Abstract
A strong hereditary influence on smoking has been demonstrated. As one of the candidate genes in relation to smoking, the serotonin transporter gene (5-HTTLPR) has been suggested, however with conflicting results. In recent studies, it has been shown that genotypic and environmental (G*E) factors interact in the shaping of a variety of phenotypic expressions. The objective of the present study was to investigate the interaction between a variation in the 5-HTTLPR and family environment in relation to smoking habits, nicotine dependence, and nicotine and cotinine levels in hair samples.
A random Swedish adolescent population sample (n = 785), from which 200 individuals were stratified regarding behaviour, was genotyped for 5-HTTLPR and assessed with semi-structured interviews, a questionnaire, and hair analyses of nicotine and cotinine.
The 5-HTTLPR gene interacted with a poor family environment to predict smoking habits, as well as nicotine and cotinine levels. The risk of being a smoker was increased 13 times for an individual with a combination of the 5-HTTLPR LS genotype and a poor family environment in comparison with the Homozygous Long-Long (LL) genotype and a good family environment.
Introduction
Tobacco consumption and smoking are the most important and preventable causes of mortality and morbidity in Western societies. Globally the tobacco consumption is rising because of increased consumption in many low-income countries Citation[1]. Smoking behaviour is influenced by both genetic and environmental factors Citation[2–5]. The genetic influence on smoking is reported to be approximately 60% (46%–84%) whereas about 20% of the variation comes from shared and 20% from individual environmental influences Citation[5], Citation[6]. However, among those who develop nicotine dependence, genetic factors appear to account for about 70% while shared environmental influences are close to negligible Citation[6].
Different polymorphisms have been identified in the promoter region of the serotonin transporter, one of which is an insertion/deletion polymorphism in the upstream regulatory region (5-HTTLPR) Citation[7]. It has been demonstrated that the long (L) and short (S) variants of this 5-HTT gene-linked polymorphic region (5-HTTLPR) have different transcriptional activities. Functionality studies of the 5-HTT gene transcription of 5-HTTLPR have shown that the short variant is associated with lower expression of 5-HTT and lower 5-HT reuptake activity in lymphoblasts and placental choriocarcinoma (JAR) cells Citation[8], Citation[9]. The 5-HTTLPR polymorphism has been related to smoking, with conflicting results, however. In two studies, the long variant was more frequent among smokers Citation[10], Citation[11]. Gerra et al. 2005 on the other hand, reported an over-representation of the short variant among adolescent smokers. Although some studies failed to find any association Citation[12], Citation[13], a meta-analysis found modest effects of 5-HTTLPR on smoking cessation Citation[14]. Additional links between the 5-HTTLPR gene and smoking can be hypothesized as adolescent smokers experience more severe withdrawal symptoms upon smoking cessation than do adults, even when daily smoking has occurred for only a short period or with low levels of consumption Citation[15], Citation[16]. Among adolescent rats in comparison with adults, withdrawal was accompanied by a significant and persistent loss of striatal 5-HT synaptic activity. In support of this there was an initial decline in turn-over followed by a reduction in 5-HT content without a compensatory increase in turn-over points, suggesting a potential for serotonin-specific reuptake inhibitors as alternatives to nicotine replacement therapy for smoking cessation in adolescents Citation[17]. Another link between the 5-HTTLPR gene and smoking could be that selective serotonin reuptake inhibitors like fluoxetine antagonize the ability of nicotine to evoke hippocampal noradrenaline release in vitro Citation[18].
During recent years, links between serotonergic function and environmental factors have been shown in several reports. Gene–environmental (G*E) models of interaction between environment and 5-HTTLPR for the risk of periods of depression Citation[19–21] and between environment and the MAO-A promoter polymorphism for the risk of antisocial behaviour Citation[22–25] have been reported. Furthermore, a G*E model of smoking initiation (serotonin receptor HTR6, lifetime traumatic experiences, and novelty-seeking), has been suggested Citation[26].
The objectives of the present study were to investigate 5-HTTLPR gene–environmental interactions in relation to smoking behaviour, nicotine dependence, and levels of nicotine and cotinine in hair samples. Our hypothesis was that 5-HTTLPR would interact with an unfavourable family environment to predict increased risk for smoking, nicotine dependence, and nicotine and cotinine levels in hair samples.
Material and methods
Subjects
All ninth-grade students in primary school (n = 2987, mean age 16.0 years) and third-grade students in secondary school (n = 2186, mean age 19.2 years) in Västmanland, a medium-sized county of Sweden, comprised the target population for the ‘Survey of Adolescent Life in Vestmanland (SALVe)’. In total, 2611 16-year-olds and 1649 19-year-olds, 87% and 75%, respectively, completed the questionnaire. Depending on risk behaviour reported in the questionnaire, all students were classified with a risk index (based on risk behaviours related to alcohol, narcotics, sex, property offences, and violent offences, please see Nilsson Citation[27] p. 64–65) and divided into four groups accordingly. Randomized samples of 400 students, matched for age, gender, and risk behaviour, were drawn from those students who accepted to take part in further investigations. A total of 81 boys and 119 girls gave blood and hair samples and took part in an interview. There were no differences between the group interviewed and those students responding to the initial questionnaire with regard to smoking habits (29% versus 33%, P = 0.300). Three years after the initial interview and the sampling of blood and hair, a follow-up questionnaire on smoking and smoking habits was sent to the participants; 65 boys and 112 girls completed the follow-up study and comprised our study group. The study design was approved by the human ethical committee at Uppsala University.
5-HTTLPR gene analysis
DNA was extracted from venous blood and analysed for 5-HTTLPR polymorphism. Polymerase Chain Reaction (PCR)-based genotyping was performed according to a modified protocol by Collier Citation[8]. In order to confirm that the correct regions of the 5-HTT gene were amplified, PCR products representing all genotypes were sequenced using BigDye® Terminator chemistry (Applied Biosystems) and analysed by an automated ABI PRISMTM (Perkin Elmer, Foster city, CA, USA). The DNA fragments were analysed using the SequencerTM 3.1.1 software.
Nicotine and cotinine hair analysis
Analysis of nicotine and cotinine in hair was performed as previously described Citation[28]. Briefly, approximately 20 mg of hair were incubated in 0.5 mL of methanol:acetonitrile:buffer (10:10:80) at 37°C for 18 hours. A 150-µL aliquot was transferred to an autosampler vial, 10 µL were injected into a liquid chromatography tandem mass spectrometry (LC-MS-MS) system, and the analytes detected in multiple reaction mode.
Interview structure design
Definitions of family functioning
The psycho-social variables were measured by the questions, ‘Could you describe your family?’, ‘What is good about your family, your mother/father and siblings?’, ‘What is not so good about your family?’, ‘What was it like in your family when you were seven, … thirteen?’, and ‘Have there ever been any tough or hard periods within your family?’ If the respondent described controversies within the family, these were followed up. The family relations were first coded on a five-point scale from very good (=1) to very poor (=5). The second stage of the coding process comprised the psycho-social variable ‘quality of relations within the family’ (1–2 = good, and 3–5 = poor). Inter-rater reliability (Cohen's κ) of family functioning, assessed by two independent raters who listened to the audiotaped interviews, was 0.7.
For a further description of participants, procedure, and coding, see Citation[27], Citation[29], Citation[30].
Smoking and nicotine tolerance/dependence
In the present study, smokers were defined as individuals who smoke daily or occasionally, whereas non-smokers were defined as individuals who do not smoke at all, i.e. had never started or had stopped smoking. A questionnaire on smoking and smoking habits including the modified version of the Fagerstrom Tolerance Questionnaire (FTQ) was used. The findings of the FTQ provide preliminary evidence that the modified FTQ scale is valid and applicable to adolescent smokers Citation[31], Citation[32]. The FTQ has been proved to be highly reliable Citation[32].
Statistical analysis
The Mann-Whitney and Kruskal-Wallis non-parametric tests were used to analyse differences in mean rank of the smokers versus non-smokers, nicotine and cotinine levels and gene, environment and gene–environment subgroups. To analyse the relationship between the results of the Fagerstrom Tolerance Questionnaire (FTQ), nicotine and cotinine, we used Spearman's r. The gene–environment interaction model of smoking, nicotine, and cotinine (below and above median) was analysed with logistic regression.
Results
Descriptive results
Among the participants, 80 (45%) were smokers (daily or occasional), of which 29 were boys and 51 were girls, with equal distribution of smokers in both sexes. However, 21 boys (32%) and 26 girls (23%) were occasional smokers. A total of 125 adolescents (71%) reported good quality of relations, comprising 45 (69%) boys and 80 (71%) girls. The frequencies of the different genotypes of the 5-HTTLPR gene were as follows: homozygosity for the short allele variant (SS) = 38 (21.5%), heterozygosity (LS) = 84 (47.5%), and homozygosity for the long variant (LL) = 55 (31%). Among the boys, the corresponding figures were SS = 13 (20%), LS = 27 (42%), and LL = 25 (38%); and among the girls SS = 25 (22%), LS = 57 (51%), and LL = 30 (27%). There were no differences according to the Hardy-Weinberg equilibrium (P = 0.451; boys, P = 0.407; girls, P = 0.477). The Fagerstrom Tolerance Index was significantly correlated to both nicotine (r = 0.511, P < 0.001) and cotinine levels (r = 0.363, P < 0.001) in hair samples. The relationship between nicotine and cotinine was r = 0.616, P < 0.001.
5-HTTLPR and family relations in association with smoking
The proportion of smokers was higher in adolescents from families with poor relations compared to those with good relations, 70% versus 35% (P < 0.001), and there was a significantly higher proportion of smokers among heterozygous 5-HTTLPR (LS) than homozygous individuals (SS = 25%, LS = 70%, and LL = 33%, P < 0.001). The same pattern was also found among boys and girls analysed separately.
Adolescents from families with poor relations scored higher in the Fagerstrom Tolerance Index (P < 0.001) and had higher nicotine and cotinine levels in hair samples (P = 0.003, and P = 0.008, respectively). 5-HTTLPR heterozygous individuals also scored higher in the Fagerstrom Tolerance Index (P < 0.001), but a similar association with nicotine and cotinine levels in hair samples was not found. However, when considering gene–environment subgroups there were differences in the Fagerstrom Tolerance Index (P < 0.001), nicotine levels (P = 0.033), and cotinine levels (P = 0.008).
The gene–environment interaction in relation to the Fagerstrom Tolerance Index is shown in . As a group, individuals with poor family relations showed higher tolerance scores, regardless of the 5-HTTLPR variant. When at environmental risk, 5-HTTLPR heterozygous individuals showed higher Fagerstrom Tolerance Indexes than both groups of homozygotes.
Figure 1. A line-chart of the relationship between the serotonin transporter gene and the quality of family relations in association with the Fagerstrom Nicotine Dependence Index. Homozygous for the long allele (LL), Heterozygous (LS), Homozygous for the short allele (SS).
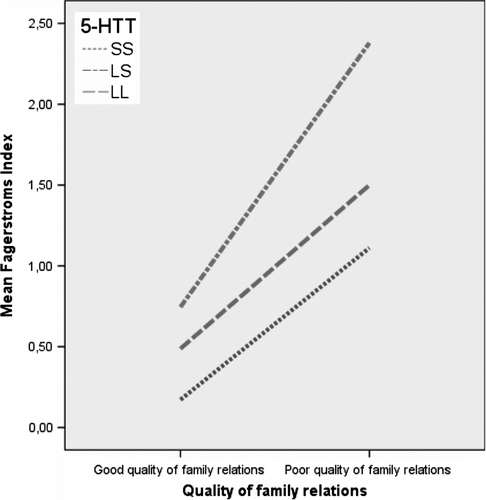
Using logistic regression (), both the quality of family relations and the 5-HTTLPR genotype were independently and significantly related to smoking in separate models (models 1 and 2), and adjusted for each other in the same model (model 3). A heterozygous individual had about three times elevated odds ratio (OR) of being a smoker. Poor family relations resulted in somewhat increased odds. However, individuals carrying the 5-HTTLPR LS variant and reporting poor family relations had elevated odds of 13 times (95% CI for OR, 4.9–42.8) for being a smoker compared to LL individuals with good family relations. Moreover, the explained variance of the G*E interaction was over 23%.
Table I. Logistic regression models of 1) 5-HTTLPR genotype, 2) quality of family relations, 3) model adjusted for 5-HTTLPR and quality of family relations, and 4) gene and environment interaction in relation to smoking among adolescents.
Likewise, there was a significant gene–environment interaction in relation to nicotine and cotinine levels (below versus above the median) tested with logistic regression (). The explained variance (Nagelkerke R2) using the nicotine and the cotinine levels model was 7% and 12%, respectively, with elevated odds (OR 3.2–4.3) comparable with that in the smoking model () for LS individuals from a family with poor relations. The gene–environment interaction in relation to cotinine levels in the analysed hair samples is illustrated in . It can be observed that the group of 5-HTTLPR SS from families with good quality of relations has a higher proportion with cotinine levels higher than the median compared to LL individuals with a similar family background (P = 0.07 in the regression model). However, among the 5-HTTLPR LL and LS individuals there was a markedly higher proportion with cotinine levels above the median, when poor family relations were considered (P = 0.03 and 0.009, respectively).
Figure 2. A line-chart of the relationship between the serotonin transporter gene and the quality of family relations in association with proportion of individuals with cotinine levels above the median in the total sample.
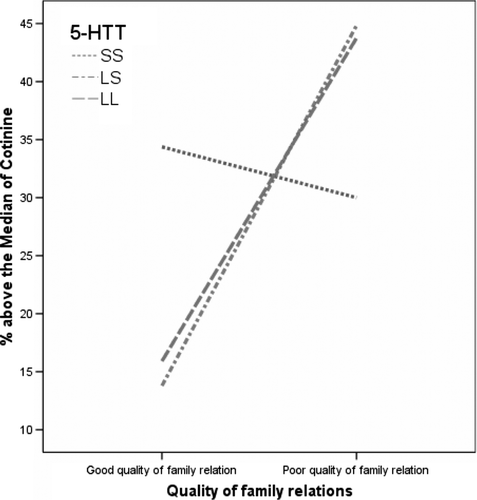
Table II. Logistic regression models of gene and environment interaction in relation to nicotine and cotinine among adolescents.
Discussion
The present study shows an association between 5-HTTLPR and smoking. This association was considerably amplified by interaction with the environment, where 5-HTTLPR heterozygous (LS) individuals with poor family relations had the highest risk of smoking with an OR of 13 compared to individuals homozygous for the long allele (LL) with good family relations. A similar, although with lower OR, gene–environment interaction pattern was found in relation to levels of nicotine and cotinine contents in hair samples. However, snuff is a commonly used tobacco product in Sweden. The nicotine and cotinine levels in the hair samples might have been influenced by snuff-taking. That could be a reason that lower OR was found for the gene–environment interaction in relation to levels of nicotine and cotinine contents in hair samples.
There was a relatively high proportion of smokers in our study (45%), which included daily and occasional smokers. The proportion of daily smokers (12% among boys and 22% among girls) did not, however, diverge from that expected according to previous findings in Swedish adolescent populations Citation[33].
In recent studies on humans, acute tryptophan depletion heightened the attentional salience of cigarette-related cues, perhaps by triggering reward and motivational mechanisms underlying nicotine dependence Citation[34]. The present study proposes a G*E model of smoking habits involving a functional variation of a gene (5-HT), which has direct implications on serotonergic neurotransmission Citation[35].
Studies on non-human primates with an orthologous variation in the 5-HTT promoter region (rh5-HTTLPR) have shown that childhood maternal deprivation interacts with the presence of the S allele of rh5-HTTLPR to predict an increased limbic-hypothalamic-pituitary-adrenal axis (HPA) activity Citation[36], Citation[37]. These animals also had lower levels of the serotonin metabolite 5HIAA in the cerebrospinal fluid (CSF), indicating a lower serotonin turn-over Citation[38]. Therefore, also humans with low serotonergic capacity might have higher arousal, which in an insecure family environment might predispose for nicotine use as a substance to control arousal.
Two explanations of gene–environment interaction are often proposed. The first explanation implies that internal and external stimuli, hormones, stress, learning, and social interaction alter the binding of the transcriptional regulators through epigenetic mechanisms Citation[39], Citation[40]. Recent studies have shown that 5-HTTLPR polymorphisms have significant effects on 5-HTT mRNA expression in lymphoblast cell lines and that the average methylation was higher among short alleles with trends for linearity with heterozygotes as an intermediate Citation[41], Citation[42]. These findings may indicate that basic gene effects on the transcriptional level of mRNA expression have to be reconsidered in favour of different environment-induced methylation processes according to individual genotypes.
The other explanation implies that the template function and the transcriptional functions are stable and that an individual's specific expression of, for example, serotonin pathways therefore is stable. Thus, a person with the short allelic variant should have a lower expression of 5-HTT and lower 5-HT reuptake activity Citation[8]. Such stable expression levels would then interact with the environment, and a person with a weak serotonergic system would be at a higher risk of negative experiences in a stressful environment.
Polymorphisms of the 5-HTTLPR gene have been associated with smoking in several studies, although with conflicting results. Such relationships have been found regarding the long variant Citation[10], Citation[11] and the short variant Citation[43]. In other cases, no relationship could be demonstrated Citation[12], Citation[13]. In the present study, 5-HTTLPR heterozygous individuals had an increased risk of smoking in comparison with either of the homozygous genotypes. Such a heterosis effect has been found in up to 50% of all gene association studies Citation[44–46]. At a molecular level, such a heterosis effect is not to be expected. At a molecular level, such a heterosis effect is not to be expected and different explanations for the phenomenon have been proposed. The first explanation is based on an inverted U-shaped response curve in which too little or too much gene expression is unfavourable Citation[46], Citation[47]. The second explanation suggests greater plasticity in heterozygous individuals, e.g. in their responses to stress, due to a broader range of gene expression when compared to individuals homozygous for either the short or the long allele Citation[46]. In the case of 5-HTTLPR, LS individuals have been shown to have fewer serotonin transporter binding sites in several mid-brain regions Citation[48], higher proportions of females with seasonal affective disorder and premenstrual dysphoric disorder Citation[49], elevated triglyceride and cholesterol levels, angina and heart disease Citation[50]. Therefore, a reverse heterosis functionality is plausible; the adolescent homozygous individuals are more ‘vulnerable’ and perhaps also more sensitive to environmental exposure. Consequently, LS individuals might be more susceptible to and more affected by artificial (nicotine) elevations of striatal 5-HT synaptic activity Citation[17].
The major limitation of the present study is the low number of subjects. It has often been proposed that a lack of significant results in genotype–phenotype studies is due to small sample sizes, and that small genetic effects need large sample sizes to be detected Citation[51]. Explanations of why some gene–environment studies of small samples show significant results, whereas other large studies fail to do so may be sample age and sex composition, and/or assessment methods of the environment and phenotypic expression Citation[52]. However, the present study demonstrates that statistically significant results on genetic contributions to smoking behaviour, measured by self-reports, can be observed if models are adjusted for environmental factors. Moreover, biological markers of smoking, such as nicotine and especially cotinine, are definitely more stable measures than self-reports of smoking and give solid additional support for the results of the present study.
Another important limitation of our study is that it relies on self-reports on family relations. Such reports regarding potentially ambiguous circumstances that may be affected by subjective retrospective interpretations and reconstructions, such as whether a person has experienced poor parenting, should be interpreted with caution Citation[53–55].
A third limitation is that our data are based on association, which means that conclusions regarding the directions of cause and effect must be considered tentative. Thus, even though we found that self-reported poor family relations may predict smoking behaviour, it may be objected that what we have actually measured might be a tendency of an individual to experience or report his/her family as more negative in terms of its relations. Such tendency might be that an adolescent who smokes has parents who strongly disapprove of the life-style and smoking habits of their offspring, and consequently quarrels or punishments make the adolescent report poor family relations.
The 5-HTTLPR gene has repeatedly been related to anxiety Citation[56] and to depression in G*E models Citation[19–21]. People with current or past depression are more likely to have been smokers at some point in their lives Citation[57]. This could be a major confounder in the present study. If there is a causal relation between 5-HTTLPR, poor family relations, and depression, then depression could act as a mediator in relation to smoking. However, the explained variance in G*E–depression studies seldom reaches above 5% Citation[19–21], whereas this study shows a substantially higher explained variance. One intriguing possibility could therefore be that the G*E interaction firstly shows relation to smoking, in which nicotine and/or other toxic compounds modulate the serotonergic synaptic activity in some individuals, leading to depression Citation[58].
The results of the present study suggest a heterosis effect of 5-HTTLPR in the same way as previously reported in relation to high alcohol consumption Citation[29]. However, both reports are based upon the same study population. Further G*E studies in relation to smoking, nicotine dependence, and nicotine and cotinine compounds in hair samples are therefore needed before any confident conclusions can be drawn.
Acknowledgements
Grants from the following funds and organizations are acknowledged: Vetenskapsrådet (VR) 4021, Systembolagets råd för alkoholforskning (SRA), Swedish Brain Foundation, AFA insurance, Fredrik and Ingrid Thurings foundation, the Regional Research Council of the Uppsala-Örebro region and the County Council of Västmanland. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Slama K. Global perspective on tobacco control. Part I. The global state of the tobacco epidemic. Int J Tuberc Lung Dis. 2008; 12: 3–7
- Arinami T, Ishiguro H, Onaivi ES. Polymorphisms in genes involved in neurotransmission in relation to smoking. Eur J Pharmacol. 2000; 410: 215–26
- True WR, Heath AC, Scherrer JF, Waterman B, Goldberg J, Lin N, et al. Genetic and environmental contributions to smoking. Addiction. 1997; 92: 1277–87
- Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking–-a study of male twins. N Engl J Med. 1992; 327: 829–33
- Batra V, Patkar AA, Berrettini WH, Weinstein SP, Leone FT. The genetic determinants of smoking. Chest. 2003; 123: 1730–9
- Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1 Suppl 2:S51–7; discussion S69–70.
- Heils A, Teufel A, Petri S, Seemann M, Bengel D, Balling U, et al. Functional promoter and polyadenylation site mapping of the human serotonin (5-HT) transporter gene. J Neural Transm Gen Sect. 1995; 102: 247–54
- Collier DA, Stober G, Li T, Heils A, Catalano M, Di Bella D, et al. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol Psychiatry. 1996; 1: 453–60
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996; 274: 1527–31
- Ishikawa H, Ohtsuki T, Ishiguro H, Yamakawa-Kobayashi K, Endo K, Lin YL, et al. Association between serotonin transporter gene polymorphism and smoking among Japanese males. Cancer Epidemiol Biomarkers Prev. 1999; 8: 831–3
- Kremer I, Bachner-Melman R, Reshef A, Broude L, Nemanov L, Gritsenko I, et al. Association of the serotonin transporter gene with smoking behavior. Am J Psychiatry. 2005; 162: 924–30
- Gerra G, Garofano L, Zaimovic A, Moi G, Branchi B, Bussandri M, et al. Association of the serotonin transporter promoter polymorphism with smoking behavior among adolescents. Am J Med Genet B Neuropsychiatr Genet. 2005; 135: 73–8
- Lerman C, Shields PG, Audrain J, Main D, Cobb B, Boyd NR, et al. The role of the serotonin transporter gene in cigarette smoking. Cancer Epidemiol Biomarkers Prev. 1998; 7: 253–5
- Trummer O, Koppel H, Wascher TC, Grunbacher G, Gutjahr M, Stanger O, et al. The serotonin transporter gene polymorphism is not associated with smoking behavior. Pharmacogenomics J. 2006; 6: 397–400
- Munafo MR, Clark T, Flint J. Does measurement instrument moderate the association between the serotonin transporter gene and anxiety-related personality traits? A meta-analysis. Mol Psychiatry. 2005; 10: 415–9
- DiFranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990; 51: 130–5
- O'Loughlin J, DiFranza J, Tyndale RF, Meshefedjian G, McMillan-Davey E, Clarke PB, et al. Nicotine-dependence symptoms are associated with smoking frequency in adolescents. Am J Prev Med. 2003; 25: 219–25
- Slotkin TA. Nicotine and the adolescent brain: insights from an animal model. Neurotoxicol Teratol. 2002; 24: 369–84
- Hennings EC, Kiss JP, Vizi ES. Nicotinic acetylcholine receptor antagonist effect of fluoxetine in rat hippocampal slices. Brain Res. 1997; 759: 292–4
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003; 301: 386–9
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004; 9: 908–15
- Sjoberg RL, Nilsson KW, Nordquist N, Ohrvik J, Leppert J, Lindstrom L, et al. Development of depression: sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. Int J Neuropsychopharmacol. 2006; 9: 443–9
- Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, et al. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Arch Gen Psychiatry. 2004; 61: 738–44
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002; 297: 851–4
- Nilsson KW, Sjoberg RL, Damberg M, Leppert J, Ohrvik J, Alm PO, et al. Role of monoamine oxidase A genotype and psychosocial factors in male adolescent criminal activity. Biol Psychiatry. 2006; 59: 121–7
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006; 11: 903–13
- Lerer E, Kanyas K, Karni O, Ebstein RP, Lerer B. Why do young women smoke? II. Role of traumatic life experience, psychological characteristics and serotonergic genes. Mol Psychiatry. 2006; 11: 771–81
- Nilsson KW. Gene-environment interaction in adolescent deviant behaviour. Uppsala University, Uppsala 2006
- Kronstrand R, Nystrom I, Strandberg J, Druid H. Screening for drugs of abuse in hair with ion spray LC-MS-MS. Forensic Sci Int. 2004; 145: 183–90
- Nilsson KW, Sjoberg RL, Damberg M, Alm PO, Ohrvik J, Leppert J, et al. Role of the serotonin transporter gene and family function in adolescent alcohol consumption. Alcohol Clin Exp Res. 2005; 29: 564–70
- Nilsson KW, Sjoberg RL, Wargelius HL, Leppert J, Lindstrom L, Oreland L. The monoamine oxidase A (MAO-A) gene, family function and maltreatment as predictors of destructive behaviour during male adolescent alcohol consumption. Addiction. 2007; 102: 389–98
- Prokhorov AV, De Moor C, Pallonen UE, Hudmon KS, Koehly L, Hu S. Validation of the modified Fagerstrom tolerance questionnaire with salivary cotinine among adolescents. Addict Behav. 2000; 25: 429–33
- Etter JF, Pelissolo A, Pomerleau C, De Saint-Hilaire Z. Associations between smoking and heritable temperament traits. Nicotine Tob Res. 2003; 5: 401–9
- Currie C, Hurrelmann K, Settertobulte W, Smith R, Todd J. Health and health behaviour among young people. WHO Policy Series: Health policy for children and adolescents Issue 1. International report. Copenhagen: World Health Organization; 2000.
- Hitsman B, Spring B, Pingitore R, Munafo M, Hedeker D. Effect of tryptophan depletion on the attentional salience of smoking cues. Psychopharmacology (Berl) 2007; 192: 317–24
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006; 59: 888–97
- Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, et al. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci U S A. 2004; 101: 12358–63
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004; 55: 733–8
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, et al. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002; 7: 118–22
- Kandel ER. A new intellectual framework for psychiatry. Am J Psychiatry. 1998; 155: 457–69
- Cloninger CR. Feeling good: the science of well-being. Oxford University Press, Inc, New York 2004
- Bradley SL, Dodelzon K, Sandhu HK, Philibert RA. Relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. Am J Med Genet B Neuropsychiatr Genet. 2005; 136: 58–61
- Philibert R, Madan A, Andersen A, Cadoret R, Packer H, Sandhu H. Serotonin transporter mRNA levels are associated with the methylation of an upstream CpG island. Am J Med Genet B Neuropsychiatr Genet. 2007; 144: 101–5
- Comings DE. Why different rules are required for polygenic inheritance: lessons from studies of the DRD2 gene. Alcohol. 1998; 16: 61–70
- Comings DE. Molecular heterosis as the explanation for the controversy about the effect of the DRD2 gene on dopamine D2 receptor density. Mol Psychiatry. 1999; 4: 213–5
- Comings DE, MacMurray JP. Molecular heterosis: a review. Mol Genet Metab. 2000; 71: 19–31
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999; 46: 1309–20
- Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, et al. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry. 1998; 155: 207–13
- Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, et al. Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry. 2002; 59: 613–20
- Comings DE, MacMurray JP, Gonzalez N, Ferry L, Peters WR. Association of the serotonin transporter gene with serum cholesterol levels and heart disease. Mol Genet Metab. 1999; 67: 248–53
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet. 2004; 127B: 85–9
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008; 13: 131–46
- Offer D, Kaiz M, Howard KI, Bennett ES. The altering of reported experiences. J Am Acad Child Adolesc Psychiatry. 2000; 39: 735–42
- Widom CS, Weiler BL, Cottler LB. Childhood victimization and drug abuse: a comparison of prospective and retrospective findings. J Consult Clin Psychol. 1999; 67: 867–80
- Sjoberg RL, Lindblad F. Limited disclosure of sexual abuse in children whose experiences were documented by videotape. Am J Psychiatry. 2002; 159: 312–4
- Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry. 2004; 9: 197–202
- Brody CL, Hamer DH, Haaga DA. Depression vulnerability, cigarette smoking, and the serotonin transporter gene. Addict Behav. 2005; 30: 557–66
- Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev. 2007; 17: 259–73
