Abstract
Objective
To understand the preferences of healthcare providers (HCPs) in Switzerland for pediatric hexavalent vaccine attributes.
Methods
A discrete-choice experiment included a series of choices between 2 hypothetical pediatric hexavalent vaccines with varying attributes: device type (including preparation time and risk of dosage errors), proportion of infants seroprotected against Haemophilus influenzae type b (Hib) at 11–12 months (pre-booster), packaging size, years on the market, and the thermostability at room temperature. Odds ratios (ORs) and conditional relative attribute importance (CRAI) were calculated using random-parameters logit.
Results
HCPs (150 pediatricians and 40 nursing staff) in Switzerland were unlikely to choose a vaccine conferring 50% (OR 0.00; 95% CI 0.00–0.00) or 70% (OR 0.01; 95% CI 0.00–0.01) of infants with Hib seroprotection at 11-12 months (pre-booster) compared with a vaccine conferring 90% seroprotection. The odds of choosing a vaccine available on the market for more than 3 years were nearly 5 times the odds of choosing a vaccine available on the market for less than 1 year (OR 4.76; 95% CI 1.87–7.65). The odds of choosing a vaccine in a prefilled syringe were nearly 3 times the odds of choosing a reconstituted vaccine (OR 2.77; 95% CI 1.39–4.15), and the odds of choosing a vaccine with a smaller package size were nearly 2 times the odds of choosing a vaccine with larger package size (OR 1.89; 95% CI 1.23–2.55). HCPs were equally likely to choose vaccines that can stay at room temperature for 6 versus 3 days (OR 1.07; 95% CI 0.73–1.42). According to CRAI, the most important attribute was Hib seroprotection, followed by years on the market, device type, and packaging size.
Conclusion
Hib seroprotection at 11–12 months was the most important hexavalent vaccine attribute to HCPs in this study.
Introduction
Childhood vaccination is a highly effective public health intervention to prevent infectious diseasesCitation1. Combination vaccines offer a number of advantages that promote vaccination compliance, including fewer injections, a simplified vaccination schedule, and improved parental acceptance, in addition to offering operational efficiencies for healthcare providers (HCPs) Citation2–6. Pediatric hexavalent vaccines are combination vaccines that help protect against diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B (HepB), and invasive disease due to Haemophilus influenzae type b (Hib) and have been recommended for administration in Europe since 2000Citation5,Citation7. Pediatric hexavalent vaccines can be given to both full-term and premature children, though further research may be needed to confirm their use in very premature childrenCitation8–11.
In Switzerland, hexavalent vaccines are recommended for routine administration to infants at ages 2, 4, and 12 monthsCitation12–13. There are 2 pediatric hexavalent vaccines currently licensed in Switzerland: DTaP5-IPV-HepB-Hib (Vaxelis, manufactured by MCM Vaccine B.V., the Netherlands) and DTaP3-IPV-HepB/Hib (Infanrix hexa, manufactured by GlaxoSmithKline Biologicals S.A., Belgium)Citation8,Citation9,Citation14. DTaP5-IPV-HepB-Hib is a fully liquid, ready-to-use vaccine containing 5 acellular pertussis components as well as a Hib component that consists of polyribosylribitol protein (PRP) conjugated to the outer membrane protein (OMP) of N. meningitidis (PRP-OMP) among its components. In contrast, DTaP3-IPV-HepB/Hib needs to be reconstituted prior to use, contains 3 acellular pertussis components, and its Hib component has PRP conjugated to tetanus toxoid (PRP-TT) Citation8,Citation9. In clinical trials, DTaP5-IPV-HepB-Hib has demonstrated higher Hib seroprotection rates at 11 to 12 months of age (i.e. prior to the booster dose) than has DTaP3-IPV-HepB/Hib: for example, 91% of children who received 2 primary doses of DTaP5-IPV-HepB-Hib at 2 and 4 months showed anti-PRP antibodies over the threshold of ≥ 0.15 µg/mL at that age, versus 48% of children who received DTaP3-IPV-HepB/HibCitation15.
Although both hexavalent vaccines have similar safety and tolerability profilesCitation7, they differ in vaccine administration features that may be associated with process and practice efficienciesCitation5. Unlike DTaP5-IPV-HepB-Hib, the Hib component of DTaP3-IPV-HepB/Hib must be reconstituted before administration by adding the entire contents of the prefilled syringe to the vial containing the Hib powderCitation8,Citation9. In a time-and-motion study conducted in Belgium, the use of a hexavalent vaccine requiring reconstitution was associated with a higher incidence of vaccination errors related to handling and dosage, and with longer preparation times than a fully liquid prefilled vaccineCitation16. Previous studies also have found that HCPs in several countries prefer prefilled, ready-to-use vaccines over vaccines requiring reconstitutionCitation17–20, both to simplify the vaccination process and to reduce risk of vaccination errors. These attributes may have financial implications: cost-minimization analyses have shown that prefilled pediatric hexavalent vaccines are associated with reduced vaccine wastage and reduced costsCitation21–22. However, little is known about which hexavalent vaccine attributes drive HCPs’ choices in Switzerland.
We present findings from a discrete-choice experiment (DCE)—which is a survey-based method for eliciting stated preferences—evaluating the preferences of HCPs in Switzerland for the attributes of pediatric hexavalent vaccines. We also explore preference heterogeneity in the respondent sample.
Methods
Study design
A DCE survey was designed to elicit preferences of HCPs (pediatricians and nursing staff) in Switzerland for different hexavalent vaccine attributes. DCEs have been used to evaluate HCPs’ preferences in a range of therapeutic areasCitation23–24. In a DCE, respondents are presented with a series of choice questions, each asking them to choose between 2 or more hypothetical, experimentally designed intervention profiles (e.g. vaccines) comprising a combination of attributes, each with varying levels. The pattern of a respondent’s choices between the hypothetical profiles reveals their preferences for the included attributes and levels.
The study design and statistical analyses followed good research practice guidelines published by ISPORCitation25–27. The study protocol was reviewed by the institutional review board (IRB) of RTI International, which determined the study to be exempt from IRB review. Respondents were informed about the study purpose, procedures, and risks before agreeing to participate in the survey. Respondents were blinded to the study sponsor to minimize bias, and a deidentified dataset was used for the analysis. Respondents received a reasonable honorarium, within a fair market value range, for their time.
Sample size calculations represent a challenge in choice experiments. Minimum sample size depends on a number of criteria, including the question format, the complexity of the choice task, the desired precision of the results, and the need to conduct subgroup analysesCitation26,Citation28; most published DCEs have a sample size of 100 to 300 respondentsCitation29. As the study feasibility limited the sample size to approximately 150 pediatricians and 40 nursing staff who prescribe and/or administer pediatric vaccines in Switzerland, the DCE was designed to elicit sufficient information to identify preferences for all attributes and levels with acceptable precision.
Survey development
Development of the DCE survey instrument was informed by a targeted review of the literatureCitation15–20,Citation30 to identify a preliminary list of attributes that characterize available hexavalent vaccines. Once a draft set of attributes was identified, qualitative interviews were conducted with 10 HCPs in Switzerland to (1) identify attributes that were most influential in their choice of which hexavalent vaccine to order or administer and (2) assess the attribute levels and attribute descriptions to be used in the DCE survey instrument. The interviews were conducted with both German-speaking participants (4 pediatricians and 2 nursing staff) and French-speaking participants (2 pediatricians and 2 nursing staff). In the qualitative interviews, HCPs noted that the following attributes were important: type of device, risk of dosage errors, immunogenicity of the Hib component, preparation time, years the vaccine has been available in the country, risk of needle stick injuries during reconstitution, and thermostability at room temperature.
The survey instrument was drafted based on the results from the qualitative interviews as well as input from the study team. A French-language and a German-language survey were developed and then pretested with 15 HCPs in Switzerland (8 pediatricians and 7 nursing staff). During pretesting, all participants accepted the hypothetical scenarios presented in the DCE and understood the questions. Minor refinements to the survey structure and attribute descriptions were made based on the pretest interview results.
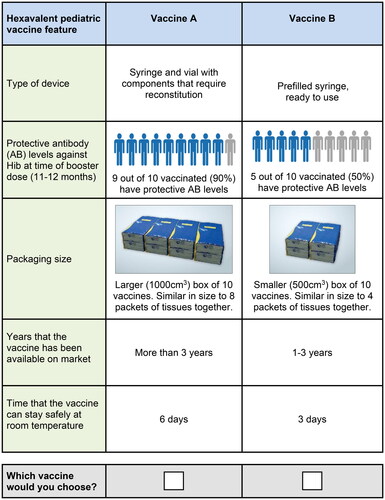
For the final online survey, the 5 attributes were as follows: (1) the type of device (ready-to-use prefilled syringe vs. syringe and vial with components requiring reconstitution), with the associated preparation time and risk of errors; (2) the percentage of infants with seroprotective Hib antibodies at 11 to 12 months of age (i.e. pre-booster dose) after receiving 2 primary doses (50% vs. 70% vs. 90%); (3) the package or box size for 10 vaccines (500 vs. 1,000 cm3); (4) the years that the vaccine has been available on the market (< 1 vs. 1–3 vs. > 3 years); and (5) the time the vaccine can stay safely at room temperature (up to 25 degrees Celsius) (6 vs. 3 days). Given the high collinearity among the 3 attributes in the survey pretests (i.e. type of device [ready-to-use prefilled syringe vs. syringe and vial with components requiring reconstitution], risk of dosage errors, and preparation time), these 3 attributes were combined into a single device-type attribute.
For the final online survey, each respondent was presented with a series of 12 choice questions in which they were asked to choose between 2 hypothetical pediatric hexavalent vaccines characterized by the 5 attributes with varying levels (). Respondents were asked to assume that both hypothetical vaccines were similar with regard to other attributes, including safety, efficacy, and cost to the provider, unless otherwise specified.
Table 1. Attributes and levels used in the discrete-choice experiment.
The combination of levels used to define each vaccine profile, the set of profiles in each choice question (), and the full set of choice questions in a DCE is known as the experimental design. A commonly accepted algorithm was used to construct the optimal fractional factorial experimental design using STATA 17 (STATA, College Station, TX) Citation26,Citation31–35. The experimental design comprised 48 DCE questions divided into 4 blocks of 12 questions. Respondents were assigned randomly to 1 of the 4 blocks of questions.
Study sample
The study sample of HCPs who prescribe or administer hexavalent vaccines in Switzerland was recruited by Global Perspectives, a research firm specializing in recruiting, survey programming and online hosting, and data collection. To be eligible, respondents had to be pediatricians or nursing staff working in Switzerland, be aged 21 years or older, prescribe or administer pediatric hexavalent vaccines to infants, have 10 or more infants vaccinated on average per month in their practice, be able to read and speak German or French, and provide online informed consent. The study team aimed to have at least 20% to 30% of respondents practicing in non-German speaking regions (called “cantons” in Switzerland), 30% to 50% experienced with DTaP5-IPV-HepB-Hib, and 50% to 70% experienced with DTaP3-IPV-HepB/Hib in the past year.
Statistical analyses
A random-parameters logit (RPL) model, specified with effects-coded variables and assuming all parameters to be normally distributed, was estimated using STATA 17 to analyze the DCE data. For each attribute level, the log-odds preference-weight, which is a measure of the relative effect of an attribute level on utility, was estimated from the RPL model. In addition, the conditional relative importance of each attribute was calculated and estimates were rescaled to sum to 100%. Therefore, the conditional relative importance of each attribute represented the proportion of total utility gained by switching that attribute from its least-preferred level to its most-preferred level. The standard errors and 95% confidence intervals (CIs) were calculated using the delta methodCitation36. Odds ratios (ORs) for each attribute level (and 95% CIs) were calculated using the estimated preference weights. Analyses were conducted for subgroups found to have significantly different preferences using a Wald test of joint equivalence and a P value < 0.05.
Results
Respondent characteristics
The survey was administered online between 23 November 2022 and 20 February 2023. In total, 190 eligible HCPs (82% of the 232 HCPs who accessed the link) completed the survey (). A majority of respondents were female (73.7%), were aged 31 to 50 years (58.4%), practiced in an urban setting (64.7%), and had been in practice for 10 years or more (73.7%). The survey was taken in German by 78.9% of respondents (118 pediatricians and 32 nursing staff) and in French by 21.1% of respondents (32 pediatricians and 8 nursing staff). Among the two types of HCPs, nurses were more often involved in vaccine preparation (80.0%) and storage (87.5%), while pediatricians (94.7%) were more often involved in administering them.
Table 2. Respondent experience and demographic characteristics and other questions.
Odds ratios
Figure S1 in the Supplementary Appendix presents the RPL preference-weight estimates, which were used to calculate ORs.
summarizes the ORs for each attribute’s levels relative to its reference attribute level. The ORs represent the change in the likelihood that an HCP would choose a vaccine with the given attribute level rather than the reference level, assuming all other attributes remained the same. All ORs were statistically significant except for the OR related to the time that the vaccine can stay safely at room temperature. Vaccines that provide protective Hib antibody levels at the time of the booster dose in 50% or 70% of infants were highly unlikely to be preferred over vaccines that provide protective Hib antibody levels at the time of booster dose in 90% of infants (OR, 0.00; 95% CI, 0.00–0.00; and OR, 0.01; 95% CI, 0.00–0.01, respectively). Survey respondents nearly always chose a vaccine providing 90% protection; thus, the odds of selecting vaccines providing 50% or 70% protection were almost zero.
Table 3. Random-parameters logit model odds ratios: full sample (N = 190).
The odds of HCPs choosing a vaccine that was commercially available for more than 3 years were nearly 5 times the odds of choosing a vaccine that was commercially available for less than 1 year (OR, 4.76; 95% CI, 1.87–7.65). Similarly, the odds of choosing a vaccine that was commercially available for 1 to 3 years were almost 3 times the odds of choosing a vaccine available for less than 1 year (OR, 2.83; 95% CI, 1.57–4.08). The odds of HCPs choosing a ready-to-use vaccine available in a prefilled syringe were almost nearly 3 times the odds of choosing a vaccine available in a syringe-and-vial combination with components requiring reconstitution (OR, 2.77; 95% CI, 1.39–4.15). The odds of choosing a vaccine in a smaller packaging size were almost twice that of choosing one in a larger packaging size (500 vs. 1,000 cm3 for a box of 10 vaccines; OR, 1.89; 95% CI, 1.23–2.55). Given the attributes and levels included in the study, the HCPs were indifferent to the time that the vaccines could stay safely at room temperature (6 vs. 3 days) (OR, 1.07; 95% CI, 0.73–1.42).
Conditional relative importance
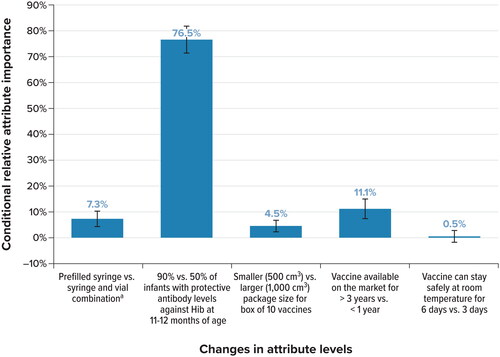
summarizes the conditional relative importance of each attribute. Given the range of attributes and levels included in the study, the seroprotection rate against Hib at the time of the booster dose was the most important attribute, associated with 76.5% of the total utility gained by switching from the least- to the most-preferred levels of an attribute included in the DCE. The next most important attribute was the time that the vaccine had been available on the market (associated with 11.1% of total utility gained), followed by the type of device (7.3% of total utility gained), and the packaging size (4.5% of total utility gained). Finally, the relative importance of the time the vaccine could be kept safely at room temperature was not statistically different from zero, indicating that respondents did not differentiate among these attribute levels when making vaccine choices in the survey.
Figure 2. Conditional relative importance of attributes: full sample (N = 190). Hib: Haemophilus influenzae type b; vs. = versus.
Note: Conditional relative attribute importance is the proportion of total utility gained by switching from the least-to the most-preferred levels of attributes, rescaled to sum to 100% across all attributes.
a With their respective associated preparation time and risk of errors.
Subgroup analyses
Significantly different preferences (p < 0.05, Wald test) were found for 2 sets of subgroups: pediatricians (n = 150) versus nursing staff (n = 40) (p = 0.002), and users of DTaP3-IPV-HepB/Hib only in the past year (n = 68) versus users of DTaP5-IPV-HepB-Hib only or users of both vaccines in the past year (n = 122) (p = 0.004) ().
The ORs show that both pediatricians and nursing staff were extremely unlikely to prefer vaccines that confer lower Hib seroprotection rates (). The odds of pediatricians choosing a ready-to-use vaccine available in a prefilled syringe were 3 times the odds of them choosing a vaccine available in a syringe-and-vial combination with components requiring reconstitution (OR, 2.99; 95% CI, 1.12–4.86). The odds of pediatricians choosing a vaccine with a smaller packaging size were 2 times that of choosing one with a larger packaging size (OR, 1.92; 95% CI, 1.17–2.67). Pediatricians were more likely to prefer a vaccine that has been available for more than 1 year than a vaccine available for less than 1 year (1–3 years vs. less than 1 year: OR, 2.32; 95% CI, 1.18–3.46; more than 3 years vs. less than 1 year: OR, 5.22; 95% CI, 1.62–8.81). Meanwhile, nursing staff showed preferences in the same direction as the pediatricians for these attributes, but the ORs were not statistically significant, except for Hib seroprotection. However, the small sample size for nursing staff may have contributed to the lack of statistically significant results. presents the conditional relative importance estimates for this subgroup analysis; for both pediatricians and nursing staff, the rate of Hib seroprotection was the most important attribute.
Figure 3. Conditional relative importance of attributes: subgroup analyses. A. Type of healthcare provider B. Experience with vaccine in the past year. Hib, Haemophilus influenzae type b; vs., versus.
a With their respective associated preparation time and risk of errors.
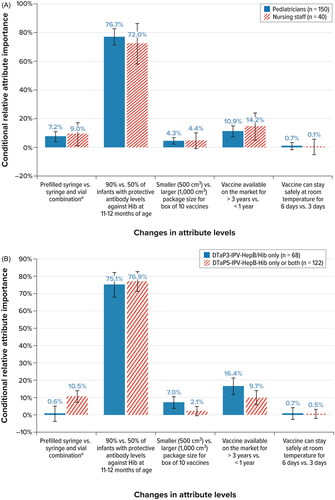
Table 4. Random-parameters logit model odds ratios, subgroup analysis: type of healthcare provider (N = 190).
The ORs also show that all users, regardless of vaccine experience in the past year, were highly unlikely to prefer vaccines that confer lower Hib seroprotection rates (). Although exclusive DTaP3-IPV-HepB/Hib users in the past year were indifferent to the device type, the odds of HCPs who had experience with either DTaP5-IPV-HepB-Hib or both vaccines in the past year choosing a ready-to-use vaccine available in a prefilled syringe were nearly 5 times the odds of them choosing a vaccine available in a syringe-and-vial combination with components requiring reconstitution (OR, 4.73; 95% CI, 1.65–7.81) (). For exclusive DTaP3-IPV-HepB/Hib users in the past year, the odds of choosing a vaccine that had been on the market for more than 3 years were almost 11 times that of choosing a vaccine on the market for less than 1 year (OR, 10.95; 95% CI, 1.07–20.82); for users of DTaP5-IPV-HepB-Hib or both vaccines in the past year, the odds of choosing a vaccine that was on the market for more than 3 years were 4 times the odds of choosing a vaccine on the market for less than 1 year (OR, 4.22; 95% CI, 1.10–7.34). Exclusive DTaP3-IPV-HepB/Hib users were more likely to prefer a vaccine in a smaller packaging size over a vaccine in a larger packaging size (OR, 2.81; 95% CI, 1.31–4.31), whereas other users were indifferent to the size of packaging, as indicated by the ORs. presents the conditional relative importance estimates for this subgroup analysis; the rate of Hib seroprotection again was the most important attribute for both groups, contributing to more than 75% of total utility.
Table 5. Random-parameters logit model odds ratios, subgroup analysis: experience with vaccine (N = 190).
Discussion
To our knowledge, this is the first study evaluating the preferences of HCPs in Switzerland for attributes of pediatric hexavalent vaccines. Our findings suggest that, when presented with vaccine alternatives conferring different pre-booster Hib seroprotection rates, the overall sample of pediatricians and nursing staff in Switzerland would nearly always prefer a vaccine conferring higher seroprotection rates (90%) over a vaccine conferring lower seroprotection rates (50% and 70%). The odds of HCPs choosing a vaccine that was commercially available for more than 3 years were nearly 5 times the odds of choosing one available for less than 1 year; the odds of selecting a ready-to-use vaccine available in a prefilled vaccine were nearly 3 times the odds of selecting a vaccine requiring reconstitution; and the odds of choosing a vaccine with a smaller packaging size were nearly twice that of choosing one with a larger packaging size. No strong preference was observed for a vaccine that can stay safely at room temperature for 6 versus 3 days.
Overall, a high early Hib seroprotection rate was the most important attribute to HCPs, accounting for 76.5% of the conditional relative importance of attributes. Subgroup analyses revealed that Hib seroprotection remained the most important driver of preferences, irrespective of the type of HCP or the type of hexavalent vaccine used in the past year. Hib seroprotection contributed > 70% of the conditional relative importance for each subgroup, with ORs strongly in favor of higher Hib seroprotection. Additionally, physicians were significantly more likely to choose a vaccine that was ready-to-use, had a smaller packaging size, and was available on the market for longer than 1 year. HCPs who exclusively used DTaP3-IPV-HepB/Hib in the past year had a higher likelihood of choosing smaller packaging size, whereas HCPs who use either DTaP5-IPV-HepB-Hib or both vaccines had a higher likelihood of choosing a prefilled ready-to-use vaccine versus one that needed reconstitution; both groups preferred vaccines that had been on the market for more than 1 year.
When considering the attributes that characterize current hexavalent vaccines available in Switzerland, the results show that HCPs in Switzerland strongly value early and robust protection against Hib. Hib is responsible for 95% of invasive H. influenzae infections in unimmunized populations and may result in severe complications, such as pneumonia and meningitisCitation37. Children aged 2 years and younger are at the highest risk of invasive H. influenzae diseases, which may be severe and potentially fatal. Although routine immunization has resulted in a considerable decline in serious Hib disease, early protection is key: most cases occur before 2 years of age, with Hib incidence peaking between the ages of 10 and 12 months in the European Union, mainly among the unimmunizedCitation38.
The importance of early protection against Hib may have gained greater urgency among HCPs due to the observed recent uptick of Hib cases among children in some European countries, including the Netherlands and FranceCitation39–Citation42. In the Netherlands, a small increase in the incidence of Hib was observed in 2020-2021, with 40% of Hib cases occurring in children aged younger than 5 years and mainly in under-vaccinated or unvaccinated individualsCitation39. In contrast, the incidence of other respiratory diseases declined during this interval as a result of pandemic-era measures, such as quarantining, school lockdowns, and use of face masksCitation40,Citation41. Similarly, during the 2020–2021 period, increases in Hib incidence were reported among children aged younger than 5 years in FranceCitation42,43, possibly due to changes in the hexavalent vaccination schedule (from 3 + 1 to a 2 + 1 schedule). In light of the observed increase in Hib cases among young children, the superior protection offered by DTaP5-IPV-HepB-Hib after 2 primary doses, and which is sustained up to the booster dose, may be of special interest. Data from several trials show that the Hib component with the PRP conjugated to OMP results in higher and more robust Hib responses after the primary series compared with the Hib component with PRP conjugated to TTCitation15,Citation44–46.
Although this study is the first to evaluate pediatricians’ and nursing staff’s perspectives on the importance of Hib immunogenicity as a hexavalent vaccine attribute, respondents’ preferences for the other attributes evaluated are broadly consistent with existing evidence that HCPs in Europe and the United States value efficient administration and avoidance of vaccination errorsCitation17–20,Citation30. The overall sample of HCP respondents in this study strongly preferred a ready-to-use vaccine available in a prefilled syringe to a vaccine available in a syringe-and-vial combination that required reconstitution. Avoiding dosage errors and reducing preparation time may be potential drivers of this preference, as shown in previous studiesCitation17–20. In a DCE in Germany, Lloyd and colleaguesCitation19 evaluated physicians’ and nurses’ preferences for hexavalent vaccines based on five attributes: type of device, years of experience with the vaccine, preparation time, probability of handling errors, and probability of dosage errors. All five attributes were important for decision-making; the probability of dosage errors was the most important attribute relative to other attributes included in the study. Similarly, studies conducted in Italy, Spain, and France have found that HCPs’ preferences for vaccines available as a prefilled, ready-to-use syringe are related to reduced preparation time, reduced risk of vaccination error, and reduced likelihood of needle contamination or injuryCitation17,Citation18,Citation20. The preference for smaller packaging size may reflect HCP preferences for smaller boxes that allow additional vaccines to be stored in the refrigerator. The indifference to longer thermostability at room temperature was unexpected; however, power outages lasting longer than a couple of days are rare in Switzerland. It is possible that this factor may be more important among HCPs in countries facing power supply issues.
The study has several strengths derived from the use of best practices in its design and analysisCitation25–27,Citation47. Selection of the attributes was informed by a literature review and qualitative research. Nonetheless, limitations are noted. As with all voluntary survey studies, the results are subject to potential selection bias and response bias, and the respondent sample may not be representative of the broader populations of physicians and nursing staff in Switzerland. Of the 190 respondents, only 40 (21.1%) were nursing staff, which limited our ability to explore preferences specific to this population. The series of attributes and levels included in the DCE may have influenced respondents’ DCE choices. Not all attributes that are relevant to HCPs who administer hexavalent vaccines could be included in the DCE. Typically, 5 to 7 attributes are assessed in any given DCE survey. We selected the attributes carefully to avoid collinearity between the type of vaccine device and closely related attributes (e.g. preparation time and risk of vaccination errors). In addition, respondents were asked to assume that the efficacy, safety, and cost of the hypothetical hexavalent vaccine profiles were similar, unless stated otherwise. The preference data are based on hypothetical choice profiles, which simulate possible clinical or administrative decisions but may not have the same consequences as actual real-world decisions.
Conclusions
This study is the first, to our knowledge, to have evaluated the preferences of HCPs in Switzerland for the attributes of pediatric hexavalent vaccines. When presented with hypothetical pediatric hexavalent vaccine profiles, pediatricians and nursing staff in Switzerland considered Hib seroprotection to be the most important attribute. Time on the market, device type, and packaging size were also considerations for HCPs when choosing pediatric hexavalent vaccines.
Transparency
Declaration of financial/other relationships
SS, TM, and TP are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), and may own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. CP and PC are employees of RTI Health Solutions, which received funding from MCM for this study. MB was an employee of RTI Health Solutions when this research was conducted. EL and SO are employees of Sanofi and may hold stock and/or hold stock options in the company.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
SS, SO, TM, CP, PC, EL, TP, and MB are responsible for the work described in this paper. All authors were involved in conception, design of work or acquisition, analysis, and interpretation of data and were involved in drafting the manuscript or reviewing the manuscript for important intellectual content. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplemental Material
Download MS Word (709.7 KB)Acknowledgements
The authors thank Bianca Chun of MSD and Kimberly Moon of RTI Health Solutions for providing project management for this study. Kate Lothman of RTI Health Solutions provided writing and editorial support, with funding from MSD.
Additional information
Funding
References
- World Health Organization (WHO). Immunization coverage. July 14, 2022. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage. Accessed July 11, 2023.
- Maman K, Zöllner Y, Greco D, et al. The value of childhood combination vaccines: from beliefs to evidence. Hum Vaccin Immunother. 2015;11(9):2132–2141. doi: 10.1080/21645515.2015.1044180.
- Centers for Disease Control and Prevention. Combination vaccines. August 1, 2019. https://www.cdc.gov/vaccines/parents/why-vaccinate/combination-vaccines.html. Accessed July 11, 2023.
- Orsi A, Azzari C, Bozzola E, et al. Hexavalent vaccines: characteristics of available products and practical considerations from a panel of Italian experts. J Prev Med Hyg. 2018;59(2):E107–E119.
- Obando-Pacheco P, Rivero-Calle I, Gómez-Rial J, et al. New perspectives for hexavalent vaccines. Vaccine. 2018;36(36):5485–5494. doi: 10.1016/j.vaccine.2017.06.063.
- Kurosky SK, Davis KL, Krishnarajah G. Effect of combination vaccines on completion and compliance of childhood vaccinations in the United States. Hum Vaccin Immunother. 2017; 13(11):2494–2502. doi: 10.1080/21645515.2017.1362515.
- Wilsdon T, Lawlor R, Li L, et al. The impact of vaccine procurement methods on public health in selected European countries. Expert Rev Vaccines. 2020;19(2):123–132. doi: 10.1080/14760584.2020.1717952.
- European Medicines Agency (EMA). European Public Assessment Report: vaxelis. February 20, 2023a. https://www.ema.europa.eu/en/medicines/human/EPAR/vaxelis. Accessed July 11, 2023.
- European Medicines Agency (EMA). European Public Assessment Report: infanrix hexa. May 24, 2023b. https://www.ema.europa.eu/en/medicines/human/EPAR/infanrix-hexa. Accessed July 11, 2023.
- Wilck MB, Xu ZJ, Stek JE, et al. Safety and immunogenicity of a fully-liquid DTaP-IPV-Hib-HepB vaccine (vaxelis™) in premature infants. Hum Vaccin Immunother. 2021; 17(1):191–196. doi: 10.1080/21645515.2020.1756668.
- Knuf M, Charkaluk ML, The Nguyen PN, et al. Penta- and hexavalent vaccination of extremely and very-to-moderate preterm infants born at less than 34 weeks and/or under 1500 g: a systematic literature review. Hum Vaccin Immunother. 2023;19(1):2191575. doi: 10.1080/21645515.2023.2191575.
- World Health Organization (WHO). Vaccination schedule for Switzerland. 2023. https://immunizationdata.who.int/pages/schedule-by-country/che.html. Accessed July 11, 2023.
- Swiss Confederation. Swiss vaccination schedule 2023. 2023. https://www.bag.admin.ch/bag/de/home/gesund-leben/gesundheitsfoerderung-und-praevention/impfungen-prophylaxe/schweizerischer-impfplan.html. Accessed August 15, 2023.
- Swissmedic. 2023. https://www.swissmedicinfo.ch/. Accessed July 11, 2023.
- Silfverdal SA, Icardi G, Vesikari T, et al. A phase III randomized, double-blind, clinical trial of an investigational hexavalent vaccine given at 2, 4, and 11-12 months. Vaccine. 2016; 34(33):3810–3816. doi: 10.1016/j.vaccine.2016.05.054.
- De Coster I, Fournie X, Faure C, et al. Assessment of preparation time with fully-liquid versus non-fully liquid paediatric hexavalent vaccines. A time and motion study. Vaccine. 2015;33(32):3976–3982. doi: 10.1016/j.vaccine.2015.06.030.
- Icardi G, Orsi A, Rosati GV, et al. Preferences of healthcare professionals regarding hexavalent pediatric vaccines in Italy: a survey of attitudes and expectations. J Prev Med Hyg. 2020;61(3):E424.
- Esteve IC, Fernández Fernández P, López Palacios S, et al. Health care professionals’ preference for a fully liquid, ready-to-use hexavalent vaccine in Spain. Prev Med Rep. 2021; 22:101376. doi: 10.1016/j.pmedr.2021.101376.
- Lloyd AJ, Nafees B, Ziani E, et al. What are the preferences of health care professionals in Germany regarding fully liquid, ready-to-use hexavalent pediatric vaccine versus hexavalent pediatric vaccine that needs reconstitution? Patient Prefer Adherence. 2015;9:1517–1524. doi: 10.2147/PPA.S87229.
- Bakhache P, Virey B, Bienenfeld C. Knowledge and practices regarding infant vaccination: results of a survey of French physicians. Eur J Pediatr. 2019; 178(4):533–540. doi: 10.1007/s00431-018-03314-3.
- Mathijssen DA, Heisen M, Clark-Wright JF, et al. Budget impact analysis of introducing a non-reconstituted, hexavalent vaccine for pediatric immunization in the United Kingdom. Expert Rev Vaccines. 2020;19(12):1167–1175. doi: 10.1080/14760584.2020.1873770.
- Lang J, Bencina G, Samant S, et al. Cost implication of introducing a fully liquid ready to use pediatric hexavalent vaccine in the United Kingdom (UK) and Switzerland Poster presented at European Society For Paediatric Infectious Diseases 2021 Virtual Meeting; May 24–29, 2021.
- Clark M, Determann D, Petrou S, et al. Discrete choice experiments in health economics: a review of the literature. Pharmacoeconomics. 2014;32(9):883–902. doi: 10.1007/s40273-014-0170-x.
- Soekhai V, de Bekker-Grob EW, Ellis AR, et al. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–226. doi: 10.1007/s40273-018-0734-2.
- Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413. doi: 10.1016/j.jval.2010.11.013.
- Johnson FR, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis discrete-choice experiment experimental design good research practices task force. Value Health. 2013;16(1):3–13. doi: 10.1016/j.jval.2012.08.2223.
- Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis experimental design task force. Value Health. 2016;19(4):300–315. doi: 10.1016/j.jval.2016.04.004.
- Louviere JJ, Hensher DA, Swait JD. Stated choice methods: analysis and applications. New York: Cambridge University Press; 2000.
- Marshall D, Bridges JFP, Hauber AB, et al. Discrete-choice experiment applications in health—how are studies being designed and reported? An update on current practice in the published literature between 2005 and 2008. Patient. 2010; 3(4):249–256. doi: 10.2165/11539650-000000000-00000.
- Samant S, Petigara T, Aggarwal J, et al. Physician preferences for attributes of pediatric combination vaccines in the United States. Curr Med Res Opin. 2022;38(11):2003–2009. doi: 10.1080/03007995.2022.2079262.
- Carlsson F, Martinsson P. Design techniques for stated preference methods in health economics. Health Econ. 2003;12(4):281–294. doi: 10.1002/hec.729.
- Cook RD, Nachtrheim CJ. A comparison of algorithms for constructing exact D-optimal designs. Technometrics. 1989;22(3):315–324. doi: 10.1080/00401706.1980.10486162.
- Hole AR. DCREATE: stata module to create efficient designs for discrete choice experiments. Statistical software components S458059. Boston (MA): Boston College Department of Economics; 2015.
- Kuhfeld W, Tobias F, Garratt A. Efficient experimental design with marketing research applications. J Marketing Res. 1997;31(4):545–557. doi: 10.1177/002224379403100408.
- Sándor Z, Wedel M. Designing conjoint choice experiments using managers’ prior beliefs. J Mark Res. 2001;38(4):430–444. doi: 10.1509/jmkr.38.4.430.18904.
- Hensher DA, Rose JM, Greene WH. Applied choice analysis. Cambridge, UK Cambridge University Press; 2005.
- World Health Organization (WHO). Haemophilus influenzae type b (Hib) Vaccination Position Paper. July 27, 2013. https://www.who.int/publications/i/item/who-wer8839-413-426. Accessed August 15, 2023.
- European Centre for Disease Prevention and Control. Factsheet about invasive Haemophilus influenzae disease. 2023. https://www.ecdc.europa.eu/en/invasive-haemophilus-influenzae-disease/facts. Accessed July 11, 2023.
- Steens A, Stanoeva KR, Knol MJ, et al. Increase in invasive disease caused by Haemophilus influenzae b, The Netherlands, 2020 to 2021. Euro Surveill. 2021; 26(42):2100956. doi: 10.2807/1560-7917.ES.2021.26.42.2100956.
- Brueggemann AB, Jansen van Rensburg MJ, Shaw D, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the invasive respiratory infection surveillance initiative: a prospective analysis of surveillance data. Lancet Digit Health. 2021; 3(6):e360–e370. doi: 10.1016/S2589-7500(21)00077-7.
- Middeldorp M, van Lier A, van der Maas N, et al. Short term impact of the COVID-19 pandemic on incidence of vaccine preventable diseases and participation in routine infant vaccinations in The Netherlands in the period March-September 2020. Vaccine. 2021;39(7):1039–1043. doi: 10.1016/j.vaccine.2020.12.080.
- Deghmane AE, Taha MK. Changes in invasive Neisseria meningitidis and Haemophilus influenzae infections in France during the COVID-19 pandemic. Microorganisms. 2022;10(5):907. doi: 10.3390/microorganisms10050907.
- Hong E, Terrade A, Denizon M, et al. Haemophilus influenzae type b (hib) seroprevalence in France: impact of vaccination schedules. BMC Infect Dis. 2021;21(1):715. doi: 10.1186/s12879-021-06440-w.
- Vesikari T, Becker T, Vertruyen AF, et al. A phase III randomized, double-blind, clinical trial of an investigational hexavalent vaccine given at two, three, four and twelve months. Pediatr Infect Dis J. 2017;36(2):209–215. doi: 10.1097/INF.0000000000001406.
- Wilck MB, Jin Xu Z, Stek JE, et al. Protective immune responses against Haemophilus influenza type b elicited by a fully-liquid DTaP-IPV-Hib-HepB vaccine (VAXELIS™). Vaccine. 2021;39(9):1428–1434. doi: 10.1016/j.vaccine.2021.01.046.
- EudraCT. 2019-002988-10. May 24, 2023. https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-002988-10/results. Accessed August 17, 2023.
- Ho MP, Gonzalez JM, Lerner HP, et al. Incorporating patient-preference evidence into regulatory decision making. Surg Endosc. 2015;29(10):2984–2993. doi: 10.1007/s00464-014-4044-2.