Abstract
Objective
The investigation of the real-world use of the extemporaneous combination of nebivolol and amlodipine (NA-EXC) in adult patients diagnosed with hypertension in Europe.
Methods
Retrospective analysis of data extracted from seven databases of patient medical records and prescriptions from Italy, Germany, France, Hungary, and Poland, to determine the prevalence and incidence of NA-EXC use and to estimate the number of patients potentially eligible for a single-pill combination of the two antihypertensives. Secondary objectives included: the description of the population of NA-EXC users and the assessment of their adherence to treatment based on the proportion of days covered.
Results
The use of NA-EXC was found to be common in Europe and ranged between 2.9% to 9.9% of all patients identified in the databases with a prescription of nebivolol and/or amlodipine. The estimated numbers of patients potentially eligible in 2019 for a single-pill combination of nebivolol and amlodipine in Italy and Germany were, respectively, 178,133 and 113,240. Users of NA-EXC were mostly aged 70–79 years, had metabolic disorders and other comorbidities; >70% of them had received ≥2 concomitant medications before starting NA-EXC. Adherence to NA-EXC was defined as high only in 15.6% to 35% of patients.
Conclusions
The extemporaneous combination of nebivolol and amlodipine is commonly prescribed in Europe, however adherence to the therapy is poor. The development of a single-pill combination of nebivolol and amlodipine may improve adherence by reducing the number of pills administered to patients and thus simplifying treatment regimens.
Introduction
More than one billion of people are affected by hypertension worldwide and prevalent cases are increasingCitation1. In central and Eastern Europe, the prevalence of hypertension is estimated to be greater than 150 million peopleCitation1,Citation2.
Hypertension remains the leading modifiable risk factor for cardiovascular (CV) disease and premature death, with a significant clinical and economic burden on healthcare systemsCitation3–6. This pathological condition is treated with a combination of lifestyle interventions and blood pressure (BP)-lowering drugsCitation2,Citation5,Citation7. Five classes of antihypertensives are currently recommended: angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), β-blockers, calcium channel blockers (CCB), and thiazide diureticsCitation2.
Combination of BP-lowering drugs is recommended by all international guidelinesCitation2,Citation8. Hypertension is a multifactorial condition, thus the combination of agents with complementary action on different physiological pathways can maximize the clinical benefitsCitation9. Moreover, the combination of two drugs from any class was associated with a 5-fold BP reduction and a limitation of the occurrence of side effect compared with doubling the dose of a single drugCitation7,Citation10. According to the ESC/ESH guidelines, with the exception of ACEi and ARB, all five drug classes can be safely combinedCitation2.
Despite the demonstrated efficacy of several treatment strategies including pharmacotherapy and lifestyle modifications, suboptimal BP control remains a pervasive problem in clinical practiceCitation11,Citation12. One of the barriers associated with poor BP control is adherenceCitation13. Notably, some studies have highlighted a strong and inverse correlation between adherence and the number of pills prescribedCitation14,Citation15. Therefore, several guidelines have clearly recommended a simplification of the treatment by using single-pill combinations (SPCs)Citation2.
Nebivolol, a third generation β1-adrenergic receptor antagonist, possesses long-acting properties and a high degree of selectivity. Nebivolol is approved in Europe for managing hypertension and, for patients ≥70 years, for chronic heart failure regardless of the level of ejection fractionCitation16. The pharmacological profile of nebivolol is peculiar as, in addition to its cardioselectivity mediated via β1 receptor blockade, it induces nitric oxide-mediated vasodilation by stimulating endothelial nitric oxide synthase via β3 agonism. Studies on nebivolol have shown that it has more favorable effects on central BP, aortic stiffness, and endothelial functionCitation17,Citation18.
Nebivolol does not negatively influence glucose or lipid metabolism and is well-tolerated in patients with chronic obstructive pulmonary diseaseCitation19. Moreover, it does not exert any detrimental effect on erectile function in hypertensive patientsCitation17.
Amlodipine, a CCB, is indicated alone or in combination, for the management of hypertension and chronic stable angina, and it has been shown to possess a favorable safety profile. Its mechanism of action involves inhibiting the influx of calcium into vascular smooth muscle cells, which results in reduced peripheral vascular resistance. In hypertensive patients, amlodipine showed a sustained and gradual onset of antihypertensive effects, measured by 24-h ambulatory blood pressure monitoring. The recommended starting dose is usually 5 mg, and the maximum daily dose should not exceed 10 mgCitation20.
Nebivolol and amlodipine are currently administered as an extemporaneous combination in clinical practice, since a SPC is not available. Currently, a prospective clinical trial is evaluating the effect of nebivolol 5 mg with amlodipine 5 or 10 mg in hypertensive patients (NCT05513937).
A fundamental principle in combination therapy is that the mechanisms of action must be complementary. The combination of a β-blocker (BB) and a dihydropyridine CCB meets, in fact, this criterionCitation21. Different guidelines recommend the combination of a BB with a dihydropyridine CCBCitation2, as it yields an enhanced blood pressure reduction and is, in general, well toleratedCitation22.
With the goal to assess if the use of the extemporaneous combination of nebivolol 5 mg and amlodipine 5/10 mg is a consolidated therapeutic approach in clinical practice, we gathered real-world data from five European countries. In addition, we estimated the number and clinical profile of patients potentially eligible for SPC of the two antihypertensives.
Methods
Study design and objectives
This was a retrospective analysis of real-world data extracted from seven databases of patient medical records and prescriptions from Italy, Germany, France, Hungary, and Poland. The primary objectives of the study were: 1) to determine the number of prevalent users of the extemporaneous combination of nebivolol and amlodipine (NA-EXC); 2) to determine the number of incident users of NA-EXC. Secondary objectives included: a description of the population of incident users of the combination therapy (demographic and clinical characteristics); an estimation of the adherence to treatment in the population of incident users; and a stratification of incident users of NA-EXC by amlodipine dosage (5 mg, 10 mg, or both) during follow-up (defined as the six-month period from the index date, see below).
Source of data
The following databases were the sources of the analyzed data: Italian IQVIA Longitudinal Patient Database (LPD), Italian IQVIA LifeLink Treatment Dynamics (LRx) Database, German IQVIA Disease Analyzer (DA), German IQVIA LRx Database, French IQVIA LRx Database, Hungarian National Insurance Fund (NHIF) Database, and Polish IQVIA LRx Database. The Italian IQVIA LPD, established in 1998, comprises data from computer-based patient records registered by roughly 900 general practitioners, uniformly distributed throughout the whole national territory and it has proven to be useful for several studiesCitation23,Citation24. The approximately 1,200,000 patients recorded are representative of the Italian general population in terms of age and gender. The information reported in IQVIA LPD includes demographic data, medical records (diagnoses, tests, tests results, hospital admissions) and information on drug prescriptionsCitation23,Citation24. Diseases are classified according to the International Classification of Diseases - Ninth Revision (ICD-9) (Table S1), while drugs are coded according to Anatomical Therapeutic and Chemical (ATC) classification system (Table S2).
The German IQVIA DA is based on a representative sample of >3,500 resident physicians in Germany and collects treatment-related data such as diagnoses, laboratory values, referrals, sick leaves, and prescriptions from statutory and private health insurances. Diagnoses are recorded according to the International Classification of Diseases - Tenth revision (ICD-10), while the drugs follow the ATC codification (Tables S1 and S2).
Table 1. Patient attrition for inclusion in the european cohorts of prevalent and incident users of the extemporaneous combination of nebivolol and amlodipine (NA-EXC).
Table 2. Clinical characteristics of incident users of the extemporaneous combination of nebivolol and amlodipine in Italy and Germany.
The IQVIA Italian, German, French and Polish LRx databases contain patient information about the prescriptions collected from retail pharmacy computers. An important advantage of these databases, which have proven to be valuable in different drug utilization studies, is their broad coverage of the territory’s pharmacies, thus allowing to capture prescriptions coming from both specialist physicians and GPsCitation24–27.
The Hungarian NHIF database contains detailed provision data (medicine, out- and inpatient services) from the whole Hungarian population of 9,730,772 subjects (Hungarian Central Statistical Office data, 2021), collected since 2010Citation28, and encompasses also demographic, diagnostic (ICD), and treatment-related data [according to the Anatomical Therapeutic Chemical (ATC) classification] (Table S2).
Cohort definitions
Prevalent and incident users
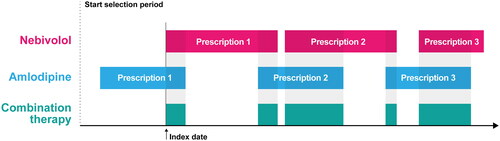
Adult patients (≥ 18 years for IQVIA LPD, DA, German and French LRx, and NHIF; >20 years for IQVIA Italian and Polish LRx) prescribed with 5 mg nebivolol and 5 mg/10 mg amlodipine (identified via the ATC code C07AB12 and C08CA01, respectively) during the selection period were considered. Selection periods differed among the databases. For the analyses of the Italian IQVIA LPD and German IQVIA DA, the selection period started on July 1st 2011 and lasted until June 30th 2020 (9 years) and, to be included in the final cohorts, patients had to present with a hypertension diagnosis (ICD-9 codes 401.xx and 402.xx; ICD-10 codes I10.x and I11.xx). For the analyses of the IQVIA LRx databases and the Hungarian NHIF database, the selection period extended from July 1st 2017 to June 30th 2020 (3 years) ().
The index date, for each patient, was defined as the date of the first prescription of the second drug that overlapped with the duration of the first drug of the combination during the selection period.
As described in detail in a previous study of similar design and objectivesCitation24, the cohort of prevalent users of NA-EXC included both patients starting the treatment during the selection period, as well as patients who received the treatment during the preselection period (i.e. during the six-month period prior to the index date). Quantifying this cohort was useful as it provided an estimate of the numbers of potential users of a fixed-dose combination of nebivolol and amlodipine in the European country considered (in this study, Italy, and Germany).
Incident users of NA-EXC included patients starting the treatment during the selection period. Patients who had a previous prescription of NA-EXC during the six-months period before the index date were excluded.
Potential eligibility for the SPC nebivolol-amlodipine
To determine the number of patients who may be eligible for a potential nebivolol-amlodipine SPC we focused on the data collected in 2019. This selection aimed to minimize potential biases resulting from the impact of the COVID-19 pandemic, which affected the diagnoses and the implementation of new treatmentsCitation29. The estimation was performed as previously describedCitation23, on the basis of adult active patients within the Italian IQVIA LPD and German IQVIA DA databases in 2019 and the adult hypertensive population of both countries, according to the Società Italiana dell’Ipertensione Arteriosa (SIIA), the Istituto Nazionale di Statistica (ISTAT), the NCD Risk Factor Collaboration and the Database of the Federal Statistical Office of Germany for Italy and Germany, respectivelyCitation30–32.
Patient characteristics
The demographic characteristics of incident users of NA-EXC were extracted from the databases as previously describedCitation23. Clinical characteristics (body mass index, comorbidities, concomitant medications, and referrals to cardiologic visits) were retrieved from the Italian IQVIA LPD and the German IQVIA DA. Comorbidities and co-prescriptions were defined according to Volpe et alCitation23. Comorbidities were searched during the pre-selection period using the recording of the corresponding ICD-9-CM or ICD-10-WHO code, while co-prescriptions were searched during the pre-selection and the follow-up periods, separately using the record of at least one prescription of the corresponding ATC code (Tables S1 and S2).
Adherence to treatment
Adherence to treatment, defined as the extent to which patients comply with the antihypertensive treatment regimen prescribed by their physicians, was assessed based on the proportion of days covered (PDC) over the follow-up periodCitation23. The PDC corresponds to the total days of medication supply over the follow-up period and is expressed as percentage. Total days of medication supply were calculated by dividing the total amount of prescribed medication by the recommended daily dose. Treatment adherence was classified as low when PDC was < 40%, intermediate when PDC was included between 40% and 79%, and high when PDC was ≥ 80%.
Statistical analysis
Data were analyzed by descriptive statistics. Categorical variables were reported as frequencies and percentages, while continuous variables were reported as mean values and standard deviation (±SD), and median values with first (Q1) and third (Q3) quartiles. All the analyses were performed using the SAS® software version 9.4.
Results
Number of NA-EXC users
The total number of patients prescribed nebivolol and/or amlodipine in the seven databases during the selection period were: 165,853 in the Italian LPD database, 161,154 in the German DA database (patients from these databases were also diagnosed with hypertension), 3,427,449 in the Italian LRx database, 5,294,053 in the German LRx database, 2,108,760 in the French LRx database, 1,321,990 in the Hungarian NHIF database, and 2,200,449 in the Polish LRx database.
reports the number of patients with at least one prescription of NA-EXC during the selection period from the different databases. Patients without a diagnosis of hypertension could be identified from the Italian LPD and German DA and were 1,212 (8.9%) and 1,3475 (20.3%), respectively.
The numbers of prevalent and incident users of NA-EXC in the various databases considered are also summarized in . Over the period investigated, the prevalent users of NA-EXC were 12,041 (7.5% of all patients of the database diagnosed with hypertension and prescribed with nebivolol and/or amlodipine during the selection period) from the Italian LPD database, 5,288 (3.3%) from the German DA database, 257,234 (7.5%) from the Italian LRx database, 155,205 (2.9%) from the German LRx database, 84,428 (4.0%) from the French LRx database, 186,461 (7.2%) from the Polish LRx database, and 131,392 (9.9%) from the Hungarian NHIF database. Incident users, starting the treatment within the selection period, were 10,222 (6.2%) from the Italian LPD, 4,604 (2.9%) from the German DA database, 186,471 (5.4%) from Italian LRx the Italian LRx database, 97,095 (1.8%) from the German LRx database, 64,764 (3.1%) from the French LRx database, 47,498 (4.9%) from the Hungarian NHIF and 155,152 (6%) from the Polish LRx database.
In the Italian LPD database, there were 4,008 prevalent users of NA-EXC in 2019. This number translated into an estimated 178,133 adult patients treated in Italy with NA-EXC over 2019, which can account for the Italian population of hypertensive patients that are potentially eligible for a SPC of nebivolol and amlodipine.
In the German DA there were 1,745 prevalent users of NA-EXC in 2019, leading to an estimated number of 113,240 adult patients treated with NA-EXC in 2019, thus providing an estimate of the population that is potentially eligible for a SPC of nebivolol and amlodipine in Germany.
Demographic characteristics of incident NA-EXC users and prescribed amlodipine doses
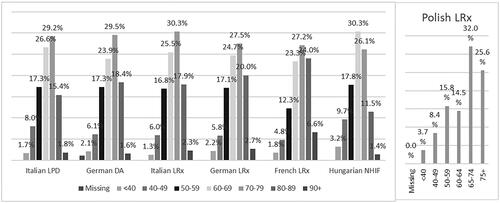
Incident users of NA-EXC were slightly predominantly female across all databases, with the exception of the German DA and French LRx databases (). The stratification of incident users of NA-EXC by age showed that approximately three-quarters of patients starting treatment with NA-EXC in the selection period were aged >60 years in Italy, Germany, and France, while Polish and Hungarian data revealed a slightly younger population of incident NA-EXC users ().
Figure 2. Incident users of the extemporaneous combination of nebivolol and amlodipine stratified by sex.
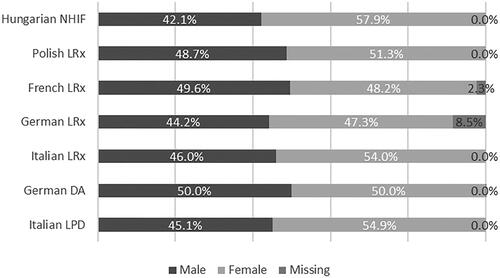
Figure 3. Incident users of the extemporaneous combination of nebivolol and amlodipine stratified by age group.
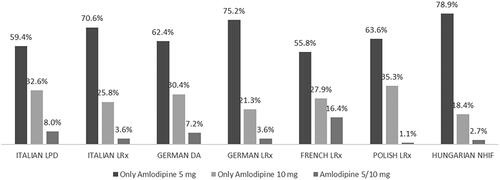
Regarding the amlodipine dosage prescribed to incident users of NA-EXC over the six-month follow-up period, the proportion of patients receiving amlodipine 5 mg/day ranged from 55.8% (French LRx) to 78.9% (Hungarian NHIF), while the proportion of patients receiving amlodipine 10 mg/day were lower and ranged from 18.4% to 35.3% (from Hungarian NHIF and Polish LRx, respectively) (). A small percentage of patients (1.1% to 16.4% in Polish LRx and French LRx, respectively) changed the amlodipine dosage during the six-month follow-up period ().
Clinical characteristics of incident NA-EXC users
summarizes the clinical characteristics of incident NA-EXC users in Italy and Germany.
Among incident NA-EXC users with a recorded BMI value, mean BMI was 28.8 (±5.3 SD) kg/m2 for Italian patients and 29.5 (±5.7 SD) kg/m2 for German patients. Overweight and obese patients were 40.8% and 35.9% in Italy, and 37.1% and 42.3% in Germany.
Diabetes mellitus was the most common comorbidity (22.2% of Italian LPD and 23.1% of German DA), followed by dyslipidemia (19.0% of Italian LPD and 21.5% of German DA), cardiac dysrhythmias (7.4% of Italian LPD and 12.6% of German DA) and other forms of chronic ischemic heart disease (6.6% of Italian LPD and 13.2% of German DA). Other comorbidities affecting the Italian incident users of NA-EXC with a frequency ≥ 3% were gout, chronic kidney disease, acquired hypothyroidism, occlusion and stenosis of precerebral arteries, and asthma. For German incident users of NA-EXC, other comorbidities with a frequency ≥ 3% included chronic kidney disease, acquired hypothyroidism, occlusion and stenosis of precerebral arteries, asthma, chronic airway obstruction not elsewhere classified, heart failure, simple and unspecified goiter, overweight and obesity, other peripheral vascular disease, and edema (including peripheral edema).
The concomitant medications prescribed before starting treatment with NA-EXC (i.e. in the six-month period before the index date) were agents acting on the renin-angiotensin system (73.5% and 70.0%), followed by antithrombotics (38.5% and 31.7%), lipid-lowering agents (34.6% and 30.3%), non-steroidal anti-inflammatory drugs (NSAIDs) (25.0% and 17.4%), diuretics (22.4% and 29.4%) and antihyperglycemic drugs (19.9% and 20.1%) in Italian LPD and German DA, respectively (). Of note, more than 70% of Italian and German patients had received ≥2 concomitant medications before starting treatment with NA-EXC.
The most frequently concomitant medications prescribed during the six-month period after starting NA-EXC treatment were agents acting on the renin-angiotensin system (73.9% of Italian LPD and 78.7% of German DA), followed by antithrombotics (44.4% of Italian LPD and 42.0% of German DA), lipid-lowering agents (39.9% of Italian LPD and 38.8% of German DA), diuretics (26.3% of Italian LPD and 39.8% of German DA), NSAIDs (23.4% of Italian LPD and 16.7% of German DA) and antihyperglycemic drugs (21.4% of Italian LPD and 23.0% of German DA).
The percentage of patients who had at least one referral for a cardiologic visit during the six-month period following the start of the treatment with NA-EXC was lower than the one reported during the six-month period preceding the index date (12.0% versus 19.3%, respectively; data available only for the Italian population). Overall, the majority of Italian patients (60.3%) did not have any cardiologic visit referral over the entire period of time considered.
Adherence to treatment
The results of the evaluation of the adherence to prescribed treatments are summarized in . Mean PDC (i.e. the proportion of days effectively covered by NA-EXC) was 41.9% (SD = 29.1) in Italian incident users of NA-EXC and 62.2% (SD = 28.8) in German patients. On average patients were taking both antihypertensives for 75 days of the 180 days of follow-up in Italy, and for 112 days of the 180 days of follow-up in Germany. From Italian LPD, low adherence (PDC < 40%) to NA-EXC was found in 54.5% of patients, while treatment adherence could be classified as moderate and high in, respectively, 29.8% and 15.6% of patients. A substantially different picture of adherence to NA-EXC emerged from the analysis of German data, according to which 41.9% of patients showed intermediate adherence, 35.0% showed high adherence, and only 23.1% had low adherence.
Table 3. Adherence to treatment of incident users of the extemporaneous combination of nebivolol and amlodipine in Italy and Germany.
Discussion
This real-world evidence study performed on databases of patient medical records and prescriptions, covering a population of 14 million adult patients across five European countries, shows that the use of NA-EXC is a consolidated practice in clinical routine. To the best of our knowledge, this is the first real-world study on drug-utilization of the combination of nebivolol and amlodipine. According to the Italian data, the patients prescribed with NA-EXC during the 9-year selection period were 12,401, corresponding to 7.5% of the total number of hypertensive adult patients with at least one prescription of nebivolol and/or amlodipine. In Germany, the data show 5,288 patients using NA-EXC, accounting for 3.3% of the total number of hypertensive adult patients prescribed nebivolol and/or amlodipine. Similar prescription rates of NA-EXC, were found by the analyses of the Italian, German, French and Polish LRx databases and Hungarian NHIF database, reinforcing the use of NA-EXC in Europe. The quantification of prevalent users of NA-EXC allowed us to estimate the number of hypertensive patients who may be eligible for a fixed-dose combination of nebivolol and amlodipine to 178,133 patients in Italy and 113,240 patients in Germany.
Incident users of NA-EXC were slightly females, and the predominant age group was that of 70–79 years. The demographic profile of patients starting NA-EXC was quite consistent across all databases analyzed, corroborating the robustness of our findings. Clinical characteristics extracted from the Italian LPD and German DA databases suggested a significant prevalence of overweight and obesity. Other CV risk factors, including diabetes and dyslipidemia, were also common. Given that conventional BBs are contraindicated in patients with metabolic disorders and taking into consideration the incident users of NA-EXC described in this study, it appears that the prescribing physicians were aware of the more favorable safety profile of nebivolol versus other members of the same drug classCitation19. Furthermore, the low percentage of patients among incident users of NA-EXC with atherosclerotic events suggests that nebivolol was given, not only to patients with heart disease, but also to patients without CV problems. This decision might be prompted by the BP-lowering properties of nebivololCitation19.
Overall, the clinical characteristics of the users of NA-EXC described here are similar to those reported in the literatureCitation33–36 and suggest that our cohorts were composed by patients receiving primary CV prevention, as well as patients with an established CV disease receiving secondary prevention. The high prevalence of older patients in the analyzed cohorts highlights that this population is a target of anti-hypertensive treatments in real-world. Effective interventions aiming to a target systolic blood pressure <130 mmHg in this population showed a reduced incidence of cardiovascular eventsCitation37. The NA-EXC is a potential treatment that may allow a target-achieved thus preventing CV risk.
Another relevant clinical characteristic of the patients starting treatment with NA-EXC was the elevated number of concomitant medications prescribed in the six-month period following the index date. Both in Italy and Germany, about 80% of patients had ≥ 2 concomitant prescriptions following the start of antihypertensive treatments with NA-EXC. The use of several pills might have complicated the treatment schedules and have induced a negative effect on the adherence to the prescribed antihypertensive drugs. Indeed, the analysis of adherence to the prescribed NA-EXC showed that only 16% of Italian patients and 35% of German patients had PDC values ≥80%. We are currently unable to explain the numerical differences in adherence between Italian and German patients. Notably, the low adherence rate to the combination reported in the present study is similar to that reported in previous real-world studies that compared patient adherence to extemporaneous versus SPC of the same antihypertensive agentsCitation33–36.
By simplifying treatment, the development of a SPC of nebivolol-amlodipine may reduce the number of pills and improve adherence, as demonstrated by several studies in hypertension and other chronic diseasesCitation38–41. This approach can also enhance the clinical benefits by optimizing BP controlCitation9. The National report on the use of medicines in Italy for the year 2021 shows that approximately 67% of the patients ≥65 years of age are prescribed with at least 5 different agents, highlighting the practical need of reducing the number of pills to be administeredCitation42. A SPC of nebivolol and amlodipine would likely simplify the therapeutic regimen for a relevant number of patients.
In the light of the multifactorial nature of hypertension, it is not surprising that monotherapy is often not effective in comparison to combination therapyCitation43. The fundamental principle in choosing which anti-hypertensive medications should be combined is that their mechanisms of action must be complementary. The combination of BBs and dihydropyridine CCBs meets this criterionCitation21. In this regard, it is worth mentioning the relevant proposed role of BBs in current guidelines due of their favorable effects in several clinical conditions including various cardiac diseases (related or not to hypertension), vascular conditions and non-cardiovascular diseasesCitation2. Among BBs, nebivolol offers some central hemodynamic effects that differ from non-vasodilating β1-blockers due to its unique mechanism of action that includes a vasodilatory mechanism. Moreover, it reduces oxidative stress and platelet volume and aggregation, leading to a decrease in heart rate, blood pressure, and vascular resistance, while increasing stroke volume and ejection fraction. These characteristics point to nebivolol having a potentially wide range of applications in the management of hypertension and chronic heart failure. In adults with mild to moderate hypertension, nebivolol demonstrated a similar effectiveness to ACEis, ARBs, and CCBs in reducing DBP and SBPCitation17.
Dihydropyridines CBBs such as amlodipine prevent vasoconstriction, and several studies have showed that while they may be less effective in preventing heart failure, they may be more beneficial in stroke preventionCitation44. When directly compared, nebivolol and amlodipine showed similar effectiveness in treatment of mild-to-moderate hypertension in elderly patients, highlighting the complementary mechanisms of action with potentially fewer side effects. This suggests that the nebivolol-amlodipine combination may offer improved tolerability compared to amlodipine alone, which is beneficial for older patientsCitation45.
Nebivolol in combination with amlodipine showed a significant reduction in central and peripheral BP with minor and tolerable side effects in a recent studyCitation46, proving to be an appropriate and effective treatment option for hypertensive patients that do not tolerate traditional BBsCitation47.
The clinical trial (BOTTICELLI study), evaluating the efficacy and tolerability of an extemporaneous combination of nebivolol 5 mg plus amlodipine 5/10 mg in hypertensive patients with uncontrolled BP after monotherapy, has been recently completed (Eudra-CT 2021-005077-10)Citation48. Previous research underlined that, considering the demonstrated efficacy and the favorable tolerability profile of nebivolol, is recommendable to explore the development of a new SPC of nebivolol with calcium antagonists to optimize the treatment of patients with hypertension who are on BBsCitation17,Citation47. Adherence to hypertensive treatment may depend on several factors including treatment acceptance by the patients, their understanding of their health situation and possible risks, collaborative relationships between patients and health professionals in the management of the condition. Indeed, an appropriate choice of antihypertensive agents and active involvement of the patient in the treatment of hypertension is extremely useful in achieving satisfactory blood pressure control in patientsCitation49. An empathetic approach which includes the explanation of the therapeutic choice, in addition to the availability of the appropriate SPC, may indeed improve treatment acceptance and outcomes.
Limitations
This study has different limitations that are typical of real-world evidence studies based on medical record and prescription databases. First, the estimates of the number of NA-EXC users were based on written prescriptions (Italian IQVIA LPD and German IQVIA DA) or on dispensed prescriptions (Italian IQVIA LRx, German IQVIA LRx, French IQVIA LRx, Hungarian NHIF and Polish IQVIA LRx). By assuming that any written or dispensed prescription was used by patients, we might have overestimated the adherence to treatment. Secondly, the Italian IQVIA LPD database does not include information on drugs prescribed by physicians working outside of the National Health System. However, as antihypertensive drugs are reimbursed by the National Health System when they are prescribed by general practitioners, we believe that the information required for an accurate estimate was generally available. Third, a direct comparison of patients initiating NA-EXC versus patients using the corresponding SPC was not possible, as this combination is not available. Additionally, the percentage of patients needing a cardiologic visit might have been underestimated, since visits performed outside of the National Health System are not registered in the databases analyzed. Finally, the European IQVIA LRx and Hungarian NHIF databases do not include information on diagnoses; it was therefore impossible to select patients based on a diagnosis of hypertension. However, the proportion of patients prescribed NA-EXC with no diagnosis of hypertension in the Italian IQVIA LPD and German IQVIA DA databases, resulted in the range of 9–20%. This suggests an estimation of the patients, from the European databases included in the present analysis, who were affected by hypertension. We believe that the size of the population potentially benefitting from a simplified treatment is substantial and warrants specific efforts to develop a SPC nebivolol-amlodipine.
Conclusions
The present study highlighted that the extemporaneous combination of nebivolol and amlodipine is commonly prescribed by European physicians in the current practice. Patients receiving this combination included in the evaluated cohorts often had multiple comorbidities and received concomitant medications. In addition, the analysis showed a proportion of patients with a low adherence. Combining nebivolol and amlodipine in an SPC offers a potential promising route to improve hypertension treatment options due to its clinical and pharmacological effects, ameliorated adherence, and increased potential patient eligibility.
Transparency
Author contributions
All authors take responsibility for the integrity of the work as a whole and have given final approval for the version to be submitted.
Supplementary material.docx
Download MS Word (20.2 KB)Acknowledgements
Editorial support, funded by Menarini, was provided by Lorenza Lanini on behalf of Health, Publishing and Services s.r.l., according to Good Publication Practice.
Declaration of financial/other relationships
R.C., V.P. have disclosed that they are employees of IQVIA. C.R. worked for IQVIA as an external consultant. G.D. reported personal fees from Menarini Corporate, Bayer, Servier and AlfaSigma. M.M., S.M., M.G. and P.F. has disclosed that he/she is currently an employee of Menarini Group and was employed at the time of study conduct. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Zhou B, Carrillo-Larco RM, Danaei G, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957–980.
- Mancia Chairperson G, Kreutz Co-Chair R, Brunstrom M, et al. 2023 ESH guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023;41:1874–2071.
- Vaduganathan M, Mensah GA, Turco JV, et al. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80(25):2361–2371. doi: 10.1016/j.jacc.2022.11.005.
- Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066.
- Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2.
- Tsioufis C, Thomopoulos C. Combination drug treatment in hypertension. Pharmacol Res. 2017;125(Pt B):266–271. doi: 10.1016/j.phrs.2017.09.011.
- Gradman AH, Basile JN, Carter BL, et al. Combination therapy in hypertension. J Clin Hypertens (Greenwich). 2011;13(3):146–154. doi: 10.1111/j.1751-7176.2010.00397.x.
- Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122(3):290–300. doi: 10.1016/j.amjmed.2008.09.038.
- Schutte AE, Jafar TH, Poulter NR, et al. Addressing global disparities in blood pressure control: perspectives of the international society of hypertension. Cardiovasc Res. 2023;119(2):381–409. doi: 10.1093/cvr/cvac130.
- Corrao G, Parodi A, Nicotra F, et al. Better compliance to antihypertensive medications reduces cardiovascular risk. J Hypertens. 2011;29(3):610–618. doi: 10.1097/HJH.0b013e328342ca97.
- Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012;125(9):882–887 e1. doi: 10.1016/j.amjmed.2011.12.013.
- Jung O, Gechter JL, Wunder C, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013;31(4):766–774. doi: 10.1097/HJH.0b013e32835e2286.
- Tomaszewski M, White C, Patel P, et al. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart. 2014;100(11):855–861. doi: 10.1136/heartjnl-2013-305063.
- Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26(3):215–225. doi: 10.1093/eurheartj/ehi115.
- Fongemie J, Felix-Getzik E. A review of nebivolol pharmacology and clinical evidence. Drugs. 2015;75(12):1349–1371. doi: 10.1007/s40265-015-0435-5.
- Mason RP, Giles TD, Sowers JR. Evolving mechanisms of action of beta blockers: focus on nebivolol. J Cardiovasc Pharmacol. 2009;54(2):123–128. doi: 10.1097/FJC.0b013e3181ad207b.
- Ferri C. The role of nebivolol in the management of hypertensive patients: from pharmacological profile to treatment guidelines. Future Cardiol. 2021;17(8):1421–1433. doi: 10.2217/fca-2021-0048.
- Fares H, DiNicolantonio JJ, O'Keefe JH, et al. Amlodipine in hypertension: a first-line agent with efficacy for improving blood pressure and patient outcomes. Open Heart. 2016;3(2):e000473. doi: 10.1136/openhrt-2016-000473.
- Cohen BJ. A fixed-dose combination of bisoprolol and amlodipine for hypertension: a potential benefit to selected patients. Clin Pharmacol Drug Dev. 2017;6(1):6–8. doi: 10.1002/cpdd.325.
- Sever PS, Messerli FH. Hypertension management 2011: optimal combination therapy. Eur Heart J. 2011;32(20):2499–2506. doi: 10.1093/eurheartj/ehr177.
- Volpe M, Pegoraro V, Peduto I, et al. Extemporaneous combination therapy with nebivolol/zofenopril in hypertensive patients: usage in Italy. Curr Med Res Opin. 2022;38(10):1673–1681. doi: 10.1080/03007995.2022.2096352.
- Volpe M, Pegoraro V, Heiman F, et al. Extemporaneous combination therapy with amlodipine/zofenopril in hypertensive patients: a real-world data analysis in Italy. Curr Med Res Opin. 2023;39(12):1593–1601. doi: 10.1080/03007995.2023.2192607.
- Bergmann KC, Skowasch D, Timmermann H, et al. Prevalence of patients with uncontrolled asthma Despite NVL/GINA step 4/5 treatment in Germany. J Asthma Allergy. 2022;15:897–906. doi: 10.2147/JAA.S365967.
- Joumaa H, Sigogne R, Maravic M, et al. Artificial intelligence to differentiate asthma from COPD in medico-administrative databases. BMC Pulm Med. 2022;22(1):357. doi: 10.1186/s12890-022-02144-2.
- Rathmann W, Czech M, Franek E, et al. Regional differences in insulin therapy regimens in five european countries. Int J Clin Pharmacol Ther. 2017;55(5):403–408. doi: 10.5414/CP202906.
- Bekele BB, Harsha N, Kőrösi L, et al. Is prescription nonredemption a source of poor health Among the roma? Cross-sectional analysis of drug consumption data From the national health insurance fund of Hungary. Front Pharmacol. 2021;12:616092. doi: 10.3389/fphar.2021.616092.
- IQVIA. Osservatorio sull’impatto della pandemia COVID-19 sull’accesso alle cure. Periodo dati: 2019-2020 2021. [cited 2022 24 October]. Available from: https://www.farmindustria.it/app/uploads/2021/03/Osservatorio-IQVIA-sullimpatto-pandemia-sullaccesso-alle-cure_Marzo-2021.pdf.
- SIIA. SIdIA. Ipertensione: i numeri in Italia 2023. [cited 2023 31 May]. Available from: https://siia.it/per-il-pubblico/ipertensione/ipertensione-i-numeri-in-italia/.
- ISTAT., Statistica INd. Popolazione residente al 1° gennaio 2023. 2023. [cited 2023 31 May]. Available from: http://dati.istat.it/Index.aspx?QueryId=42869.
- STATIS. DotFSOoGD. Popolazione residente al 1° gennaio 2023. 2023. [cited 2023 31 May]. Available from: https://www-genesis.destatis.de/genesis/online?operation=sprachwechsel&language=en.
- Levi M, Pasqua A, Cricelli I, et al. Patient adherence to olmesartan/amlodipine combinations: fixed Versus extemporaneous combinations. J Manag Care Spec Pharm. 2016;22(3):255–262. doi: 10.18553/jmcp.2016.22.3.255.
- Yang W, Chang J, Kahler KH, et al. Evaluation of compliance and health care utilization in patients treated with single pill vs. free combination antihypertensives. Curr Med Res Opin. 2010;26(9):2065–2076. doi: 10.1185/03007995.2010.494462.
- Mazzaglia G, Ambrosioni E, Alacqua M, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120(16):1598–1605. doi: 10.1161/CIRCULATIONAHA.108.830299.
- Del Pinto R, Desideri G, Ferri C, et al. Real-world antihypertensive treatment patterns, treatment adherence, and blood pressure control in the elderly: an Italian awareness-raising campaign on hypertension by senior Italia FederAnziani, the Italian Society of Hypertension and the Italian Federation of General Practitioners. High Blood Press Cardiovasc Prev. 2021;28(5):457–466. doi: 10.1007/s40292-021-00465-7.
- Deng Y, Bai J, Yang X, et al. Achieved systolic blood pressure and cardiovascular outcomes in 60-80-year-old patients: the strategy of blood pressure intervention in the elderly hypertensive patients (STEP) trial. Eur J Prev Cardiol. 2023;30(10):1017–1027. doi: 10.1093/eurjpc/zwad142.
- Conn VS, Ruppar TM, Chase JA, et al. Interventions to improve medication adherence in hypertensive patients: systematic review and meta-analysis. Curr Hypertens Rep. 2015;17(12):94. doi: 10.1007/s11906-015-0606-5.
- Rao S, Jamal Siddiqi T, Khan MS, et al. Association of polypill therapy with cardiovascular outcomes, mortality, and adherence: a systematic review and meta-analysis of randomized controlled trials. Prog Cardiovasc Dis. 2022;73:48–55. Jul- doi: 10.1016/j.pcad.2022.01.005.
- Yusuf S, Joseph P, Dans A, et al. Polypill with or without aspirin in persons without cardiovascular disease. N Engl J Med. 2021;384(3):216–228. doi: 10.1056/NEJMoa2028220.
- Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190. doi: 10.3390/pharmaceutics12020190.
- AIFA. National Report on Medicines Use in Italy. Year 2021. 2022 [cited 2023 31 May]. Available from: https://www.aifa.gov.it/documents/20142/1740782/Rapporto-OsMed-2021_EN.pdf.
- International Statistical Classification of Diseases and Related Health Problems 10th Revision. 2019. [cited 2023 18 July]. Available from: https://icd.who.int/browse10/2019/en.
- Gottwald-Hostalek U, Sun N, Barho C, et al. Management of hypertension with a fixed-dose (single-Pill) combination of bisoprolol and amlodipine. Clin Pharmacol Drug Dev. 2017;6(1):9–18. doi: 10.1002/cpdd.309.
- Mazza A, Gil-Extremera B, Maldonato A, et al. Nebivolol vs amlodipine as first-line treatment of essential arterial hypertension in the elderly. Blood Press. 2002;11(3):182–188. doi: 10.1080/080370502760050421.
- Sultan EM, Rabea H, Elberry AA, et al. Effect of amlodipine/nebivolol combination therapy on Central BP and PWV compared to amlodipine/valsartan combination therapy. Egypt Heart J. 2022;74(1):15. doi: 10.1186/s43044-022-00254-0.
- Cicero AFG, Kuwabara M, Borghi C. A critical review of nebivolol and its fixed-dose combinations in the treatment of hypertension. Drugs. 2018;78(17):1783–1790. doi: 10.1007/s40265-018-0999-y.
- EUDRACT. [cited 2023 18 July]. Available from: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2021-005077-10.
- Trimarco V, Izzo R, Mone P, et al. Therapeutic concordance improves blood pressure control in patients with resistant hypertension. Pharmacol Res. 2023;187:106557. doi: 10.1016/j.phrs.2022.106557.