expect a long-term prognosis with high-intensity chemotherapy alone, for patients who relapse, or for those who do not respond to high-intensity chemotherapy [Citation5]. In spite of chemotherapy’s long use as the preferred treatment for AML, the overall cure rate of chemotherapy is approximately 30%–50% in AML patients [Citation6].
Furthermore, even with the introduction of allo-HSCT and supportive care, the overall 5-year survival rate in older patients (age >60 years) with advanced AML remains low (5%–15%) [Citation4]. Additionally, approximately 30% of patients with AML have confirmed mutations in the FMS-like tyrosine kinase 3 (FLT3) gene (FLT3m+), and those with FLT3 internal tandem duplications (FLT3-ITD) typically have a much lower prognosis with a higher chance of relapse and a shorter overall survival (OS) when compared with patients without the mutation [Citation7]. For example, patients with relapsed FLT3m + AML have a particularly low prognosis with a hazard ratio of 2.05 (95% confidence interval [CI]: 1.20–3.51) compared with patients with relapsed AML but without the FLT3 mutation [Citation8].
FLT3 inhibitors (FLT3i) are indicated for relapsed/refractory (R/R) cases with FLT3m + AML [Citation9]. Two FLT3i, gilteritinib and quizartinib, have been approved for R/R FLT3m + AML patients in Japan. Gilteritinib was approved in Japan in September 2018 [Citation10] and quizartinib in June 2019 [Citation11]. The efficacy of gilteritinib was established in the Phase 3 randomized ADMIRAL trial, which compared treatment with gilteritinib to salvage chemotherapy (SC). The ADMIRAL trial reported that gilteritinib significantly improved patient survival outcomes, with 1-year survival rates of 54.3% compared with 26.3% for SC in a Japanese subpopulation [Citation12]. Additionally, among the overall population, the median OS for patients receiving gilteritinib was significantly longer when compared with patients receiving SC (9.3 months versus 5.6 months, respectively; hazard ratio for death = 0.64; p < 0.001), and a higher proportion of patients treated with gilteritinib achieved complete remission (CR) or CR with partial hematologic recovery (CRh) compared to those treated with SC (34.0% versus 15.3%, respectively) [Citation13]. Comparable results were obtained in the Japanese subgroup, where the median OS for patients receiving gilteritinib was longer when compared with patients receiving SC (14.3 months versus 9.6 months, respectively) [Citation12]. Although the use of FLT3i is expected to become more prevalent in the treatment of R/R FLT3m + AML and its potential has been shown in clinical trials, the implementation of treatment strategies for this disorder is influenced by numerous factors, such as age, pre-existing medical conditions, performance status, and genetic and molecular characteristics, all of which can impact treatment response and OS [Citation14]. However, the actual clinical usage of FLT3i is still unclear. Furthermore, the financial burden of treatment with high-intensity induction chemotherapy and HSCT could be substantial for Japan’s rapidly aging society, as AML incidence among the elderly is higher compared to the younger population [Citation15]. In the United States, Pandya et al. (2020) reported that total mean episode costs were highest for R/R episodes (US dollars [USD] 439,104), followed by HSCT (USD 329,621) and high-intensity induction chemotherapy (USD 198,657) [Citation16]. Although some previous studies reported the expenditures or costs for AML treatments [Citation17,Citation18], data about real-world costs for treating R/R FLT3m + AML in Japan are scarce.
Taking into consideration the lack of data as mentioned above, this study aimed to provide a comprehensive understanding of the real-world management of FLT3m + AML in Japan, including treatment patterns, patient characteristics, survival outcomes, and health care resource utilization (HCRU) and costs. The primary objectives of this study were to gain insight into the treatment patterns and to provide a detailed description of the baseline demographic and clinical characteristics of patients with FLT3m + AML in a real-world setting in Japan. The secondary objectives of the study aimed to describe the demographic and clinical characteristics of patients with R/R disease, to examine the survival time following FLT3i treatment, to determine the duration of FLT3i treatment, and to describe the HCRU and costs associated with FLT3m + AML patients.
Methods
Study design and data source
A retrospective cohort study of Japanese AML patients was conducted using data extracted from a national hospital-based claims database provided by Medical Data Vision Co. Ltd. (MDV; Tokyo, Japan). The MDV database comprises administrative data from 416 hospitals in Japan that provided care to approximately 32 million people at the time of this study and contains diagnostic and hospital visit (outpatient and inpatient) information, alongside laboratory information for a proportion of the patients.
According to Japanese guidelines, informed consent was not necessary for this study, as the study used secondary data and patient records were anonymized by allocating unique identifiers for each patient and maintained. This study used de-identified patient records, and did not involve the collection, use or transmittal of individually identifiable data, therefore Institutional Review Board approval to conduct the study was not necessary.
Study population
The cohort was identified from the MDV database, spanning from April 2008 to April 2021 inclusive (). The population of interest was extracted for the above period (herein referred to as the “identification period”) from the MDV database. The study population consisted of AML patients who met the inclusion/exclusion criteria. Patients meeting the inclusion criteria were followed up from their initial definitive identification of AML (index date) until whichever of the following occurred first: the end of the study period (April 2021), death, or loss to follow-up according to .
Figure 1. Identification period and follow-up period. *Index date is defined as the date of first appearance of diagnosis of AML in the database during the identification period. †Loss-to follow-up is defined as the date of the individual patient’s last claim. Abbreviations: AML, acute myeloid leukemia, R/R, relapsed/refractory.

The inclusion criteria were adult patients aged ≥18 years at the index date and patients who satisfied either of the following conditions: (a) having at least one claim with a confirmed diagnosis of AML based on the International Classification of Diseases 10th Revision (ICD-10) (ICD-10: C92.0, C92.5, C92.6, C92.8, C93.0, C94.0, C94.2, C94.6), and at least one prescription claim of FLT3i during the identification period; or (b) having at least one claim with a confirmed diagnosis of AML FLT3m+ (disease code: 8849858) during the identification period. The exclusion criteria were: (a) patients with a diagnosis of acute promyelocytic leukemia (APL) (ICD-10: C92.4) during the identification period, (b) patients with a diagnosis of solid cancer after the initial identification of AML (ICD-10: C00-C75), and (c) patients who did not have any claims after the index date.
Statistical analysis
Descriptive statistics were summarized for demographics, baseline clinical characteristics, and the outcomes of interest. Frequencies and percentages were reported as categorical variables, while means, standard deviations (SD), medians, minimum and maximum values, and 25 and 75 percentile values were reported as continuous variables. The Kaplan–Meier method was used to describe OS. To capture the trends and features visually, treatment patterns were represented using Sankey plots. The analysis for treatment patterns was summarized based on the following time frames: from index date to follow-up end, from index date to R/R, from R/R to follow-up end, and after HSCT to follow-up end. The time point of R/R is defined as the date when: (i) the recurrence flag was assigned in the diagnostic procedure combination (DPC) Form 1; (ii) AML treatment was restarted after 90 days; (iii) a regimen was switched from high-intensity chemotherapy (HIC) to low-intensity chemotherapy (LIC), or the opposite, regardless of a gap period for the treatment switching; (iv) the date of the first FLT3i treatment when this treatment was the first treatment for AML during the follow-up period; and (v) the date when HIC, LIC, or FLT3i treatment was started after HSCT.
The duration of FLT3i treatment was defined as the period from the first prescription date of FLT3i to the last prescription date of FLT3i. These durations were reported for those within the follow-up period and for those within the period after HSCT to follow-up end. The FLT3i treatment after HSCT was limited to the treatments applied within 60 days after HSCT. OS after initiating FLT3i treatment was defined from the date of initiating FLT3i treatment to the date of death within the follow-up period. However, for patient without death record, follow-up end was censored. HCRU including length of hospital stay, number of outpatient visits, number of emergency room (ER) visits, number of HSCTs, number of chemotherapies prescriptions other than FLT3i and number of FLT3i prescriptions, and costs including inpatient, outpatient, HSCT, chemotherapy (other than FLT3i), FLT3i, and transfusion costs were summarized and calculated by per-patient-per-month (PPPM) units. The costs in Japanese Yen (JPY) were converted into USD using the exchange rate (USD 1= JPY 111.84) at the time when the analysis was conducted (1 April 2021).
Algorithms to capture treatment patterns
Treatment patterns were employed in the main process and post-process, which are presented in the Supplementary materials. The main process was defined by 6 criteria that identified the medication combination patterns and the post-process was defined by 8 criteria that specifically identified each regimen including careful manual review. The following regimens were categorized into LIC: azacitidine (AZA), cytarabine, aclarubicin/cytarabine, aclarubicin and granulocyte colony-stimulating factor (G-CSF), gilteritinib, quizartinib, venetoclax (VENE), low-dose cytarabine, and AZA + VENE combination, while other regimens without pre-HSCT treatment of busulfan, melphalan, and cyclophosphamide were categorized into HIC. In this study, low-dose cytarabine defined in the post-process of treatment patterns was listed as “LoDAC”, high-dose cytarabine defined in the post-process of treatment patterns was listed as “HiDAC”, and other regimens containing low-dose cytarabine or high-dose cytarabine were listed as their combination of drug names.
Results
Patient demographic and clinical characteristics
A total of 360 patients were included in this study’s analysis and are referred to as the study population. The baseline demographic and clinical characteristics at the time of initial identification of AML are listed in . The majority of the study population was comprised of older patients aged ≥65 years, with a mean age of 62.4 years. More than half of the population was male (56.9%), and the mean weight was 59.6 kg. The most common comorbidities across the study population were infectious diseases (70.8%), followed by liver dysfunction/hepatitis (19.7%) and heart failure (17.5%), and the mean Charlson Comorbidity Index (CCI) score was 3.2.
Table 1. Demographic and clinical characteristics at initial diagnosis of AML.
Treatment patterns starting from the initial identification of AML
A Sankey plot was used to illustrate the treatment flow of patients from first-line (1 L) to sixth-line (6 L) treatment and from the index date to the end of follow-up (). Of the 349 1 L-treated patients, 41.3% (n = 144) received cytarabine + anthracyclines. This was followed by FLT3i (24.9% [n = 87]), CA/CAG (12.3% [n = 43], and AZA (8.9% [n = 31]). Second-line (2 L) therapy was received by 85.7% (n = 299) of 1 L-treated patients. FLT3i was received by 37.1% (n = 111), followed by cytarabine + anthracyclines (24.4% [n = 73]), HSCT (12.4% [n = 37]), HiDAC (7.4% [n = 22]), and CA/CAG (6.0% [n = 18]). Third-line (3 L) therapy was received by 67.9% (n = 237) of 1 L-treated patients. Of note, 36.3% (n = 86) received FLT3i followed by cytarabine + anthracyclines (21.9% [n = 52]), CA/CAG (8.4% [n = 20]), HSCT (8.0% [n = 19]), AZA (5.5% [n = 13]), and combination of mitoxantrone, etoposide, and cytarabine (MEC) (5.1% [n = 12]). Among the study population, patients who did not have any treatment records were not examined (n = 11). Many patients who received FLT3i treatment reached the end of follow-up. Also, there were notable variations in the pre-treatment regimens for FLT3i.
Figure 2. Sankey plot of treatment flow for the period from index date to follow-up end (first line to sixth line). Abbreviations: ACR, aclarubicin; Ara-c, high-dose cytarabine; Aza, azacitidine; BHAC, behenoyl cytarabine; CA/CAG, cytarabine, aclarubicin/cytarabine, aclarubicin and G-CSF; cytarabine + anthracycline, 7 + 3, 7 + 5 and 5 + 3; DNR, daunorubicin; ETP, etoposide; FLAG, fludarabine + high-dose cytarabine + G-CSF; FLT3i, FMS-like tyrosine kinase 3 inhibitor; GO, gemtuzumab ozogamicin; HAM, high-dose cytarabine and mitoxantrone; HiDAC, high-dose cytarabine; HSCT, hematopoietic stem-cell transplantation; LoDAC, low-dose cytarabine; MEC, mitoxantrone/etoposide/cytarabine; MIT, mitoxantrone; Vene, venetoclax.

The number of patients by treatment regimens, regardless of the treatment line from the index date to the end of follow-up, is shown in . Overall, FLT3i was the most prescribed regimen (95.7% [n = 334]), followed by cytarabine + anthracyclines (45.8% [n = 160]), HSCT (26.6% [n = 93]), and CA/CAG (23.2% [n = 81]).
Table 2. Number of patients by treatment regimens from index date to follow-up end.
Among patients who have R/R, for the index date period to R/R, cytarabine + anthracyclines was the most common regimen in the 1 L (48.2%, [n = 134]), accounting for treatment in about half of the patients and the main treatment across all treatment lines (Supplementary Figure 1). For the period of R/R to follow-up end, FLT3i was the most common regimen in the 1 L-post R/R, accounting for 68.4% [n = 212], and FLT3i was the main treatment for all treatment lines (Supplementary Figure 2).
For the period from the first HSCT to the end of follow-up, among patients after HSCT as 1 L-post HSCT, FLT3i was the main regimen for this study population, and many of these patients reached the end of follow-up (Supplementary Figure 3).
Patient demographic and clinical characteristics at the time of R/R disease
The patients’ demographic and clinical characteristics at the time of R/R disease are presented in Supplementary Table 1. The majority of the study population was comprised of older patients aged ≥65 years with a mean age of 61.5 years. More than half of the population were male (56.5%), and the mean weight was 58.2 kg. The most common comorbidities were infectious diseases (70.2%), followed by liver dysfunction/hepatitis (20.3%) and heart failure (16.2%). The mean CCI score was 3.2 (Supplementary Table 1).
Survival time after initiating FLT3i treatment
The OS for the study population after initiating FLT3i treatment is presented in and . The median OS duration was 394 (95% CI:336–508) days, and patients older than 65 years old showed a lower survival rate (Supplementary Figure 4).
Figure 3. Kaplan–Meier curve after initiating FLT3i treatment for study population. Abbreviation: FLT3i, FMS-like tyrosine kinase 3 inhibitor.

Table 3. OS after initiating FLT3i treatment from date of initiating FLT3i treatment to follow-up end.
Treatment duration of FLT3i treatment
Among patients treated with FLT3i, the median treatment duration from the date of initiating FLT3i to the end of FLT3i treatment was 88.5 (min–max: 1.0–989.0) days. After transplantation, the use of FLT3i as post-transplantation maintenance therapy administered within 60 days was also analyzed. The results showed that patients who received FLT3i within this time frame had a median treatment duration of 66.0 (min–max: 8.0–597.0) days ().
Table 4. Duration of FLT3i in study population.
HCRU and costs in FLT3m + AML patients
Analysis of the HCRU based on PPPM in FLT3m + AML patients from index date to the end of follow-up showed that the mean length of hospital stay was 15.5 days for the study population. Patients of outpatient visits and ER visits were reported 1.5 and 0.1 days, respectively. Additionally, compared to patients who were prescribed FLT3i, the mean number of chemotherapies prescribed for patients excluding FLT3i was higher (10.7 and 14.5, respectively) (). Furthermore, analysis of the cost in JPY (USD)/PPPM of treatment for the study population showed that the mean monthly total cost for the study population was JPY 2,009,531.7/PPPM (USD 17,967.9/PPPM). For the study population, the mean cost of chemotherapy was higher than the cost of FLT3i treatment (JPY 578,841.5/PPPM [USD 5,175.6/PPPM] and JPY 498,236.7/PPPM [USD 4,454.9/PPPM], respectively). Additionally, the mean inpatient, HSCT, and transfusion costs in the study population were JPY 1,644,923.0/PPPM (USD 14,707.8/PPPM), JPY 18,574.5/PPPM (USD 166.1/PPPM), and JPY 26,094.2/PPPM (USD 233.3/PPPM), respectively, while the mean outpatient cost was JPY 364,615.1/PPPM (USD 3,260.1/PPPM) ().
Table 5. HCRU of study population from index date to follow-up end.
Table 6. Costs of study population from index date to follow-up end.
Discussion
Treatment strategies for patients with FLT3m + AML may have changed following the launch of FLT3is in 2019 in Japan. However, no studies have examined the clinical practices and treatment strategies for FLT3M + AML. This is the first study that examined the real-world management of FLT3m + AML in Japan, including treatment patterns, patient characteristics, survival outcomes, HCRU, and costs. While analyzing treatment patterns, including HSCT aggregated by prescribed antineoplastic agents, our study results suggested that cytarabine + anthracyclines, which is categorized as HIC, was the most common (41.3%) 1 L treatment for FLT3m + AML patients for the period from the index date to the end of follow-up. Since cytarabine + anthracyclines is recommended as the first standard treatment in the clinical guidelines including other regions [Citation5,Citation19,Citation20], the result could be considered to be reflective of the clinical practice of treatment for patients with AML. In contrast, even though FLT3i is not approved for newly diagnosed AML in Japan, the proportion of use was second to cytarabine + anthracyclines (24.9%). One reason for that would be the nature of database. For example, since this database does not have traceability for patients transferred to other hospitals, there might be patients who had been diagnosed with AML and treated at other medical institutions who could have been transferred to the facility which is subjected to this database and treated with FLT3i immediately. Therefore, it can be assumed that a certain number of 1 L FLT3i were not the actual 1 L treatments in some patient histories. From the 2 L onwards, FLT3i was the most prescribed treatment, followed by cytarabine + anthracyclines. Furthermore, a high proportion of treatment with LIC (CA/CAG, AZA) was observed and it could be due to the high percentage of elderly patients aged 65 years old or more in the study population (58.1%). Considering the current identification period and the approval of VENE in Japan was in March 2021, this study expected to find few data for VENE + AZA. Although treatment with LIC showed a high percentage in this study, it is expected that LIC will be replaced by VENE + AZA in the future. For the period from R/R to follow-up end, FLT3i was again the most common 1 L-post R/R for FLT3m + AML patients, accounting for 68.4%, and being the main treatment for all treatment lines. For the period of the first HSCT to follow-up end, FLT3i was the primary treatment. Additionally, the patients under the age of 65, which composed 41.9% (n = 151) of the study population, could be eligible for transplantation therapy, and 26.6% (n = 93) of the patients in the study population received transplantation therapy. Thus, this might indicate that more than 60% of transplant-eligible patients were able to undergo transplantation, which was considered to reflect the real-world clinical situation.
In Japan, gilteritinib and quizartinib are FLT3i that can be used for R/R FLT3m + AML patients, and some cases were treated with FLT3i as sequential treatment. According to a study by Loschi et al. (2021), FLT3i increased the OS of patients with previously low prognosis. These medications represent a significant advancement in terms of efficacy, having fewer side effects, and are revolutionizing the way FLT3m + AML patients are treated at every stage, from induction to post-allo-HSCT maintenance [Citation21]. This study using real-world data also suggests that FLT3i has become established as a standard treatment (including use before and after transplantation) for R/R FLT3m + AML in Japan, as seen in prior studies.
The median OS in this study for the study population after initiating FLT3i treatment was 394 days (approximately 13 months); this result was comparable to that of the ADMIRAL trial (median OS: 14.3 months) [Citation12], which is a well-controlled clinical trial different from real-world settings. However, the median age of patients in the ADMIRAL study was 62.0 years, whereas the median age in this study population was 68.0 years, making an older population comparison with the ADMIRAL study population. Despite such a discrepancy between the study populations, it could be noteworthy that the generalizability of the treatment efficacy of FLT3i for patients with FLT3m + AML was strengthened with the result of this study. In this study, whereas the median treatment duration of FLT3i was 88.5 days, that of the Japanese post-marketing surveillance study of all cases was 98 days, and the result of our study was close to the surveillance result [Citation22]. On the other hand, our result was lower than the median duration of treatment in the Japanese subpopulation (154 days) in the ADMIRAL trial [Citation12]. It is possible that this difference occurred because the difference between a nature of well-controlled clinical trial and real-world clinical setting, or the patient population in this study, which included a certain number of patients with low income due to loss of productivity with the disease and difficulties continuing long-term treatment under real-world clinical practice, was different from that in the clinical trial. As one of the findings in the ADMIRAL trial, 9% of patients had Eastern Cooperative Oncology Group status ≥2 as a baseline characteristic [Citation12], compared with 24% in the Japanese post-marketing surveillance [Citation22]. Additionally, in this study, an analysis was also conducted on patients who started FLT3i treatment within 60 days after transplantation which was regarded as post-transplantation maintenance. According to this analysis, it was 66.0 days for patients treated with FLT3i as the first treatment after HSCT which was shorter than what was found from all patients treated with FLT3i on this study. This might be attributed to the small number of cases for this analysis, as there is a possibility that cases with various conditions, such as non-remission transplantation and early recurrence, were also included. It is also possible because physicians were unfamiliar with the use of FLT3i after transplantation, particularly just after the market launch of FLT3i.
In recent years, the development of therapeutic drugs has progressed, and the number of treatment options has increased. Consequently, in Japan, due to the effects of the rapidly aging population and the emergence of expensive treatment methods, awareness of medical resources and medical costs has been rising. Therefore, this study also investigated the HCRU and its costs for patients with FLT3m + AML. The study results showed that the overall mean monthly total treatment cost was JPY 2,009,531.7/PPPM (USD 17,967.9/PPPM). Additionally, the mean chemotherapy cost was higher than the cost of FLT3i treatment for the study population (chemotherapy: JPY 578,841.5/PPPM [USD 5,175.6/PPPM]; FLT3i: JPY 498,236.7/PPPM [USD 4,454.9/PPPM]). Although FLT3i is more expensive than other chemotherapy drugs for AML treatment in Japan, considering the number of medical resources used, FLT3i could be comparable or cheaper than chemotherapeutic drugs in terms of medical expenses per month per patient in clinical practice. Moreover, medical resources other than drugs would be required for chemotherapy such as hospitalization and cost for outpatient chemotherapy. Consequently, there is a likelihood that the overall cost incurred by patients undergoing chemotherapy would be higher.
Limitations of present study
This study used the MDV database, which comprises claims data from acute care hospitals with a large number of beds across Japan. This allows for representation of Japan’s general population. However, the MDV database did not contain detailed clinical information to identify AML patients by disease severity or disease progression. Also, information such as the FLT3 mutation subtype, and cytogenetic risk status were not available in the MDV database. Furthermore, deaths were only captured through patients’ hospital discharge records, and deaths outside the hospitals were not captured. Therefore, such characteristics and database limitations might cause or lead to bias in the results of this study. In particular, the variety of baseline characteristics of patients would have a significant impact on the estimates. Patient follow-up was limited during the study duration, as MDV could not capture the subsequent patient journey once patients left the hospital or when treatments were provided outside the hospital setting. Patients who were transferred to other health care facilities were not traceable. In fact, this research contained a number of cases where FLT3i was used in the first line from the index date whereas FLT3i is approved for patients with R/R FLT3m + AML. Furthermore, if the patient’s relocated facility is subject to data accumulation, transferred patients are considered as new patients with new medical records. The diagnosis date was the date of the first claim recorded in the diagnosis year-month, which might differ from the actual date. The MDV database recorded diagnoses only if they were linked to insurance claims, therefore leading to underreporting of comorbidities, and the whole patient journey could not be captured if patients visited non-DPC hospitals or a doctor’s office. Also, out-of-pocket transactions or off-label use of drugs and procedures were not included. Finally, some results might not reflect a complete picture of the real clinical practice due to the limited sample size.
Conclusion
This observational study is the first to describe real-world treatment patterns in patients with FLT3m + AML in Japan. The study showed treatment patterns and features in patients with FLT3m + AML. FLT3i was the most prescribed treatment across the study period. The study suggests that FLT3i has become established as a standard treatment, including use before and after transplantation, for R/R FLT3m + AML in Japan. The median OS duration for the study population after initiating FLT3i treatment was more than a year, which is comparable to the ADMIRAL trial’s result. The study also found various insights in terms of the clinical characteristics, clinical outcomes, HCRU, and costs of patients with FLT3m + AML in Japan. Although some limitations exist due to the nature of the database, the study results can constitute real-world evidence and provide descriptive information for health care providers. The findings of this study could be helpful for clinicians to optimize treatment strategies for AML in Japan.
Transparency
Declaration of funding
This study was sponsored by Astellas Pharma Inc.
Declaration of financial/other relationships
TK received grants or contracts from Daiichi-Sankyo, Janssen, Otsuka, and SymBio and payment or honoraria for lectures from Bristol-Myers Squibb, Novartis, and SymBio. MS, TM, YT, and KK are employees of Astellas. KY and SS are employees of IQVIA Solutions Japan KK. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
TK, MS, YT, KK, and YK made substantial contributions to the study design; TK, MS, TM, YT, KK, YK, and SS acquired the study data; TK, MS, TM, YT, KK, YK, and SS analyzed the study data; TK, MS, TM, YT, KK, YK, and SS interpreted the study data.
Supplementary material.pdf
Download PDF (311.3 KB)Acknowledgements
Medical writing support was provided by Dipti Mothay and Rosario Vivek from IQVIA India, and Dilinuer Ainiwaer from IQVIA Solutions Japan. Editorial assistance was provided by Todd D. Taylor, IQVIA Solutions, Japan.
Data availability statement
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com.
For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
References
- Saultz JN, Garzon R. Acute myeloid leukemia: a concise review. J Clin Med. 2016;5(3):33. doi:10.3390/jcm5030033.
- IHME. Acute myeloid leukemia—Level 4 cause 2019. https://www.healthdata.org/results/gbd_summaries/2019/acute-myeloid-leukemia-level-4-cause.
- Miyawaki S. Clinical studies of acute myeloid leukemia in the Japan adult leukemia study group. Int J Hematol. 2012;96(2):171–177. doi:10.1007/s12185-012-1150-6.
- Miyamoto K, Minami Y. Precision medicine and novel molecular target therapies in acute myeloid leukemia: the background of hematologic malignancies (HM)-SCREEN-Japan 01. Int J Clin Oncol. 2019;24(8):893–898. doi:10.1007/s10147-019-01467-1.
- Kiyoi H, Yamaguchi H, Maeda Y, et al. JSH practical guidelines for hematological malignancies, 2018: i. leukemia-1. Acute myeloid leukemia (AML). Int J Hematol. 2020;111(5):595–613. doi:10.1007/s12185-020-02856-3.
- Yang X, Wang J. Precision therapy for acute myeloid leukemia. J Hematol Oncol. 2018;11(1):3. doi:10.1186/s13045-017-0543-7.
- Daver N, Schlenk RF, Russell NH, et al. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33(2):299–312. doi:10.1038/s41375-018-0357-9.
- Wagner K, Damm F, Thol F, et al. FLT3-internal tandem duplication and age are the major prognostic factors in patients with relapsed acute myeloid leukemia with normal karyotype. Haematologica. 2011;96(5):681–686. doi:10.3324/haematol.2010.034074.
- Capelli D, Menotti D, Fiorentini A, et al. Overcoming resistance: FLT3 inhibitors past, present, future and the challenge of cure. Cancers (Basel). 2022;14(17):4315. doi:10.3390/cancers14174315.
- Astellas Announces Approval in Japan for XOSPATA® 40 mg Tablets for the Treatment of FLT3mut + Relapsed or Refractory AML [Available from https://www.astellas.com/system/files/news/2018-09/180921_3_En.pdf.
- Vanflyta (quizartinib). Prescribing information. Daiichi Sankyo Co, Ltd; 2019. [Internet]. Available from: https://www.medicalcommunity.jp/member/certification?destination=/&
- Hosono N, Yokoyama H, Aotsuka N, et al. Gilteritinib versus chemotherapy in japanese patients with FLT3-mutated relapsed/refractory acute myeloid leukemia. Int J Clin Oncol. 2021;26(11):2131–2141. doi:10.1007/s10147-021-02006-7.
- Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728–1740. doi:10.1056/NEJMoa1902688.
- Dhakal PP, Pyakuryal B, Pudasainee P, et al. Treatment strategies for therapy-related acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2020;20(3):147–155. doi:10.1016/j.clml.2019.12.007.
- Almeida AM, Ramos F. Acute myeloid leukemia in the older adults. Leuk Res Rep. 2016;6:1–7. doi:10.1016/j.lrr.2016.06.001.
- Pandya BJ, Chen C-C, Medeiros BC, et al. Economic and clinical burden of acute myeloid leukemia episodes of care in the United States: a retrospective analysis of a commercial payer database. J Manag Care Spec Pharm. 2020;26(7):849–859. doi:10.18553/jmcp.2020.19220.
- Kogushi K, Nakamura K, Ikeda S. Medical cost estimation for elderly acute myeloid leukemia (AML) using real world data in Japan. Value in Health. 2018;21:s13. doi:10.1016/j.jval.2018.07.099.
- Pandya BJ, Yang H, Schmeichel C, et al. A budget impact analysis of gilteritinib for the treatment of relapsed or refractory FLT3 mut + acute myeloid leukemia in a US health plan. J Med Econ. 2021;24(1):19–28. doi:10.1080/13696998.2020.1851698.
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi:10.1182/blood-2016-08-733196.
- National Comprehensive Cancer Network. NCCN Guidelines for Patients: Acute Myeloid Leukemia 2023. https://www.nccn.org/patients/guidelines/content/PDF/aml-patient.pdf
- Loschi M, Sammut R, Chiche E, et al. FLT3 tyrosine kinase inhibitors for the treatment of fit and unfit patients with FLT3-Mutated AML: a systematic review. Int J Mol Sci. 2021;22(11):5873. doi:10.3390/ijms22115873.
- Astellas Pharma Inc. Xospata Tablets Interim results of general use-results survey [Data collection period: December 3, 2018 to January 31, 2022] 2022. https://amn.astellas.jp/content/jp/amn/jp/ja/common/pdfviewer.html/content/dam/jp/amn/jp/ja/di/doc/Pdfs/DocNo202311426_y.pdf
