Abstract
The recurrent outbreaks of fatal Newcastle disease (ND) in commercial poultry flocks throughout the world indicate that routine vaccinations are failing to sufficiently induce the high levels of immunity necessary to control ND. There is a need for vaccination programmes that could be initiated at 1-day-old for mass application and which would induce a long-lasting immunity, with no need for a booster vaccination at a later age. In this context, the duration of immunity delivered by a vaccination programme including a recombinant herpesvirus of turkeys expressing the F gene of ND virus (rHVT-ND) and live ND vaccine at 1-day-old was compared with a classical programme that included a conventional live and an inactivated ND vaccine at the same age in commercial layer chickens. The humoral, cell-mediated and local immunity were followed weekly and birds were challenged with a viscerotropic velogenic ND virus strain at 6 and 10 weeks of age. We determined that immunity induced by the vaccination programme involving the rHVT-ND vaccine was more protective than that provided by the conventional vaccine-based regime. This might be related to a T-helper type 1 (Th1) cellular-driven immunological response, in contrast to the T-helper type 2 (Th2) humoral-oriented immune response provided by the current conventional vaccine-based vaccination programmes.
Introduction
Newcastle disease virus (NDV), also known as avian paramyxovirus type-1, is an economically important pathogen of poultry, which quickly assumes epizootic proportions if no strict and effective control measures are implemented. In addition to good biosecurity practices, control of Newcastle disease (ND) primarily consists of preventive vaccination of flocks and the culling of infected birds and birds at risk of being infected (protection zone). Vaccination programmes are tailored to suit the prevailing disease situation and take into account other factors such as maternal immunity, additional vaccination programmes, other pathogen circulation, types of flock, available labour, climatic conditions and inferred cost (Alexander & Senne, Citation2008). Nonetheless, the recurrent outbreaks of fatal ND in commercial poultry flocks in many parts of the world indicate that routine vaccinations are failing to induce the high levels of immunity required to control ND (Van Boven et al., Citation2008). Currently vaccines and vaccination strategies protect against morbidity and mortality but do not stop either viral infection or viral excretion. In addition, in laying hens and breeders, a booster vaccination with an inactivated vaccine is required at the start of lay, in order to induce a good protective immunity during the laying period.
A variety of approaches have been investigated in commercial chicks possessing maternally derived antibody (MDA) against NDV to develop a suitable vaccination programme that results in a long-lasting protection after a single vaccination at the hatchery. It has been reported that the combination of live and inactivated ND vaccines at 1-day-old induces a higher and longer-lasting humoral immunity when compared with inactivated vaccine administered alone (Bennejean et al., Citation1978; Giambrone & Clay, Citation1986; Folitse et al., Citation1998). This programme provides clinical protection against pneumotropic velogenic NDV (vNDV) isolated in past epizootics (Bennejean et al., Citation1978; Giambrone & Clay, Citation1986; Folitse et al., Citation1998; Chansiripornchai & Sasipreeyajan, Citation2006) and has become routinely used in countries where ND is a significant problem. In this context, a new ND vaccination regimen including the concomitant use of a recombinant herpesvirus of turkey expressing the F gene of NDV (rHVT-ND) and a live ND vaccine adjuvanted (Rauw et al., Citation2010a) or not (Palya et al., Citation2008; Rauw et al., Citation2010a) with chitosan at 1-day-old was investigated. These vaccination programmes were shown to improve protection and immunity against recent viscerotropic vNDV during the first 6 weeks of life in commercial chickens when compared with live vaccine administered alone.
The duration of immunity induced by the concurrent application of the aforementioned ND vaccines, adjuvanted or not with chitosan, was evaluated in the present study and compared with a classical vaccination programme, which included the combination of live and inactivated ND vaccine administration at 1-day-old. We present the results of challenge with a recent viscerotropic vNDV strain and investigations of the immune responses induced by these three vaccination schedules.
Materials and Methods
Chickens
Commercial Isa Brown layer chickens (sex sale linked) were hatched from eggs supplied by Het Anker B.V. (Ochten, Belgium). After hatching, all birds were kept in biosecurity level 3 isolators and animal experiments were conducted under the authorization and supervision of the Biosafety and Bioethics Committees of the Veterinary and Agrochemical Research Institute, following national and European regulations.
Vaccines, adjuvant and challenge strain
The live ND vaccine (Cevac Vitapest L) was provided by Ceva Santé Animale (Ceva-Phylaxia campus, Budapest, Hungary). This vaccine is based on the apathogenic enterotropic PHY.LMV.42 strain (Meszaros, Citation1991; Meszaros et al., Citation1992; Rauw et al., Citation2009b) (intracerebral pathogenicity index (ICPI) range between 0.00 and 0.16; intravenous pathogenicity index (IVPI) = 0.00; mean death time > 168), belonging to genotype I of NDV (Czegledi et al., Citation2006). The vaccine was reconstituted in phosphate-buffered saline (PBS) to one dose in 50 µl, which corresponds to approximately 106 egg infective dose that kills 50% of eggs (EID50)/dose, and was inoculated by the oculo-nasal route at 1-day-old.
The inactivated ND vaccine (Cevac Broiler ND K) was supplied by Ceva Santé Animale. This vaccine is a La Sota strain-based water-in-oil emulsion vaccine, and was inoculated subcutaneously in the neck at one dose per 100 µl at 1-day-old.
The cryopreserved cell-associated rHVT expressing the F protein of the avirulent D26 NDV strain (Sato et al., Citation1987) (rHVT-ND, Vectomune® ND) was produced by Ceva-Biomune (Lenexa, KS, USA). One dose of recombinant vaccine was diluted in 100 µl of the corresponding vaccine diluent (Ceva-Biomune) and inoculated subcutaneously in the neck at 1-day-old.
The chitosan (chitosan hydrochloride) adjuvant was provided by Ceva Santé Animale. Chitosan is a chloride salt of an unbranched binary heteropolysaccharide consisting of two N-acetyl-d-glucosamine and d-glucosamine units. Chitosan was dissolved in PBS at a final concentration of 0.5% (w/v) and used to reconstitute and dilute the live ND vaccine to the final concentration.
The viscerotropic Chimalhuacan vNDV strain belonging to class II genotype V of NDV (ICPI = 1.89) used for challenge was isolated in Mexico (Calderon et al., Citation2005). Oculo-nasal inoculation of 105 EID50 of this strain induces 100% mortality within 3 to 6 days in specific pathogen free (SPF) and commercial broiler chickens when challenged at 3 to 6 weeks of age (personal observations).
Mitogens and Newcastle disease virus antigens
The mitogens phorbol-12-myristate-13-acetate (PMA) and ionomycin (Iono) were purchased from Sigma (Diegem, Belgium). NDV recall antigens were prepared from the NDV La Sota strain as previously described (Lambrecht et al., Citation2004; Rauw et al., Citation2009b) and named inactivated NDV and all dissociated NDV proteins (prot-NDV).
Measurement of NDV-specific cell-mediated immunity in the spleen, the peripheral blood, and the digestive and respiratory tracts
The induction of NDV-specific cell-mediated immunity (CMI) was evaluated by the production of ChIFNγ after ex vivo antigen-activation of splenocytes, peripheral blood lymphocytes (PBL), lamina propria lymphocytes of the duodenum, tracheal lymphocytes and pulmonary lymphocytes as previously described (Rauw et al., Citation2009a, Citationb, Citation2011). Briefly, spleens were removed aseptically from chickens and heparinized blood samples were layered by sedimentation of red blood cells to isolate splenocytes and PBL, respectively (Rauw et al., Citation2009a, Citationb). Lymphocytes from the digestive tract (Rauw et al., Citation2009a), the trachea and the lung (Rauw et al., Citation2011) were isolated by enzymatic digestion. Immune cells were then activated by mitogens (PMA/Iono, 1 µg/ml), as a positive control of the ex vivo activability of lymphocytes, and by NDV recall antigens (prot-NDV, 1 µg/ml). ChIFNγ production was measured by capture enzyme-linked immunosorbent assay (ELISA). Cellular immune responses were expressed as stimulation indices (S.I.). These were calculated for each bird by dividing the optical density values of mitogen-activated and antigen-activated lymphocytes by the optical density of non-activated lymphocytes (Rauw et al., Citation2009b), and the S.I. per group were calculated.
Measurement of the NDV-specific humoral and local antibody-mediated immunity
NDV-specific humoral immunity was evaluated by the haemagglutination inhibition (HI) test and NDV-specific IgG, IgM and IgA ELISA. HI tests and NDV-specific IgG ELISA were performed as previously described (Rauw et al., Citation2009b). For detection of NDV-specific IgM and IgA, MaxiSorp Nunc-Immuno F96 microwell plates (International Medical, Watermaal, Belgium) were coated overnight at 4°C with mouse antibody directed against chicken IgM or IgA (SouthernBiotech, Brussels, Belgium), respectively, diluted at 5 µg/ml in carbonate/bicarbonate pH 9.6 buffer. The following day, plates were washed three times with PBS supplemented with 0.1% Tween 80. Plates were blocked for 30 min at 37°C with PBS containing 3% bovine serum albumin (BSA) and then incubated with diluted samples, as specified afterwards, in PBS containing 0.1% Tween 80, 5% NaCl and 4% BSA for 1 h at room temperature. Inactivated NDV diluted at 1 µg/ml and biotin-labelled mouse antibody 4D6 directed against HN protein (Mast et al., Citation2006) were then added for 1 h at room temperature. Plates were incubated with streptavidin–horseradish peroxidase conjugate (Biosource Europe, Nivelles, Belgium) for 1 h at room temperature. After six washings, peroxidase activity was revealed by adding 100 µl tetra-methyl benzidine peroxidase substrate (Thermo Fisher Scientific, Erembodegem, Belgium) for 15 min in darkness, before stopping the reaction with 1 M H3PO4 buffer. Optical density was determined at 450 to 560 nm with an ELISA reader.
Local antibody-mediated immunity to NDV was measured at preferential replication sites of the vaccine by NDV-specific IgG, IgM and IgA ELISA on lung washings and supernatant of ex vivo duodenum, and trachea tissue culture. The protocols for collection of lung washings and ex vivo duodenal tissue culture were described previously (Rauw et al., Citation2009b). The culture of tracheal tissue was based on paper by Zoth et al. (Citation2008) with minor modifications. After collection of trachea, connective tissue, blood and fat in serosa were removed in PBS with 5% (w/v) gentamicin and most of the mucus was discarded by gently rubbing in one direction over the outside of the tubular part of the trachea. Tracheal tissue was then opened with scissors and cut into 1 cm length slices before washing. During this washing procedure, the tissue strips were pelleted by centrifugation for 5 min at 300 × g. Subsequently the tissue was re-suspended in 5 ml RPMI 1640 medium containing 10% FCSi. After incubation at 39°C for 48 h, the supernatant was collected by centrifugation and frozen at –20°C until the time of assay.
Measurement of virus shedding via oropharyngeal and cloacal routes after challenge
The quantification of Chimalhuacan NDV challenge virus in oropharyngeal and cloacal swabs was performed by quantitative real-time reverse transcription-polymerase chain reaction (QRRT-PCR) targeting the matrix (M) gene, as previously described (Rauw et al., Citation2010a). The sensitivity threshold of this NDV QRRT-PCR (R 2 = 0.998, efficiency = 94.17%) was determined at 1 EID50 per reaction (102.7 EID50/ml swabs), based on standard curve data. For statistical analysis, undetermined samples were considered negative and received a value of 10 EID50/ml swabs. Results were expressed as the titre of challenge strain per millilitre of swabs (log10).
In addition, quality of the sample and RNA extraction procedure were validated using avian β-actin as described previously (Van Borm et al., Citation2007).
Experimental design
Commercial layer chickens were assigned into four groups and vaccinated or not at 1-day-old. The first group was vaccinated with the rHVT-ND and live ND vaccines, while in the second group the live ND vaccine was co-administrated with chitosan. These were designated as the “rHVT-ND/live ND” and “rHVT-ND/live ND-Chitosan” groups, respectively. The third group was vaccinated with both live and inactivated ND vaccines, and designated the “inact ND/live-ND” group. The last group was left untreated and served as unvaccinated negative controls. At 1-day-old, the serum of 10 unvaccinated birds was sampled to determine the MDA level. Serum, blood, spleen, trachea, lung and duodenum samples were collected (n = 5) at 3, 4, 5, 6, 8, 10 and 12 weeks post vaccination. At 6 and 10 weeks post vaccination, 10 chickens from each group were individually tagged and challenged with 105 EID50/200 µl Chimalhuacan NDV strain by the oculo-nasal route. After challenge, chickens were monitored daily for clinical signs (swelling of the head, depression, prostration and nervous signs) and mortality over a 2-week period. Birds that showed the clinical signs typical of ND or died were considered unprotected. Oropharyngeal and cloacal swabs were taken at 2, 4, 7 and 10 days post infection (d.p.i.).
Statistical analysis
Statistical analyses of data were performed using Minitab 13 (Minitab Ltd, Coventry, UK) and STATA 10 (Stata Corp LP, Texas, USA) software (statistical programmes for Windows 2000) and differences were considered significant at P < 0.05. The analyses of the ChIFNγ production, the antibody level and the titre of viral excretion were carried out to compare the groups by one-way analysis of variance and Turkey's pair-wise comparison tests or by the non-parametric Kruskal–Wallis test, as previously described (Rauw et al., Citation2009b). The qualitative criteria “positive QRRT-PCR reaction” and “positive cell activation” were analysed by Fisher's exact test, using the Bonferroni method to adjust the risk α (Rauw et al., Citation2009b).
Results
Vaccination schedules including the rHVT-ND vaccine provided a greater and longer protection against clinical signs, mortality and virus shedding after NDV challenge
After challenge with the viscerotropic Chimalhuacan vNDV strain at 6 and 10 weeks of age, unvaccinated chickens showed typical clinical signs of ND, including swelling of the head, depression, prostration and nervous signs, from 3 d.p.i. Birds started dying on 5 d.p.i. and all birds were found dead by 6 d.p.i., which validated the challenge. Neither clinical signs nor mortality were observed among the rHVT-ND/live ND and the rHVT-ND/live ND-Chitosan vaccinated groups following either of the two challenge dates (). The chickens vaccinated with the inact ND/live ND combination were also fully protected at 6 weeks of age. At 10 weeks of age, protection against clinical signs and mortality was reduced to 70% and 90%, respectively, but the difference was not statistically significant.
Table 1. Protection against morbidity and mortality after challenge with the Chimalhuacan vNDV strain of commercial layer chickens vaccinated at 1-day-old with the live ND and the rHVT-ND or the inact ND vaccines, according to different vaccination regimens.
After challenge at 6 weeks, each of the three vaccination schedules significantly reduced challenge virus shedding at 4 d.p.i. by the oropharyngeal route (). Excretion stopped at 10 d.p.i. in both groups receiving the vaccination schedules which included the rHVT-ND vaccine. Similarly, the cloacal excretion was significantly reduced at 4 d.p.i. in each of the three investigated vaccination schedules and fewer than 40% of birds were found positive. At this age of challenge, there was no difference in virus shedding between the three vaccination programmes. Following challenge carried out at 10 weeks of age, each of the three investigated vaccination schedules significantly reduced challenge virus shedding at 4 d.p.i. by the oropharyngeal route. Interestingly, this reduction was significantly stronger at 4 and 7 d.p.i. in the rHVT-ND/live ND and rHVT-ND/live ND-Chitosan groups in comparison with the inact ND/live ND group. Moreover, both vaccination programmes including the rHVT-ND vaccine fully protected against cloacal excretion, while excreting chickens were observed at 4, 7 and 10 d.p.i. in the inact ND/live ND group.
Table 2. Shedding after challenge at 6 and 10 weeks of age with the Chimalhuacan vNDV strain on commercial layer chickens vaccinated at 1-day-old with the live ND and rHVT-ND or the inact ND vaccines according to different vaccination regimens.
The rHVT-ND/live ND vaccination schedule maintained a measurable peripheral CMI during the 12-week period of observation, as well as a greater and longer CMI in the digestive and respiratory tracts
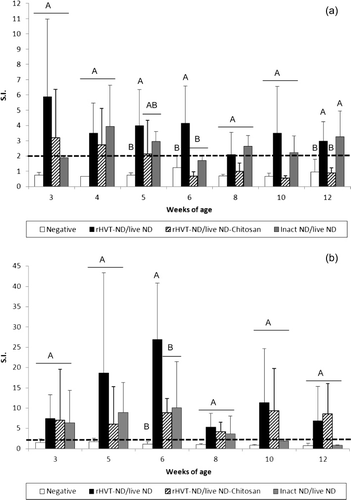
NDV-specific peripheral CMI remained above the threshold of positivity during the whole 12-week observation period in the rHVT-ND/live ND group (), and was significantly different from the unvaccinated group at weeks 5, 6 and 12. This CMI was positive only at 4, 5, 8, 10 and 12 weeks of age in the inact ND/live ND group, with significant difference from unvaccinated group arising during the last week of observation. The peripheral cellular immune response in the rHVT-ND/live ND-Chitosan group was detected only during the first 5 weeks of age.
Figure 1. NDV-specific cell-mediated immunity in the peripheral blood (1a), in the digestive tract (1b), in the lung (1c) and in the spleen (1d) of commercial layer chickens vaccinated at 1-day-old with the live ND and the rHVT-ND or inact ND vaccines, according to different vaccination regimens. Lymphocytes were stimulated with prot-NDV recall antigen (1 µg/ml), and supernatants of stimulated cells were harvested after 72 h of activation. ChIFNγ production was determined by the ChIFNγ capture ELISA. The results correspond to the mean ± standard deviation of stimulation indices at each time point (n = 5). Mean ± standard deviation at time points with no common uppercase letters differ significantly (P < 0.05). S.I., stimulation indices.All three vaccination schedules induced NDV-specific CMI in the digestive tract from the third week (). The duodenal CMI in the rHVT-ND/live ND group increased rapidly during the first 6 weeks to reach a peak level that was significantly higher at 6 weeks of age, compared with the other vaccinated and unvaccinated groups. Both vaccination programmes including the rHVT-ND vaccine were able to maintain a digestive antigen-specific cellular immunity during the 12 weeks of observation, while the digestive CMI in the inact ND/live ND group had waned after 8 weeks of age.
The rHVT-ND/live ND combination induced NDV-specific CMI in lung from 6 to 10 weeks of age and the rHVT-ND/live ND-Chitosan vaccination regimen did from 8 to 10 weeks of age (). Indeed, although difference was not significant when compared with the unvaccinated group, positive CMI was detected in the lung of 40 to 80% of vaccinated chickens. The inact ND/live ND group remained negative during the whole experiment. No NDV-specific CMI could be observed in the trachea during the 12-week period of this experiment (data not shown).
Large standard deviations and variations between experimental days were observed for CMI responses. These observations could be explained by biological variations between birds, especially in conventional animals, as observed after mitogenic activation of lymphocytes (data not shown). Experimental design with more than five chickens per group at each experimental day could probably reduce the standard deviation but would be difficult to manage with four groups to evaluate on the same day.
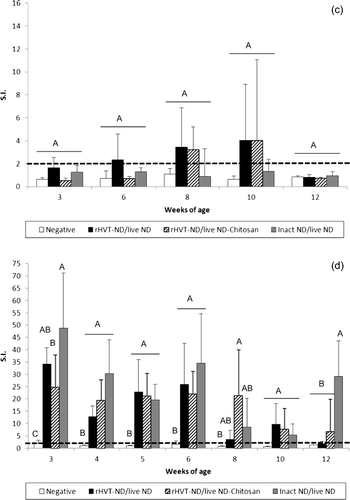
Each vaccination schedule induced a high CMI in the spleen during the first 6 weeks of age
The NDV-specific CMI in the spleen was significantly higher from the third to the sixth week of age in the three vaccinated groups when compared with unvaccinated birds (). The splenic CMI induced by the vaccination regimen including the inact ND vaccine tended to be the highest one at weeks 3, 4, 6 and 12. This superiority was significant at week 3 in comparison with the rHVT-ND/live ND-Chitosan group, and at week 12 in comparison with both vaccinated groups including the rHVT-ND vaccine. At 8 weeks of age, the NDV-specific CMI in the spleen from the rHVT-ND/live ND-Chitosan group remained at a constant and significantly higher level, when compared with the unvaccinated group, while it decreased in the two other vaccinated groups.
The inact ND/live ND vaccination regimen may induce a higher systemic antibody-mediated immunity
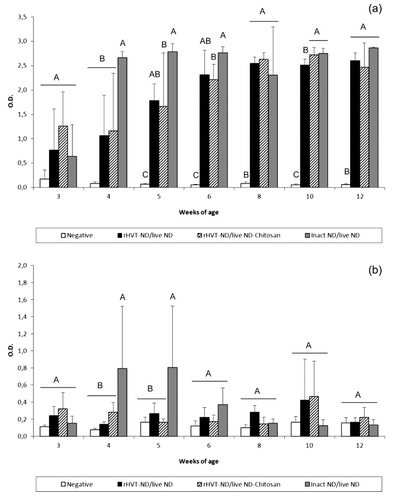
The mean HI antibody titre at 1 day old was 9.2 ± 1.2 log2 (data not shown). Subsequently, HI titres indicated a decline of passive, maternally-derived, immunity until the fourth week of age (). An active primary immune response was detected by HI tests in the three vaccinated groups starting at 3 weeks of age and was significantly higher than the unvaccinated group from the fourth week of age. The HI titres in the inact ND/live ND vaccinated chickens were significantly higher at week 4 than groups that received the vaccination schedules including the rHVT-ND vaccine. This superiority was maintained at weeks 6, 8 and 10, although not significantly. A similar tendency was observed by NDV-specific IgG ELISA during the whole experiment (data not shown). NDV-specific IgM was detected in the inact ND/live ND vaccinated chickens between 3 and 5 weeks of age and the level was significantly higher than in the unvaccinated group (). IgM appeared later, between the age of 5 and 8 weeks, in the rHVT-ND/live ND group with a significant difference with the unvaccinated group at 5 and 6 weeks of age. IgM was detected only at 5 weeks in the rHVT-ND/live ND-Chitosan group. No NDV-specific IgA was detected in the sera from any of the vaccinated groups throughout the duration of this experiment (data not shown).
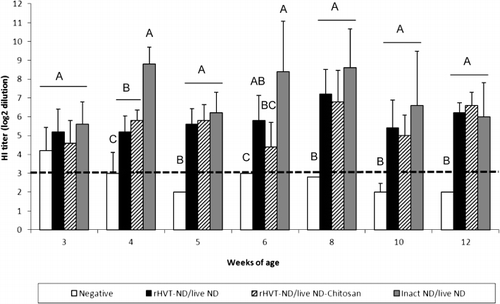
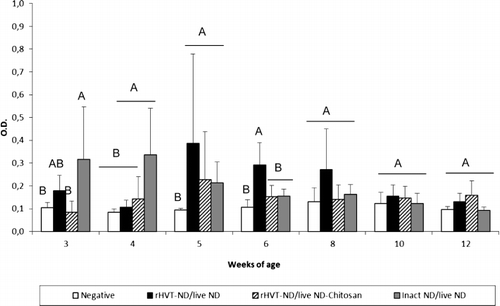
The inact ND/live ND vaccination regimen may induce a higher local antibody-mediated immunity
The three vaccination regimens induced IgG in the digestive tract from 3 weeks of age (). When compared with the unvaccinated group, the level of this antibody-mediated immunity was significantly higher from the fourth week in the inact ND/live ND group and 1 week later in the rHVT-ND/live ND and rHVT-ND/live ND-Chitosan groups. The inact ND/live ND vaccination regimen tended to induce higher IgG levels in the duodenum until 6 weeks of age, but the difference was only significant at 4 weeks of age, compared with the two other vaccination schedules. NDV-specific IgA and IgM were detected in the digestive tract of 40% of inact ND/live ND vaccinated birds at 5 weeks of age, while the two other vaccinated groups that included the rHVT-ND vaccine remained negative (data not shown).
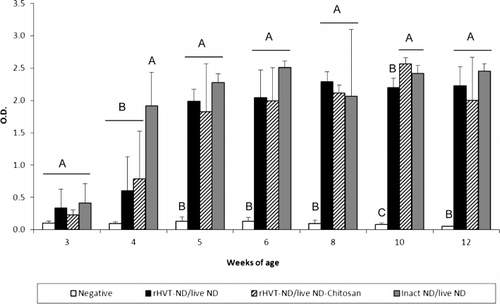
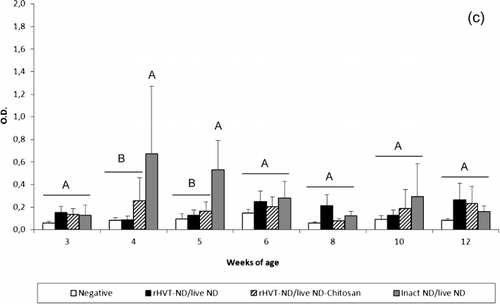
All vaccination schedules induced secretion of IgG in the trachea from 3 weeks of age (). When compared with the unvaccinated group, the NDV-specific IgG level was significantly higher from 4 weeks of age in the inact ND/live ND group, while this difference was observed one week later in the two other vaccinated groups. The tracheal IgG response of the inact ND/live ND vaccinated chickens was higher at weeks 4, 5 and 6 in comparison with the two other vaccinated groups, and the difference was significant at 4 weeks of age. The inact ND/live ND vaccination regimen also induced a significantly higher level of tracheal IgG at weeks 5 and 6 in comparison with the rHVT-ND/live ND-Chitosan vaccination programme, and at week 10 in comparison with the rHVT-ND/live ND vaccination regimen. The inact ND/live ND group showed a peak level of tracheal IgA () and IgM () at 4 and 5 weeks of age that was significantly higher compared with the unvaccinated group and the other vaccinated groups. No immunoglobulin could be detected in lung washings during the experiment (data not shown).
Figure 5. Tracheal NDV-specific IgG (5a), IgA (5b) and IgM (5c) antibody titre of commercial layer chickens vaccinated at 1-day-old with the live ND and the rHVT-ND or inact ND vaccines according to different vaccination regimens. Data represent mean ± standard deviation of absorbance values determined by ELISA at specified ages (n = 5). The immunoglobulin response was measured in 1:2 diluted supernatants of ex vivo tracheal tissue cultures. Means ± standard deviations with no common uppercase letters differ significantly (P < 0.05). O.D., optical density.Discussion
In regions where ND is enzootic and the field pressure of ND virus is high, there is much competition between field viruses and vaccine development. Therefore it is very important to induce an active immune response by vaccination as soon as possible. In addition, the typically high levels of passive immunity interfere with the efficacy of very early immunization by the commonly available ND vaccines. Moreover, in many countries, local customs or situations result in insufficient vaccination or poor timing of vaccination, which have serious consequences (Alexander & Senne, Citation2008). Thus, there is a tendency to provide very early (as soon as at 1-day-old) and frequent vaccinations of progeny with live ND vaccines. Although these vaccines in optimum conditions induce good protection related to a strong humoral immunity and CMI, they are known to be sensitive to MDA at 1-day-old (Rauw et al., Citation2009b), There is a clear need for improving vaccination programmes that could be used at 1-day-old for mass application and induce a long protective immunity that requires fewer booster vaccinations at later ages. In this context, enterotropic live ND vaccine combined with either inactivated ND or rHVT-ND vaccines and adjuvanted, or not, with chitosan was used to immunize 1-day-old commercial layer chicks. The efficacy of these three vaccination schedules was then investigated. The clinical protection obtained at 6 weeks by the inact ND and live ND vaccines administrated simultaneously at 1 day old was more enhanced than previously described (Bennejean et al., Citation1978). The inact ND/live ND vaccination programme including a live ND vaccine with enterotropic tropism was more protective against a viscerotropic vNDV at 4 weeks, in comparison with a tracheotropic live strain (Chansiripornchai & Sasipreeyajan, Citation2006). This protection against more recent viscerotropic vNDV strains may be partly explained by the use of an enterotropic strain as live ND vaccine, which has been reported to induce a higher antibody-mediated immunity in the digestive tract during the first weeks of life when inoculated at 1-day-old in SPF chickens (Rauw et al., Citation2009b). However, the inact ND/live ND vaccination schedule did not provide 100% clinical protection at 10 weeks of age and the reduction of viral excretion following challenge was moderate. On the contrary, the rHVT-ND/live ND and rHVT-ND/live ND-Chitosan vaccine combinations afforded complete protection against mortality and clinical signs after challenge at 10 weeks of age as well as prevention of viral shedding by cloacal route and a reduction in the duration of the oropharyngeal excretion. Previous studies have shown that 1-day-old subcutaneous inoculation of rHVT-ND, compared with the in ovo route, improved the protection in commercial layer chickens with the same MDA level (Rauw et al., Citation2010a). Indeed, the subcutaneous inoculation of chicks at 1-day-old appears to be less variable than an injection in ovo, as the site of injection in the embryonated egg is of crucial importance to achieve adequate replication of the vaccine virus (Johnston et al., Citation1997; Jochemsen & Jeurissen, Citation2002; Wakenell et al., Citation2002).
To explain the differences observed in protection against mortality, morbidity and viral shedding, the active immune response profiles afforded by these three vaccination programmes was analysed. Because all vaccinated groups received the same live vaccine, the higher systemic and local antibody-mediated immunity observed in the inact ND/live ND combination must be related to the inact ND vaccine, which promotes a Th2-oriented immune response. The IgG antibodies are mainly transferred from the serum to the trachea (Zoth et al., Citation2008) and the duodenum (Muir, Citation1998), which may explain their elevated levels in the respiratory and digestive tracts, respectively, while IgA and IgM are produced locally. The water-in-oil emulsion used in the inact ND vaccine is known to retain the killed antigens at the injection site and to release it progressively, thus triggering a local inflammatory response and stimulating the recruitment of antigen-presenting cells (APC) and lymphocytes (Aucouturier et al., Citation2001; Degen et al., Citation2003; Jansen et al., Citation2006, Citation2007). After antigen phagocytosis, APC travel from the injection site to secondary lymphoid organs in order to interact with naïve T and B lymphocytes (Kaspers et al., Citation2008). The sustained release of non-replicating antigens is expected to maintain a high level of antibody production by repeated exposure of B cells to antigens or persistence of long-lived plasma cells (Degen et al., Citation2003; Jansen et al., Citation2006), explaining the higher humoral immunity in the inact ND/live ND group. In the case of combined vaccination with live ND vaccine, this antigen-presenting cell migration from injection site to secondary lymphoid organs allows the enhancement of the local IgA and IgM production, as observed in the trachea and duodenum at 4 and 5 weeks of age. This local response was primed by the live ND vaccine administrated simultaneously at 1-day-old and is known to replicate during the first week in the trachea and duodenum (Rauw et al., Citation2009b).
In addition, the persistent specific ex vivo production of ChIFNγ by lymphocytes from the peripheral blood and the digestive tract during 12 weeks and the detection of pulmonary CMI in the rHVT-ND/live ND group suggest that the rHVT-ND vaccine promotes a Th1-oriented immune response. Owing to the persistent viraemia of HVT in lymphocytes for at least 30 weeks (Tsukamoto et al., Citation2002), the HVT-ND is thought to circulate throughout the body by infected PBL, which migrate to the lymphoid tissues of the digestive and respiratory tracts. The HVT-ND then delivers foreign antigens locally to the immune system over an extended period of time. Moreover, the HVT replicates in a highly cell-associated manner in lymphocytes, suggesting that this delivery system induces a high degree of CMI (Heller & Schat, Citation1987). These properties could explain the higher and longer-lasting Th1-oriented CMI observed in peripheral blood, duodenum and lungs of chickens vaccinated with the rHVT-ND/live ND combination, whereas the rHVT-ND vaccine enhances the cellular immune response primed by the live ND vaccine administrated simultaneously at 1-day-old (Rauw et al., Citation2009b). The absence of CMI in the trachea could be explained by the absence of organized lymphoid structure (Kothlow & Kaspers, Citation2008) and/or of vaccinal antigen in this organ at these times to stimulate local immune response (Rauw et al., Citation2009a). Surprisingly, the positive effect of chitosan adjuvant on antigen-specific CMI in the spleen and peripheral blood following vaccination at 1 day old with the live ND vaccine (Rauw et al., Citation2010b) was not detected when using the rHVT-ND/live ND-Chitosan vaccination schedule. However, a faster cellular immune response in the peripheral blood was observed, confirming previous findings (Rauw et al., Citation2010a). It is anticipated that the beneficial effect of this adjuvant on immunity induced by a live ND vaccine is less clear when a second vaccine also promoting a Th1-oriented immune response is used simultaneously, at 1-day-old.
The differences observed in protection at 10 weeks of age indicate that the CMI in the respiratory and digestive tracts is required to efficiently reduce viral shedding. T cells, and especially CD8+ probably cytotoxic T lymphocytes (CTL), have been shown to be essential for NDV clearance from the Harderian gland, but not B cells (Cannon & Russell, Citation1986; Russell et al., Citation1997). CTL are most probably also involved in the viral clearance from the respiratory and digestive tracts, explaining the reduction of viral shedding, which could be stimulated more by the rHVT-ND/live ND than the inact ND/live ND combination.
In conclusion, the main advantage of the rHVT-ND/live ND over the inact ND/live ND vaccination programme is the induction of a longer-lasting protection against mortality and morbidity, as well as a stronger inhibition of viral shedding, by combining the advantages of the live ND and rHVT-ND vaccines. This study also shows that, in the case of ND, a strong Th1-mediated immune response combined to a humoral response is more protective than a Th2-oriented response. The addition of chitosan adjuvant in this rHVT/ND/live ND combination had no clear benefit. Given the known long-lasting persistency of HVT in vaccinated birds, protection induced by the rHVT-ND/live ND combination will last longer than that induced by the inact ND/live ND combination, and will better protect long-living chickens such as layers. However, further investigations are necessary to confirm this.
Acknowledgements
The authors gratefully acknowledge J. F. Pirlot and S. Anbari for their technical contributions to this work and C. Delgrange and M. Vandenbroeck for bird handling and sampling assistance.
References
- Alexander, D.J. & Senne, D.A. (2008). Newcaslte disease, other avian Paramyxovirus, and Pneumovirus infections. In Y.M. Saif, A.M. Fadly, J.R. Glisson, L.R. McDougald, L.K. Nolan, & D.E. Swayne (Eds.). Diseases of Poultry 12th edn (pp. 75–115). Ames, Iowa, USA: Blackwell Publishing.
- Aucouturier, J., Dupuis, L. & Ganne, V. (2001). Adjuvants designed for veterinary and human vaccines. Vaccine, 19, 2666–2672. 10.1016/S0264-410X(00)00498-9
- Bennejean, G., Guittet, M., Picault, J. P., Bouquet, J.F., Devaux, B., Gaudry, D. & Moreau, Y. (1978). Vaccination of one-day-old chicks against Newcastle disease using inactivated oil adjuvant vaccine and/or live vaccine. Avian Pathology, 7, 15–27. 10.1080/03079457808418256
- Calderon, N.L., Galindo-Muniz, F., Ortiz, M., Lomniczi, B., Fehervari, T. & Paasch, L.H. (2005). Thrombocytopenia in Newcastle disease: haematological evaluation and histological study of bone marrow. Acta Veterinaria Hungarica, 53, 507–513. 10.1556/AVet.53.2005.4.11
- Cannon, M.J. & Russell, P.H. (1986). Secondary in vitro stimulation of specific cytotoxic cells to Newcastle disease virus in chickens. Avian Pathology, 15, 731–740. 10.1080/03079458608436335
- Chansiripornchai, N. & Sasipreeyajan, J. (2006). Efficacy of live B1 or Ulster 2C Newcastle disease vaccines simultaneously vaccinated with inactivated oil adjuvant vaccine for protection of Newcastle disease virus in broiler chickens. Acta Veterinaria Scandinavica, 48, 1–4. 10.1186/1751-0147-48-2
- Czegledi, A., Ujvari, D., Somogyi, E., Wehmann, E., Werner, O. & Lomniczi, B. (2006). Third genome size category of avian paramyxovirus serotype 1 (Newcastle disease virus) and evolutionary implications. Virus Research, 120, 36–48. 10.1016/j.virusres.2005.11.009
- Degen, W.G.J., Jansen, T. & Schijns, V.E. (2003). Vaccine adjuvant technology: from mechanistic concepts to practical applications. Expert Review of Vaccines, 2, 327–335. 10.1586/14760584.2.2.327
- Folitse, R., Halvorson, D.A. & Sivanandan, V. (1998). Efficacy of combined killed-in-oil emulsion and live Newcastle disease vaccines in chickens. Avian Diseases, 42, 173–178. 10.2307/1592591
- Giambrone, J.J. & Clay, R.P. (1986). Vaccination of day-old broiler chicks against Newcastle disease and infectious bursal disease using commercial live and/or inactivated vaccines. Avian Diseases, 30, 557–561. 10.2307/1590421
- Heller, E.D. & Schat, K.A. (1987). Enhancement of natural killer cell activity by Marek's disease vaccines. Avian Pathology, 16, 51–60. 10.1080/03079458708436352
- Jansen, T., Hofmans, M.P.M., Theelen, M.J.G., Manders, F. & Schijns, V.E.J.C. (2006). Structure- and oil type-based efficacy of emulsion adjuvants. Vaccine, 24, 5400–5405. 10.1016/j.vaccine.2006.03.074
- Jansen, T., Hofmans, M.P., Theelen, M.J., Manders, F.G. & Schijns, V.E. (2007). Dose and timing requirements for immunogenicity of viral poultry vaccine antigen: investigations of emulsion-based depot function. Avian Pathology, 36, 361–365. 10.1080/03079450701567395
- Jochemsen, P. & Jeurissen, S.H.M. (2002). The localization and uptake of in ovo injected soluble and particulate substances in the chicken. Poultry Science, 81, 1811–1817.
- Johnston, P.A., Liu, H., O'Connell, T., Phelps, P., Bland, M., Tyczkowski, J., Kemper, A., Harding, T., Avakian, A., Haddad, E., Whitfill, C., Gildersleeve, R. & Ricks, C.A. (1997). Applications in in ovo technology. Poultry Science, 76, 165–178.
- Kaspers, B., Kothlow, S. & Butter, C. (2008). Avian antigen presenting cells. In F. Davison, B. Kaspers, & K.A. Schat (Eds.). Avian Immunology 1st edn (pp. 183–202). London: Academic Press.
- Kothlow, S. & Kaspers, B. (2008). The avian respiratory immune system. In F. Davison, B. Kaspers, & K.A. Schat (Eds.). Avian Immunology 1st edn (pp 273–288). London: Academic Press.
- Lambrecht, B., Gonze, M., Meulemans, G. & van den Berg, T. (2004). Assessment of the cell-mediated immune response in chickens by detection of chicken interferon-γ in response to mitogen and recall Newcastle disease viral antigen stimulation. Avian Pathology, 33, 343–350. 10.1080/0307945042000220318
- Mast, J., Nanbru, C., Decaesstecker, M., Lambrecht, B., Couvreur, B., Meulemans, G. & van den Berg, T. (2006). Vaccination of chickens embryos with escape mutants of La Sota Newcastle disease virus induces a protective immune response. Vaccine, 24, 1756–1765. 10.1016/j.vaccine.2005.10.020
- Meszaros, J. (1991). Aerosol-vaccination against Newcastle disease. Deutsche Tierarztliche Wochenschrift, 98, 117–164.
- Meszaros, J., Szemeredi, M. & Tamasi, G. (1992). Immunization of day-old chickens against Newcastle disease. Acta Veterinaria Hungarica, 40, 121–127.
- Muir, W.I. (1998). Avian intestinal immunity: basic mechanism and vaccine design. Poultry and Avian Biology Reviews, 9, 87–106.
- Palya, V., Penzes, Z., Horváth, T., Kardi, V., Dorsey Moore, K. & Gardin, Y. (2008). Comparative efficacy of several vaccination programmes including or not recombinant HVT-ND vaccine against challenge with mexican Chimalhuacan NDV strain. Proceedings of the 57th Western Poultry Disease Conference & XXXIII Annual ANECA convention (p. 36). Puerto Vallarta, Mexico.
- Rauw, F., Anbari, S., van den Berg, T. & Lambrecht, B. (2009a). New tools to measure peripheral and local NDV cell-mediated immunity in chickens. Proceedings of the 3rd European Veterinary Immunology Workshop (EVIW) (p. 30). Berlin, Germany.
- Rauw, F., Gardin, Y., Palya, V., Van Borm, S., Gonze, M., Lemaire, S., van den Berg, T. & Lambrecht, B. (2009b). Humoral, cell-mediated and mucosal immunity induced by oculo-nasal vaccination of one-day-old SPF and conventional layer chicks with two different live Newcastle disease vaccines. Vaccine, 27, 3631–3642. 10.1016/j.vaccine.2009.03.068
- Rauw, F., Gardin, Y., Palya, V., Anbari, S., Lemaire, S., Boschmans, M., van den Berg, T. & Lambrecht, B. (2010a). Improved vaccination against Newcastle disease by an in ovo recombinant HVT-ND combined with an adjuvanted live vaccine at day-old. Vaccine, 28, 823–833. 10.1016/j.vaccine.2009.10.049
- Rauw, F., Gardin, Y., Palya, V., Van Borm, S., Gonze, M., Lemaire, S., van den Berg, T. & Lambrecht, B. (2010b). The positive adjuvant effect of chitosan on antigen-specific cell-mediated immunity after chicken's vaccination with live Newcastle disease vaccine. Veterinary Immunology and Immunopathology, 134, 249–258. 10.1016/j.vetimm.2009.10.028
- Rauw, F., Anbari, S., van den Berg, T. & Lambrecht, B. (2011). Measurement of systemic and local respiratory cell-mediated immunity after influenza infection in chickens. Veterinary Immunology and Immunopathology, 143, 27–37. 10.1016/j.vetimm.2011.05.029
- Russell, P.H., Dwivedi, P.N. & Davison, T.F. (1997). The effect of cyclosporin A and cyclophosphamide on the populations of B and T cells and virus in the Harderian gland of chickens vaccinated with the Hitchner B1 strain of Newcastle disease virus. Veterinary Immunology and Immunopathology, 60, 171–185. 10.1016/S0165-2427(97)00094-9
- Sato, H., Oh-hira, M., Ishida, N., Imamura, Y., Hattori, S. & Kawakita, M. (1987). Molecular cloning and nucleotide sequence of P, M and F genes of Newcastle disease virus avirulent strain D26. Virus Research, 7, 241–255. 10.1016/0168-1702(87)90031-1
- Tsukamoto, K., Saito, S., Saeki, S., Sato, T., Tanimura, N., Isobe, T., Mase, M., Imada, T., Yuasa, N. & Yamaguchi, S. (2002). Complete, long-lasting protection against lethal infectious bursal disease virus challenge by a single vaccination with an avian herpesvirus vector expressing VP2 antigens. Journal of Virology, 76, 5637–5645. 10.1128/JVI.76.11.5637-5645.2002
- Van Borm, S., Steensels, M., Ferreira, H.L., Boschmans, M., De Vriese, J., Lambrecht, B. & van den Berg, T. (2007). A universal avian endogenous real-time reverse transcriptase-polymerase chain reaction control and its application to avian influenza diagnosis and quantification. Avian Diseases, 51, 213–220. 10.1637/7552-033106R.1
- Van Boven, M., Bouma, A., Fabri, T.H.F., Katsma, E., Hartog, L. & Koch, G. (2008). Herd immunity to Newcastle disease virus in poultry by vaccination. Avian Pathology, 37, 1–5. 10.1080/03079450701772391
- Wakenell, P.S., Bryan, T., Schaefer, A.E., Avakian, A., Williams, C. & Whitfill, C. (2002). Effect of in ovo vaccine delivery route on herpesvirus of turkeys-SB-1 efficacy and viremia. Avian Diseases, 46, 274–280. 10.1637/0005-2086(2002)046[0274:EOIOVD]2.0.CO;2
- Zoth, S.C., Gomez, E., Carrillo, E. & Berinstein, A. (2008). Locally produced mucosal IgG in chickens immunized with conventional vaccines for Newcastle disease virus. Brazilian Journal of Medical and Biological Research, 41, 318–323. 10.1590/S0100-879X2008000400010