Abstract
Background
While obesity is recognisably associated with changes in heart rate variability (HRV), the association between skeletal muscle mass and HRV is less clear.
Aims
In this cross sectional study, we analysed the association of body fat (four parameters) and muscle mass (five parameters) with indicators of HRV activity.
Subjects and methods
Assessment of body composition and HRV was performed in n = 180 young-to-middle age healthy men exposed to high occupational physical activity, using the multi-frequency bioelectrical impedance device and the PPG-StressFlow® HRV photoplethysmography device, respectively.
Results
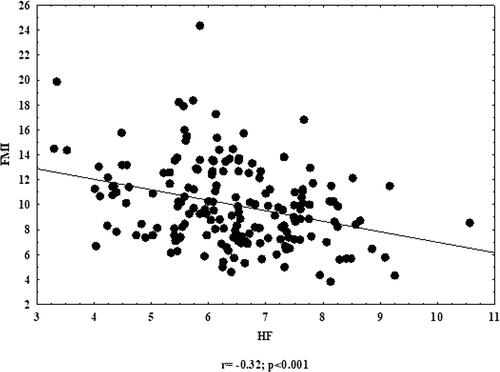
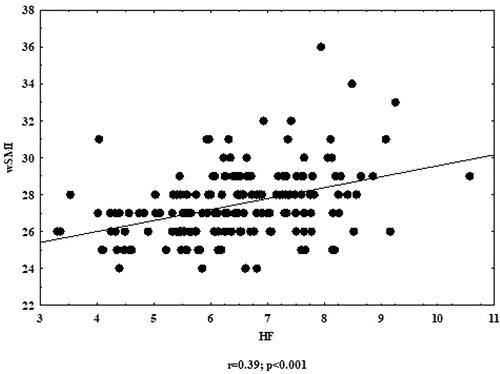
Mean values of parameters of fat tissue were above normal/reference values. Muscle tissue indicators were higher or within the reference ranges. Fat tissue parameters were significantly higher in participants with lower parasympathetic nervous system (PNS) indicators. Weight-adjusted skeletal muscle index (wSMI) was significantly lower in men with reduced PNS parameters. Fat tissue parameters were negatively correlated with PNS parameters, while wSMI was positively correlated with PNS parameters.
Conclusions
Participants with higher fat mass and lower muscle mass had poorer parasympathetic activity. Since mean values of HRV parameters indicated mild parasympathetic dominance, we conclude that physical activity and consequently good muscle mass potentially compensated for the negative interaction between fat tissue and HRV.
Introduction
The autonomic nervous system (ANS) regulates a number of physiological processes and its actions are largely involuntary. It is functionally divided into the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS), acting opposingly to complement each other (Svorc Citation2018). The SNS and PNS release neurotransmitters that bind to the appropriate receptors on the cells, resulting in different biological effects (McCorry Citation2007). Both SNS and PNS regulate heart rate variability (HRV – the variance in time between heart beats), sending opposing signals for faster or slower beats, respectively (Svorc Citation2018). Therefore, HRV is commonly used as an indicator of ANS activity with sympathetic and parasympathetic activity modifying the heart rate intervals at distinct frequencies and in opposing manners (Tokić Citation2016; Shaffer and Ginsberg Citation2017). The HRV refers to the heart’s capability to react to various physiological and environmental influences, with lower HRV generally indicating a poorer autonomic function and reduced capacity of the body to deal with different stressors (Tracey Citation2007; Shaffer and Ginsberg Citation2017; Kim et al. Citation2018).
Experimental and epidemiological studies have shown a correlation between ANS and body composition, as recently reviewed (Guarino et al. Citation2017; Ilich et al. Citation2020). Most authors agree that the overall ANS imbalance is connected to the changes in energy expenditure and consequently in body composition (Peterson et al. Citation1988; Chen et al. Citation2008; Windham et al. Citation2012). It has been proposed that SNS has a major influence on fat metabolism in a way that obesity could be associated with both elevated or reduced SNS activity (Davy and Orr Citation2009). Chronic sympathetic overactivity is known to be present in central obesity (Messina et al. Citation2013) and on the other side, the low activity to some regions (e.g. skeletal muscle and adipose tissue) may be a risk factor for obesity development (Davy and Orr Citation2009). This explains the mostly unfavourable association between obesity and HRV parameters (Windham et al. Citation2012).
While most of the research on HRV is focussed on its association with body fat, less is known about the ANS regulation of HRV regarding muscle tissue. The sympathetic effects at the muscle level mainly support muscle activity. At the onset of physical activity, activation of skeletal muscles inhibits cardiac parasympathetic activity which contributes to the increase in heart rate (Fisher Citation2014). However, frequent skeletal muscle activity and physical conditioning have beneficial effects on sympathovagal balance by increasing the parasympathetic component of heart rate variability (Khan and Sinoway Citation2000). Both body fat and muscles interact with and influence each other (Ilich et al. Citation2014), and each of them or in combination may be affected by the imbalance of the ANS. All this indicates that the balance between the SNS and the PNS is important for stable energy expenditure and a stable ratio of fat to muscle tissue.
As mentioned before, physical activity and regular training have been associated with increased parasympathetic activity and higher total HRV suggesting improved cardiac autonomic regulation (Daniela et al. Citation2022). Healthy people as well as cardiac patients showed increased high frequency (HF), as an indicator of parasympathetic dominance and decreased low frequency (LF) and LF/HF ratio, as indicators of sympathetic dominance, after training (Esco and Williford Citation2013; Besnier et al. Citation2017). Moreover, higher HRV was found to be related to greater physical activity (PA) levels in workers (Rennie et al. Citation2003), and lower work-related stress (Uusitalo et al. Citation2011).
Our objective was to analyse the association of each body composition component with the ANS activity using HRV as a proxy for ANS activities in a group of healthy, young-to-middle-age men who were engaged in high occupational physical activity. Specifically, we wanted to clarify the association of body fat and lean/muscle mass with cardiac autonomic control. We hypothesised that participants with higher muscle mass and lower fat mass will have higher parasympathetic activity. To optimise the biological homogeneity of the sample and counteract for possible confounders, we recruited only healthy, young-to-middle age men enrolled in a high level of occupational activity (namely, the logging industry). It is expected that in this population any possible disorders of the ANS could not be the consequence of some primary diseases. We also hypothesised that good muscle mass/strength, resulting from continuous physical efforts, can overcome possible negative effects of excess fat tissue affecting cardiac autonomic modulation, particularly low PNS activity.
Subjects and methods
Participants and data collection
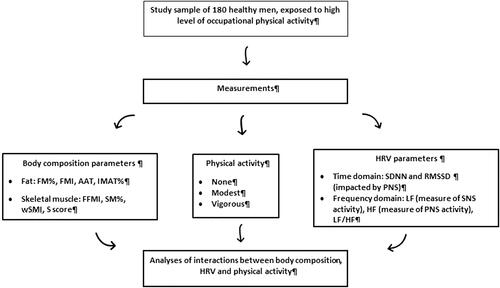
The study included 180 healthy, male workers employed in the logging industry exposed to high occupational physical activity. They had been referred to their annual medical examination and at that time recruited for body composition and ANS functions assessment by non-invasive bioimpedance measurements on a voluntary basis. The study took place between November 2019 and January 2020 during the participants’ medical exams. A total of n = 182 workers underwent a medical examination and only two of them did not agree to join the study. All participants were interviewed by the physician, and asked about their medical history, physical activity and the presence of medically unexplained symptoms (MUS), using the questionnaires integrated with the software of the bioimpedance device. The measurements were performed by a qualified technician. As per the inclusion criteria only young to middle-aged healthy men with an elevated level of physical activity were recruited. In order to warrant that condition, only men who were exposed to a high level of occupational physical effort and activity (in this case work in the logging industry) were recruited. The exclusion criteria were the presence of, and therapy for, chronic diseases (hypertension; diabetes; renal, liver or thyroid dysfunction; conditions related to chronic pain, like chronic muscle-skeletal diseases). Given that the requirement for employment and work in the logging industry is good health, no participant was excluded from the study due to the exclusion criteria.
The study was performed in accordance with the ethical standards laid down in the 1964 Helsinki declaration and its later amendments (World Medical Association Citation2001). The study was approved by the Ethics Committee of the Institute of Medical Research and Occupational Health (No.100-21/18-10). Signed informed consent was obtained from each participant.
Physical activity assessment
We used the Physical Activity Rating (PA-R) questionnaire tool (Jackson et al. Citation1990), which is incorporated in the Biotekna software, for assessing and categorising a person’s level of physical activity. The questionnaire evaluates the overall level of physical activity for the previous 6 months. It is divided into three parts (questions), which are additionally subdivided and scored as follows: 1. Does not participate regularly in programmed recreation, sport, or physical activity (scores 0 and 1); 2. Participates regularly in recreation or work requiring modest physical activity (scores 2 and 3); 3. Participates regularly in heavy physical exercise or engages in vigorous aerobic type activity (scores 4 to 7). A higher score means a higher level of physical activity.
Anthropometry and bioimpedance measurements
The measurements of body composition were performed using a multi-frequency bioelectrical impedance device BIA-ACC® (BioTekna®, Marcon-Venice, Italy). The BIA-ACC® uses algorithms to provide quantitative and qualitative assessments of body composition. Body mass index (BMI; kg/m2) was calculated from the measured weight and height. Other parameters of measurement used in this study were body fat and lean/muscle tissue. Body fat parameters were: fat mass (FM) expressed as % of total body weight (kg); abdominal adipose tissue (AAT) (cm2); intramuscular adipose tissue (IMAT) expressed as % of total body weight (kg). Lean/muscle tissue parameters were: fat-free mass (FFM) (kg); skeletal muscle (SM) mass expressed as % of fat-free mass (kg); S score (standard deviation of skeletal muscle mass with respect to healthy reference individuals between 25 and 30 years old); and skeletal muscle index (wSMI%) calculated as SM/body weight × 100. Additionally, we calculated fat mass index (FMI) (fat mass/height2) and fat-free mass index (FFMI) (fat-free mass/height2).
The measurements were performed with participants in a supine position with legs slightly spread and arms not touching the body. Two electrodes were placed on the right hand and two on the right foot. The first set of electrodes was placed in the area below the heads of the second and third metacarpal (metatarsal for foot) bone, on the dorsal side of the hand (foot), and the second set of electrodes was placed approximately 5 cm more proximal than the first set.
BIA-ACC® instrument was validated against DXA and the results have shown strong agreement with both adipose and lean tissue parameters (Peppa et al. Citation2017). Clinical validation of the instrument was also performed in large clinical studies (Tsigos et al. Citation2015; Straub et al. Citation2017). We performed additional validation against the OMRON scale (Omron BF511, OMRON Healthcare Europe B.V.). Validity was assessed using Pearson’s correlation coefficient (r) (FM%: r = 0.924; p < 0.001; AAT: r = 0.883; p < 0.001; SM%: r = 0.436; p < 0.001). Technical details about variable estimation by the instrument, including total body water, extracellular water, fat-free mass and skeletal muscle mass have already been reported (Tsigos et al. Citation2015).
Remaining variables are estimated on the basis of an algorithm developed by BioTekna srl. (Venice, Italy) (BioTekna algorithm (2011) Boschiero, D-R&D Group BioTekna Inc., Marcon, Venezia, Italy).
The PPG-StressFlow® HRV photoplethysmography device was used to determine the number of parameters to evaluate the physiological activity of the ANS and HRV and includes time-domain and frequency-domain indices. Time-domain indices of HRV quantify the amount of variability in measurements of the inter-beat interval and they include: standard deviation of the heart beat-to-beat interval (SDNN; signifying the overall health of ANS) and root mean square of the differences between adjacent heart beat intervals (RMSSD; expressing the vagal component of PNS). Frequency-domain measurements estimate the distribution of absolute or relative power into different frequency bands: high frequencies (HF) power (0.15–0.5 Hz) and low frequencies (LF) power (0.05–0.15 Hz). LF reflects the dominance of the SNS, HF reflects the dominance of the PNS, and the LF/HF ratio is considered to be an index of sympathetic-parasympathetic balance of cardiac work (Shaffer and Ginsberg Citation2017). LF, HF, SDDN and RMSSD are standard parameters for evaluating heart rate variability. These parameters are also commonly used in analysing associations of body composition or physical activities with HRV (ANS). They represent PNS activity (SDDN; RMSSD; HF), partial SNS activity (LF) and balance between SNS and PNS (LF/HF).
To obtain ANS tests, each participant was sitting in a relaxed position with two sensors attached to the tips of the second fingers of the hands. All measurements were performed in the morning and during the fasting condition.
The algorithms for HRV estimates using the PPG-StressFlow instrument were developed based on the previous literature (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Citation1996; Varon et al. Citation2012; Zong et al. Citation2003) and are the proprietary rights of BioTekna srl. Additionally, other studies have found that photoplethysmography (PPG-StressFlow) had sufficient accuracy for estimating HRV (Budidha and Kyriacou Citation2019; Kimmel et al. Citation2021).
Statistical analysis
The results are shown as mean ± standard deviation for continuous variables and as percentages for categorical variables. The distribution of variables was tested using the Kolmogorov-Smirnov test. Since most HRV variables were not normally distributed, the non-parametric functions were used in data analyses. The differences between two independent variables were tested using the Mann-Whitney test. The relationship between two variables was tested with Spearman’s correlation. Multiple regression was performed separately with each HRV variable (LF, HF, SDNN and RMSSD) as the dependent variable and with body composition variables as predictors and controlled by age. The calculations were performed with the Statistica, 13.0 (Dell Inc, Tulsa, OK) and JMP, Pro 14.0 (SAS Institute Inc., Cary, NC). The level of significance was set at p < 0.05.
A diagram of measurements in participants is shown in .
Results
A total of n = 180 men with mean age of 31.4 ± 6.7 years (range: 20-49 years) participated in the study. The whole study group was uniform according to race, ethnicity and education (of European descent, Croatian citizens, with finished secondary school). The mean duration of working in the logging industry was 5.6 ± 2.7 years. During their working hours they cut wood, carry and stack large pieces of trees. Participants reported no history of any serious chronic disease (cardiovascular, diabetes, cancer), the majority were smokers (70.5%) and almost all (98.3%) declared no presence of MUS. Out of 180 respondents, 176 (97.7%) categorised their level of physical activity as a score of 7 (“Runs more than 10 miles per week or spends more than 3 h per week in comparable physical activity”).
According to the BMI categories, 33.4% of participants were in the normal-weight category, 36.6% were overweight and 30.0% were with obesity. BMI ≥30 kg/m2 was categorised as obesity, 25≤ BMI <29.9 kg/m2 as overweight and 18≤ BMI <24.9 kg/m2 as normal weight ([WHO] World Health Organization Citation2010). However, based on the BIA analysis, almost all participants (93.3%) had high FM (>25%) and 91.7% of them had high FMI. Only a small number had low skeletal muscle mass (7.2%). Mean values for FM%, FMI, AAT and IMAT% were above normal/reference values (). Only 7 (3.8%) participants were within the recommended values for IMAT (<1.5). Almost half of the participants (47.2%) had high abdominal adipose tissue area (AAT). Mean FFM% and FFMI were below, while mean SM% was above, normal values. According to wSMI, 12 men (6.7%) were categorised as having mild sarcopenia or presarcopenia. Thirteen participants (7.2%) had an S score lower than −1. However, 20 men (11.1%) had high muscle mass (S score > 2.0 and/or wSMI > 34).
Table 1. Age and body composition parameters in participants (N = 180).
Mean values of HRV parameters were normal (), although the time domain parameters were above reference values, indicating mild parasympathetic dominance. Low HF (< 5) was found in 22 (12.2%) participants, while low LF was found only in 2 (1.1%) participants.
Table 2. Heart rate variability (HRV) assessment in participants (N = 180).
Separate analyses of body composition parameters were conducted in participants based on frequency and time domain HRV values ( and and ). FMI and AAT were significantly higher in participants with low HF compared to those with normal HF (p < 0.001). FFMI and wSMI were significantly higher in participants with normal HF compared to those with lower HF (p = 0.021 and p < 0.001, respectively). wSMI was also significantly higher in participants with normal LH compared to those with high LF (p < 0.001) (). Similar differences were found based on time domain values. Fat tissue parameters were high in participants with low SSDN and RMSSD. wSMI was significantly lower in men with lower SSDN and RMSSD ().
Figure 2. Difference in AAT between participants with normal and decreased HF, SDNN and RMSSD. AAT: abdominal adipose tissue; HF: high frequency; SDNN: standard deviation of the heart beat-to-beat interval record; RMSSD: root mean square of the differences between adjacent heartbeat intervals.
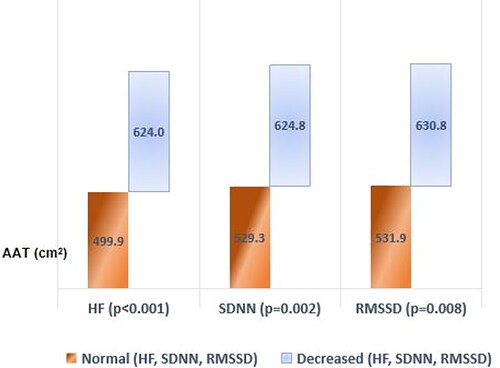
Table 3. Body composition parameters by HRV time domain parameters.
Table 4. Body composition parameters by HRV time-frequency parameters.
All fat tissue parameters (FM%, FMI, AAT and IMAT%) were significantly and mostly negatively correlated with all HRV parameters. When analysing fat-free parameters, significant positive correlation was found between wSMI and all HRV parameters ( and and ). Other body composition parameters were also negatively correlated with HRV; however, those correlations were not significant.
Table 5. Correlation coefficients (Spearman) between HRV and body composition parameters.
Multiple regression analysis was applied separately for each HRV parameter as the dependent variable, with all body composition variables as predictors. By controlling with age, the results showed significant positive association between wSMI and SDNN (p = 0.041) and between wSMI and HF (p = 0.038) (). Other HRV parameters were not significantly associated with any of the body composition parameters.
Table 6. Multiple regressions of HRV parameters as dependent variables and body composition parameters as predictors.
Discussion
Utilising bioimpedance technology, we determined the parameters of fat and muscle tissue and related them with the parameters of HRV. For each body composition component, we used four HRV parameters to assess their associations. Our results showed better HRV in subjects with normal body fat parameters (expressed as FMI, IMAT% and AAT) compared to those with high values of fat parameters. Fat tissue parameters were higher in participants in whom LF as an indicator of sympathetic modulation of ANS was higher, and in whom HF and time domain (SDNN, RMSSD) parameters were lower, implicating that abdominal obesity is pathophysiologically related to vagal withdrawal and sympathetic overactivity (Chen et al. Citation2008; El-Salamony et al. Citation2014). The results regarding the association of the sympathetic system with obesity are contradictory since some studies show higher activity (Hillebrand et al. Citation2014; Yadav et al. Citation2017; Soumya et al. Citation2022) and others lower (Millis et al. Citation2010, Windham et al. Citation2012). One postulate was due to the activity of leptin secreted by fat cells. Leptin is responsible for activating the neural pathways that increase SNS activity (Brydon et al. Citation2008). Other findings of a negative correlation between the percentage of body fat and sympathetic modulation suggest physiological adaptations that limit lipolysis and/or adipokinesis and favour greater adiposity (Millis et al. 2009). As Triggiani et al. (Citation2017) proposed, it can be assumed that the adaptive flexibility of autonomic cardiac function is generally reduced in individuals with obesity. This could explain conflicting results about the nature of autonomic activity in individuals with obesity.
Some studies have shown that central (abdominal) adiposity (not overall adiposity), accounts for obesity-related autonomic dysfunction (Poliakova et al. Citation2012; Windham et al. Citation2012), but we did not confirm those findings. In our study, total fat mass, along with FMI, and visceral and intramuscular fat, were negatively associated with HRV. Correlation analyses confirmed that lower levels of markers for adiposity and higher muscle mass predict higher levels of HRV in this population. The HRV parameters were also better in participants with higher muscle/lean mass compared to those with lower values, although only one parameter (wSMI) showed a strong association, indicating that the overall and central obesity more significantly impaired cardiac autonomic balance compared to lean tissue, although the latter two showed some benefits.
According to the BMI categories, the majority of our participants (>66%) were overweight or with obesity and based on BIA measurements, most (>90%) had higher than normal percentage of fat tissue and high FMI. This was unexpected considering their relatively younger age and high occupational physical activity; they were involved in heavy physical work on a daily basis, and they had a high muscle mass. Although it would be expected that muscular individuals with high BMI, such as athletes, have muscle weight higher than body fat weight, that was not confirmed in our participants. Their skeletal muscle tissue accounted for 27.5%, while the fat tissue accounted for 34.5% of the total body mass. Therefore, some other factors contributed to their overweight/obesity, possibly unhealthy nutritional and lifestyle habits.
Generally, during exercise, the SNS is activated in order to maintain adequate blood pressure and blood flow to the level required by the cardiac output (Bishop Citation2004; Charkoudian and Rabbitts Citation2009). However, regular physical activity may reduce sympathetic outflow (Charkoudian and Rabbitts 2019) and autonomic mechanisms like HRV can be improved, which was also reflected in our study participants presenting with higher mean values of SDNN and RMSSD. This suggests that the impact of physical activity on balancing ANS in our participants was potentially beneficial, offsetting any unfavourable effects of higher fat mass or obesity. Although occupational physical activity in some instances may exceed the physical capabilities of an individual and cause some unfavourable consequences on health (Dalene et al. Citation2021), our results are consistent with those showing health benefits for individuals engaging in high versus low occupational physical activities (Hu et al. Citation2005; Probert et al. Citation2008; Cillekens et al. Citation2020). Park et al. showed that moderate aerobic exercise can improve ANS function and prevent and treat obesity (Park et al. Citation2020), which suggested that physical activity significantly predicted greater indices of heart rate variability (May et al. Citation2017) and that regular physical exercise has strong beneficial effects on cardiac autonomic nervous function and thus appears to offset the negative effect of obesity on HRV (Felber Dietrich et al. Citation2008).
Limited data are available regarding the association of ANS and lean tissue. For example, there are only a few studies examining skeletal muscle mass (Millis et al. Citation2010; El-Salamony et al. Citation2014; Jasrotia et al. Citation2019) or fat-free mass (FFM) (Saecheea et al. Citation2019) reporting positive associations of SDNN and RMSSD as indicators of parasympathetic nervous activity with skeletal muscle mass. Those findings were corroborated in our study, but with a different indicator - wSMI, which is a diagnostic parameter for sarcopenia and reflects the skeletal muscle mass of the extremities adjusted for weight (Lim et al. Citation2010). Studies investigating the impact of muscle mass on ANS are usually aimed to analyse the association with exercise and physical activity, which presumably contributes to muscle mass gain (Esco and Williford Citation2013; Vaz et al. Citation2014; Besnier et al. Citation2017; Inthachai et al. Citation2020). Some of these studies were focussed on the physical exertion of lower limbs, which resulted in improvement of the vagal modulation of the heart rate (Heffernan et al. Citation2007), whereas whole-body training had no effect on HRV (Hu et al. Citation2005). That is consistent with our results, since wSMI is an indicator of muscle strength in extremities and was related to HRV. Although there are differences among authors regarding which HRV indices adequately present sympathetic and parasympathetic activity, there is a general opinion that physical activity has a beneficial effect on the stability of the autonomic system (Andrew et al. Citation2013; Chen et al. Citation2019). Therefore, as speculated, long-term physical activity in our participants might have potentially led to physiological adaptations, including higher parasympathetic activity and HRV.
There are some limitations to our study. It was of a cross-sectional design, thus the cause–effect relationship between HRV and body composition could not be established. Additionally, the participants were relatively young adult males, therefore, the findings could not be generalised to women and other age groups. Moreover, our participants were mostly healthy individuals, so our observations could not be generalised to individuals with poorer health. However, having a uniformed sample of biological, sex, and physical activity participants (all healthy males exposed to high occupational activity) makes it more applicable to this particular segment of the population, otherwise rarely investigated in clinical studies; they are assumed to be healthy and without problems. The results can serve as an example of the influence of occupational or any other regular physical activity in offsetting the influence of relatively unfavourable fat tissue parameters regarding ANS activities. Another limitation is a lack of data about participants’ socioeconomic status, an important predictor of lifestyle habits. However, all participants were employed in a state-owned company (Croatian Forest Industry) and their salaries were similar – which put them to the lower middle class. Additionally, all had finished middle school education. Another limitation is that HRV was not measured using an electrocardiogram or HR monitor.
We need to point out that the coefficients of correlation and regression in our study are low, despite their statistical significance. We believe that parameters which influence ANS in healthy people, like hormonal disbalance, stress, and lifestyle habits (not analysed in this study) are probably more influential in explaining HRV variability than body composition.
We used the bioimpedance technique for assessing body composition. While some other devices, e.g. DXA, MRI or CT, could provide more accurate results, they are typically more invasive, more expensive, or not available to many researchers. Moreover, the BIA-based instruments are non-invasive, portable, widely available, and many studies were performed utilising them.
Our results are consistent and in agreement with the general assumption of associations between body composition and HRV in which high-fat mass and low muscle mass are associated with lower PNS activity parameters. We also indicate that many authors have questioned the positive association of cardiac sympathetic activity with LF power, suggesting that the HRV power spectrum is mostly influenced by the parasympathetic system. Although many studies have used LF as a measure of both sympathetic and para-sympathetic activity (Millis et al. Citation2010; El-Salamony et al. Citation2014; Izumi et al. Citation2016; Jasrotia et al. Citation2019; Park et al. Citation2020), the clinical significance of connections between cardiac sympathetic activity and LF indices should be interpreted with caution. Continuing research by aiming for the validation of HRV indices will be necessary for the future.
Conclusions
This study was designed to analyse the association of ANS parameters with body fat and muscle tissue. Most of the participants were overweight and with obesity, but they were physically active and had good muscle mass parameters. Moreover, the mean values of parameters reflecting HRV were normal. Using different analyses and different parameters of both body composition and HRV, the results showed a significant association of high-fat mass, as well as reduced muscle mass with lower parasympathetic activity. Our study shows the importance of measuring HRV in younger healthy individuals and assessing the possible cardiometabolic risks, especially in those with altered body composition.
Disclosure statement
The authors declare that they have no conflict of interest.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Andrew ME, Shengqiao LI, Wactawski-Wende J, Dorn JP, Mnatsakanova A, Charles LE, Fekedulegn D, Miller DB, Violanti JM, Burchfiel CM, et al. 2013. Adiposity, muscle, and physical activity:predictors of perturbations in heart rate variability. Am J Hum Biol. 25(3):370–377.
- Besnier F, Labrunée M, Pathak A, Pavy-Le Traon A, Galès C, Sénard JM, Guiraud T. 2017. Exercise training-induced modification in autonomic nervous system: an update for cardiac patients. Ann Phys Rehabil Med. 60(1):27–35.
- Bishop VS. 2004. Exercise and the autonomic nervous system. In: Robertson D, Biaggioni I, Burnstock G, Low PA, editors. Primer on the autonomic nervous system. Academic Press; p. 183–184.
- Brydon L, O'Donnell K, Wright CE, Wawrzyniak AJ, Wardle J, Steptoe A. 2008. Circulating leptin and stress-induced cardiovascular activity in humans. Obesity (Silver Spring). 16(12):2642–2647.
- Budidha K, Kyriacou PA. 2019. Photoplethysmography for quantitative assessment of sympathetic nerve activity (SNA) during cold stress. Front Physiol. 9:1863.
- Charkoudian N, Rabbitts JA. 2009. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin Proc. 84(9):822–830.
- Chen GY, Hsiao TJ, Lo HM, Kuo CD. 2008. Abdominal obesity is associated with autonomic nervous derangement in healthy Asian obese subjects. Clin Nutr. 27(2):212–217.
- Chen LY, Zmora R, Duval S, Chow LS, Lloyd-Jones DM, Schreiner PJ. 2019. Cardiorespiratory fitness, adiposity, and heart rate variability: the coronary artery risk development in young adults study. Med Sci Sports Exerc. 51(3):509–514.
- Cillekens B, Lang M, van Mechelen W, Verhagen E, Huysmans MA, Holtermann A, van der Beek AJ, Coenen P. 2020. How does occupational physical activity influence health? An umbrella review of 23 health outcomes across 158 observational studies. Br J Sports Med. 54(24):1474–1481.
- Dalene KE, Tarp J, Selmer RM, Ariansen IKH, Nystad W, Coenen P, Anderssen SA, Steene-Johannessen J, Ekelund U. 2021. Occupational physical activity and longevity in working men and women in Norway: a prospective cohort study. Lancet Public Health. 6(6):e386–e395.
- Daniela M, Catalina L, Ilie O, Paula M, Daniel-Andrei I, Ioana B. 2022. Effects of exercise training on the autonomic nervous system with a focus on anti-inflammatory and antioxidants effects. Antioxidants. 11(2):350.
- Davy KP, Orr JS. 2009. Sympathetic nervous system behavior in human obesity. Neurosci Biobehav Rev. 33(2):116–124.
- El-Salamony GI, El-Agaty SM, Zawawi BM. 2014. The impact of body mass index and body composition on cardiac autonomic function in young adult Saudi females. J King Saud Univ Sci. 21(1):31–50.
- Esco MR, Williford HN. 2013. Relationship between post-exercise heart rate variability and skinfold thickness. Springerplus. 2(1):389.
- Felber Dietrich D, Ackermann-Liebrich U, Schindler C, Barthélémy J-C, Brändli O, Gold DR, Knöpfli B, Probst-Hensch NM, Roche F, Tschopp J-M, et al. 2008. Effect of physical activity on heart rate variability in normal weight, overweight and obese subjects: results from the SAPALDIA study. Eur J Appl Physiol. 104(3):557–565.
- Fisher JP. 2014. Autonomic control of the heart during exercise in humans: role of skeletal muscle afferents. Exp Physiol. 99(2):300–305.
- Guarino D, Nannipieri M, Iervasi G, Taddei S, Bruno RM. 2017. The role of the autonomic nervous system in the pathophysiology of obesity. Front Physiol. 8:665.
- Heffernan KS, Fahs CA, Shinsako KK, Jae SY, Fernhall B. 2007. Heart rate recovery and heart rate complexity following resistance exercise training and detraining in young men. Am J Physiol Heart Circ Physiol. 293(5):H3180–H3186.
- Hillebrand S, De Mutsert R, Christen T, Maan AC, Jukema JW, Lamb HJ, De Roos A, Rosendaal FR, Den Heijer M, Swenne CA. 2014. Body fat, especially visceral fat, is associated with electrocardiographic measures of sympathetic activation. Obesity. 22(6):1553–1559.
- Hu G, Sarti C, Jousilahti P, Silventoinen K, Barengo NC, Tuomilehto J. 2005. Leisure time, occupational, and commuting physical activity and the risk of stroke. Stroke. 36(9):1994–1999.
- Ilich JZ, Gilman JC, Cvijetic S, Boschiero D. 2020. Chronic stress contributes to osteosarcopenic adiposity via inflammation and immune modulation: the case for more precise nutritional investigation. Nutrients. 12(4):989.
- Ilich JZ, Kelly OJ, Inglis JE, Panton LB, Duque G, Ormsbee MJ. 2014. Interrelationship among muscle, fat, and bone: connecting the dots on cellular, hormonal, and whole body levels. Ageing Res Rev. 15:51–60.
- Inthachai T, Demeekul K, Sawatmongkul C, Somboonchai S, Pluksek A, Tunpha B. 2020. Effects of home-based breathing exercise on heart rate variability, cardio-ankle vascular index, respiratory muscle strength and maximum oxygen consumption in female participants with normal weight obesity. J Assoc Med Sci. 54:1–9.
- Izumi M, Manabe E, Uematsu S, Watanabe A, Moritani T. 2016. Changes in autonomic nervous system activity, body weight, and percentage fat mass in the first year postpartum and factors regulating the return to pre-pregnancy weight. J Physiol Anthropol. 35(1):26.
- Jackson AS, Blair SN, Mahar MT, Wier LT, Ross RM, Stuteville JE. 1990. Prediction of functional aerobic capacity without exercise testing. Med Sci Sports Exerc. 22(6):863–870.
- Jasrotia RB, Kanchan A, John NA, Verma MK, Gangwar V. 2019. A cross-sectional study of impact of body composition and anthropometry on heart rate variability in different age groups of adults. Natl J Physiol Pharm Pharmacol. 9(5):423–428.
- Khan M, Sinoway L. 2000. Muscle reflex control of sympathetic nerve activity failure: the role of exercise conditioning. Heart Fail Rev. 5(1):87–100.
- Kim HG, Cheon EJ, Bai DS, Lee YH, Koo BH. 2018. Stress and heart rate variability: a meta-analysis and review of the literature. Psychiatry Investig. 15(3):235–245.
- Kimmel MC, Fransson E, Cunningham JL, Brann E, Grewen K, Boschiero D, Chrousos GP, Meltzer-Brody S, Skalkidou A. 2021. Heart rate variability in late pregnancy: exploration of distinctive patterns in relation to maternal mental health. Transl Psychiatry. 11(1):286.
- Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, Kim KW, Lim JY, Park KS, Jang HC. 2010. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean longitudinal study on health and aging (KLoSHA). Diabetes Care. 33(7):1652–1654.
- May R, McBerty V, Zaky A, Gianotti M. 2017. Vigorous physical activity predicts higher heart rate variability among younger adults. J Physiol Anthropol. 36(1):24.
- McCorry LK. 2007. Physiology of the autonomic nervous system. Am J Pharm Educ. 71(4):78.
- Messina G, De Luca V, Viggiano A, Ascione A, Iannaccone T, Chieffi S, Monda M. 2013. Autonomic nervous system in the control of energy balance and body weight: personal contributions. Neurol Res Int. 2013(2013):1–5.
- Millis RM, Austin RA, Hatcher MD, Bond V, Faruque MU, Goring KL, Hickey BM, DeMeersman RE. 2010. Association of body fat percentage and heart rate variability measures of sympathovagal balance. Life Sci. 86(5–6):153–157.
- Park HY, Jung WS, Kim J, Hwang H, Lim K. 2020. Twelve weeks of aerobic exercise at the lactate threshold improves autonomic nervous system function, body composition, and aerobic performance in women with obesity. J Obes Metab Syndr. 29(1):67–75.
- Peppa M, Stefanaki C, Papaefstathiou A, Boschiero D, Dimitriadis G, Chrousos GP. 2017. Bioimpedance analysis vs. DEXA as a screening tool for osteosarcopenia in lean, overweight and obese Caucasian postmenopausal females. Hormones. 16(2):181–193.
- Peterson HR, Rothschild M, Weinberg CR, Fell RD, McLeish KR, Pfeifer MA. 1988. Body fat and the activity of the autonomic nervous system. N Engl J Med. 318(17):1077–1083.
- Poliakova N, Després JP, Bergeron J, Alméras N, Tremblay A, Poirier P. 2012. Influence of obesity indices, metabolic parameters and age on cardiac autonomic function in abdominally obese men. Metabolism. 61(9):1270–1279.
- Probert AW, Tremblay MS, Connor Gorber S. 2008. Desk potatoes: the importance of occupational physical activity on health. Can J Public Health. 99(4):311–318.
- Rennie KL, Hemingway H, Kumari M, Brunner E, Malik M, Marmot M. 2003. Effects of moderate and vigorous physical activity on heart rate variability in a British study of civil servants. Am J Epidemiol. 158(2):135–143.
- Saecheea J, Wannasamaia K, Jaimaneea P, Pinkeawb D, Chindac K, Inthachaia T. 2019. Effects of general obesity on heart rate variability in Thai people with physical inactivity. Chula Med J. 63(3):187–192.
- Shaffer F, Ginsberg JP. 2017. An overview of heart rate variability metrics and norms. Front Public Health. 5:258.
- Soumya BA, Lohitashwa R, Nadiger VM. 2022. Obesity and heart rate variability: a cross-sectional study in obese young adults. Indian J Health Sci Biomed Res. 15(1):34–37.
- Straub RH, Ehrenstein B, Günther F, Rauch L, Trendafilova N, Boschiero D, Grifka J, Fleck M. 2017. Increased extracellular water measured by bioimpedance and by increased serum levels of atrial natriuretic peptide in RA patients-signs of volume overload. Clin Rheumatol. 36(5):1041–1051.
- Svorc P. 2018. Autonomic nervous system. IntechOpen. http://doi.org/10.5772/intechopen.73119
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. 1996. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 93(5):1043–1065.
- Tokić A. 2016. The parameters of heart rate variability: indicators of the autonomic nervous system function. Med Jad. 46(3–4):73–84.
- Tracey KJ. 2007. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 117(2):289–296.
- Triggiani AI, Valenzano A, Ciliberti MAP, Moscatelli F, Villani S, Monda M, Messina G, Federici A, Babiloni C, Cibelli G. 2017. Heart rate variability is reduced in underweight and overweight healthy adult women. Clin Physiol Funct Imaging. 37(2):162–167.
- Tsigos C, Stefanaki C, Lambrou GI, Boschiero D, Chrousos GP. 2015. Stress and inflammatory biomarkers and symptoms are associated with bioimpedance measures. Eur J Clin Invest. 45(2):126–134.
- Uusitalo A, Mets T, Martinmäki K, Mauno S, Kinnunen U, Rusko H. 2011. Heart rate variability related to effort at work. Appl Ergon. 42(6):830–838.
- Varon C, Testelmans D, Buyse B, Suykens JA, Van Huffel S. 2012. Robust artefact detection in long-term ECG recordings based on autocorrelation function similarity and percentile analysis. Proceeding of the Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; p. 3151–3154.
- Vaz MS, Picanço LM, Del Vecchio FB. 2014. Effects of different training amplitudes on heart rate and heart rate variability in young rowers. J Strength Cond Res. 28(10):2967–2972.
- Windham BG, Fumagalli S, Ble A, Sollers JJ, Thayer JF, Najjar SS, Griswold ME, Ferrucci L. 2012. The relationship between heart rate variability and adiposity differs for central and overall adiposity. J Obes. 2012(2012):1–8.
- World Health Organization. 2010. Body Mass Index. A healthy lifestyle – WHO recommendations. https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle–-who-recommendations.
- World Medical Association. 2001. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 79(4):373–374.
- Yadav RL, Yadav PK, Yadav LK, Agrawal K, Sah SK, Islam MN. 2017. Association between obesity and heart rate variability indices: an intuition toward cardiac autonomic alteration – a risk of CVD. Diabetes Metab Syndr Obes. 10:57–64.
- Zong W, Heldt T, Moody GB, Mark RG. 2003. An open-source algorithm to detect onset of arterial blood pressure pulses. Comput Cardiol. 2003:259–262.