Abstract
Overcrowding stress is a reality in the poultry industry. Chickens exposed to long-term stressful situations present a reduction of welfare and immunosuppression. We designed this experiment to analyse the effects from overcrowding stress of 16 birds/m2 on performance parameters, serum corticosterone levels, the relative weight of the bursa of Fabricius, plasma IgA and IgG levels, intestinal integrity, macrophage activity and experimental Salmonella Enteritidis invasion. The results of this study indicate that overcrowding stress decreased performance parameters, induced enteritis and decreased macrophage activity and the relative bursa weight in broiler chickens. When the chickens were similarly stressed and infected with Salmonella Enteritidis, there was an increase in feed conversion and a decrease in plasma IgG levels in the stressed and Salmonella-infected birds. We observed moderate enteritis throughout the duodenum of chickens stressed and infected with Salmonella. The overcrowding stress decreased the macrophage phagocytosis intensity and increased Salmonella Enteritidis counts in the livers of birds challenged with the pathogenic bacterium. Overcrowding stress via the hypothalamic–pituitary–adrenal axis that is associated with an increase in corticosterone and enteritis might influence the quality of the intestinal immune barrier and the integrity of the small intestine. This effect allowed pathogenic bacteria to migrate through the intestinal mucosa, resulting in inflammatory infiltration and decreased nutrient absorption. The data strengthen the hypothesis that control of the welfare of chickens and avoidance of stress from overcrowding in poultry production are relevant factors for the maintenance of intestinal integrity, performance and decreased susceptibility to Salmonella infection.
Introduction
The concept of animal welfare is a reality in poultry production. Welfare assessment studies in chickens have been traditionally conducted by observing their mortality, physiology, behaviour and health, with emphasis placed on a bird's leg health and walking ability (Ekstrand et al., Citation1998; Weeks et al., Citation2000; Kestin et al., Citation2001; Martrenchar et al., Citation2002; Shepherd & Fairchild, Citation2010). Welfare concepts are being expanded to include glucocorticoid hormone release, performance parameters, immunological functions and disease susceptibility (Kaiser et al., Citation2009; Shini et al., Citation2010; Quinteiro-Filho et al., Citation2012a). Because of these findings, stressful situations—including inadequate housing conditions, overcrowding, environmental temperature extremes, nutritional deprivation and others—must be carefully considered in the practices of broiler chicken production (Dawkins et al., Citation2004; Hoerr, Citation2010; Quinteiro-Filho et al., Citation2010; Lay et al., Citation2011).
Overcrowding stress is one of the most important stressors in poultry production. Studies conducted on overcrowding stress showed decreased performance parameters; that is, decreased body weight gain, decreased feed intake and decreased food conversion in broiler chickens (Thaxton et al., Citation2005; Guardia et al., Citation2011). Mortality, leg problems, susceptibility to diseases and behavioural changes were related to stress conditions from overcrowding (Estevez, Citation2007). Few authors have considered and analysed the effects of overcrowding on the immune system activity of chickens (Dafwang et al., Citation1987; Heckert et al., Citation2002; Houshmand et al., Citation2012). Heckert et al. (Citation2002) showed that high stocking densities in broiler chickens decreased the relative weight of the bursa of Fabricius, one of the most important lymphoid organs of chickens, and they related this finding to possible immunosuppression.
Environmental stressors were shown to negatively affect a bird's immune system, compromising its performance and ability to overcome bacterial infections (Humphrey, Citation2006; Dunkley et al., Citation2007; Burkholder et al., Citation2008; Quinteiro-Filho et al., Citation2012a; Soleimani et al., Citation2012). We showed that heat stress (31°C and 36°C for 35 to 42 days) decreased performance parameters and macrophage activity and induced intestinal injury; these effects were attributed to hypothalamic–pituitary–adrenal (HPA) axis activation. Heat stress was linked to a decrease in immune activity in poultry and mammals, following invasion by pathogenic bacteria (such as Salmonella spp.) through the intestinal epithelium (Burkholder et al., Citation2008; Quinteiro-Filho et al., Citation2012a; Verbrugghe et al., Citation2012).
Salmonella enterica serovar Enteritidis is the predominant serovar associated with food-borne illness and salmonellosis worldwide (Van Immerseel, Citation2010; Barrow et al., Citation2012; Mebrhatu et al., Citation2014) and stress situations cause immunosuppression in chickens. We thus designed this study to analyse the effects of overcrowding stress on performance parameters, immune status, small intestine integrity and Salmonella invasion in broiler chickens experimentally infected with Salmonella Enteritidis.
Materials and Methods
Birds and overcrowding stress protocol
One-day-old broiler chicks (Ross®; Aviagen®, Rio Claro, Sao Paulo, Brazil) were housed in two climate-controlled rooms at the Experimental Center of Avian Pathology, School of Veterinary Medicine, University of São Paulo. A total of 360 male broiler chickens were used. The birds were obtained from a breeder hatchery and were housed in floor pens covered with sterilized and contaminant-free wood shavings. The breeder hatchery was free of Salmonella spp. and Mycoplasma spp. as certificated by the supplier and confirmed in our laboratory. Water and food (hanging feeders) were provided ad libitum to the chickens, and the birds were constantly observed for health status and behaviour. The transport boxes and housing environments were employed according to ISO 6579:2002/Amd 1:2007 protocols (ISO, Citation2007). Additionally, transport box samples, wood shavings and house environment swabs were pre-enriched in Difco tetrathionate broth (BD Difco, Sparks, MD, USA) and cultured on Difco xylose–lysine–deoxycholate agar with xylose–lysine–tergitol-4 (XLT4) (BD Difco) to detect Salmonella Enteritidis. Salmonella spp. were not detected in the transport boxes or in the bird house environment. The birds were maintained and used in accordance with the guidelines of the Committee on Care and Use of Laboratory Animal Resources of The School of Veterinary Medicine, University of São Paulo, Brazil (Protocol Number 1798/2009).
On experimental day 1 (ED1), the chickens were weighed and allocated into four groups: the control group (Group C), the positive Salmonella control group (Group PC), the negative Salmonella overcrowding stress group (Group Ov), and the positive Salmonella overcrowding stress group (Group POv). Chickens submitted to the Salmonella challenge were allocated to a different room than the non-infected chickens. From ED1 to ED42, the birds in the control groups (Groups C and PC) were maintained in groups of 10 birds/m2. The chickens in the two overcrowding-stressed groups (Groups Ov and POv) were maintained at 16 birds/m2.
Salmonella Enteritidis infection
On ED1, the birds from Groups PC and POv were challenged orally via gavage with 0.1 ml Difco brain heart infusion containing 106 colony-forming units/ml Salmonella Enteritidis. The birds in Groups C and Ov simultaneously received a similar volume of sterile brain heart infusion. After 42 days (ED42), the caecal colonization and organ invasion were analysed in the birds of Groups PC and POv using microbiological techniques. The birds were independently housed in climate-controlled rooms following the challenge.
Performance parameters
Broiler chicken performance was assessed in terms of the mortality rate, body weight gain (g/bird), feed consumption per bird (g/bird) and feed conversion throughout the experiment. The feed conversion ratio was calculated on the basis of the feed/gain ratio for each replicate. For each group, five replicates of 12 birds per box were analysed. The birds were monitored weekly for performance parameters, but statistical analysis was only applied to data collected at the end of the experiment (ED42).
Organ harvest
On ED42, 20 birds per group were randomly selected and euthanized via cervical dislocation immediately after weighing. At necropsy, the liver and spleen were harvested for Salmonella Enteritidis analysis. The lymphoid organs (the spleen and bursa of Fabricius) were harvested for the relative weight determinations (percent of body weight), and the connective tissues were removed before weighing.
Caecal colonization and organ invasion by Salmonella
To determine the bacterial organ invasion following the Salmonella Enteritidis challenge, liver and spleen samples were taken from 20 birds from each experimental group (Groups PC and POv) on ED42. The samples were aseptically removed, and the liver and spleen weights were recorded. The organs were independently homogenized. The homogenates of each organ were diluted 1:10 in a sterile solution of 0.1% peptone water, and 100 µl was spread on XLT4 agar (Difco) and incubated at 37°C for 24 h. The organ invasion was evaluated by counting the Salmonella Enteritidis colonies recovered from the liver and spleen. In the cases in which bacterial growth was detected, Salmonella spp. were confirmed by a serological test (polyvalent antisera; Promicro, Sao Paulo, Brazil). The following biochemical tests were also performed to confirm Salmonella: triple sugar iron agar, urea broth, l-lysine decarboxylase, ortho-nitrophenyl-β-galactosidase, Voges Proskauer and indole tests. To confirm the absence of Salmonella Enteritidis, the non-positive samples were placed in a tetrathionate broth (1:10), incubated at 37°C for 48 h and streaked on XLT4 agar. The absence of Salmonella Enteritidis was confirmed by analysis of bacterial colonies grown on the XLT4 agar. The birds in the negative control groups (Groups C and Ov) were similarly tested to confirm the absence of Salmonella Enteritidis.
Intestinal morphology
Light microscopy specimens were taken from several sites within the segments of the small bowel. The intestinal segments were defined by the following anatomic limits: the length of the duodenum (from the gizzard [duodenum ostium] to the beginning of the mesentery [duodenum loop]), the length of the jejunum (from the most distal point of insertion of the mesentery to 5 cm before the Meckel's diverticulum) and the length of the ileum (from 5 cm after the Meckel's diverticulum to the ileo-caecal junction). The tissues were fixed in 10% buffered formalin, embedded in paraffin, cut to a thickness of 4 to 5 µm and stained with haematoxylin and eosin for light microscopy. The slides were examined for pathological changes by two pathologists who were blind to the group affiliation; a high positive correlation was observed between their evaluations.
The following histological parameters were analysed: villous height, crypt morphology and depth, villous height:crypt depth ratio, intensity and composition of the inflammatory infiltrate in the lamina propria and the number of intraepithelial lymphocytes per 100 enterocytes. The severity of the small intestinal lesions was scored semi-quantitatively from 0 to 3, where 0 = control material, 1 = mild alteration, 2 = moderate alteration and 3 = severe alteration. The criteria used to define normal histology were those described by Hodges (Citation1974) and Riddell (Citation1987).
Serum corticosterone determination
Ten birds per group were randomly removed and gently handled to collect 2 ml blood from their brachial veins over a period of 30 sec to 1 min. The corticosterone concentrations were measured on ED42 between 08:00 and 10:00 a.m. Whole blood was used to collect the serum for the corticosterone assays, and the corticosterone serum concentrations were determined using a corticosterone enzyme-linked immunosorbent assay kit from Enzo Life Science (Farmingdale, NY, USA), as described by Tachibana et al. (Citation2007).
Total plasma IgM, IgG and IgA determination
The same birds used for the collection of blood for the corticosterone analyses were used for the total plasma IgM, IgG and IgA determinations. From each bird, 2 ml blood was collected from the brachial vein, and the plasma was extracted for immunoglobulin determination. The IgG, IgM and IgA plasma concentrations were determined using an enzyme-linked immunosorbent assay ELISA kit from Bethyl Laboratories (Montgomery, TX, USA), as described by Gao et al. (Citation2008).
Peritoneal macrophage activity
Because chickens do not have resident peritoneal macrophages, activation was performed as proposed by Qureshi et al. (Citation1986) and modified by Quinteiro-Filho et al. (Citation2010). Briefly, 3% Sephadex G-50 Fine (Sigma, St Louis, Missouri, USA) in 0.9% saline solution was injected at a dose of 5 ml/200 g body weight into the peritoneal cavity of 10 chickens from each group (Groups C, Ov, PC and POv) on ED40. Forty-eight hours after the inoculation (ED42), the birds were euthanized by cervical dislocation, and the cellular and cytometric analyses were obtained, as described by Quinteiro-Filho et al. (Citation2010).
Peritoneal macrophage activity measurement
A flow cytometer (FACS Calibur; Becton Dickinson Immunocytometry Systems, San Jose, California, USA) interfaced with a Macintosh G4 computer was used for data collection. Data from 5000 events were collected in list mode and analysed in Cell Quest (Becton Dickinson Immunocytometry Systems). The cell populations were identified based on their properties on the forward scatter/side scatter plots, mechanically sorted (FAC-Scan; Becton Dickinson Immunocytometry Systems) and evaluated by light microscopy after staining with Giemsa. The peritoneal macrophage population was confirmed by the KUL01 (Abcam, Cambridge, Massachusetts, USA) phenotype method described previously (Mast et al., Citation1998; Quinteiro-Filho et al., Citation2010). The data from the peritoneal macrophages were collected and analysed by applying gates that sorted the lymphocyte and monocyte clusters. The fluorescence data were collected on a log scale. The green fluorescence from 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) was measured at 530 ± 30 nm (FL1 detector), and the red fluorescence from propidium iodide (PI)-labelled Staphylococcus aureus was measured at 585 ± 42 nm (FL2). PI and DCFH-DA fluorescence were analysed after fluorescence compensation to correct for any crossover between the PI and DCFH-DA signals.
The quantification of the amount of phagocytosis and oxidative burst was estimated by the mean fluorescence in the PI and DCFH-DA cells, respectively. The peritoneal macrophages (2 × 106 cells/ml) collected as described above were divided into four different polypropylene tubes. Tube A contained only cells and was defined as the basal tube; tube B contained cells mixed with 200 µl DCFH-DA (0.3 mM) in phosphate-buffered saline and was considered to represent the basal oxidative burst data; tube C contained cells mixed with100 µl S. aureus (1000 bacteria:1 macrophage) and was used to assess phagocytosis; and tube D contained cells mixed with 200 µl DCFH-DA (0.3 mM) and 100 µl S. aureus, representing the oxidative burst induced by phagocytosis. The samples were incubated under agitation at 37°C for 30 min. Then, 2 ml cold ethylenediamine tetraacetic acid solution (3 mM) was added to stop phagocytosis. The samples were centrifuged (250 × g for 10 min), and the obtained cell pellets were re-suspended in 0.5 ml ice-cold phosphate-buffered saline for flow cytometry. The direct measurements of the mean fluorescence on the green and red channels were recorded as the amount of oxidative burst and phagocytosis, respectively, as proposed by Hasui et al. (Citation1989). The percentage of cells undergoing phagocytosis (the percentage of macrophages that ingested bacteria) was expressed as the number of macrophages with red fluorescence divided by the total number of cells (multiplied by 100). The intensity of the phagocytosis (the quantity of ingested bacteria) was measured directly through the intensity of fluorescence emitted by the cells that performed phagocytosis. The basal tube was used as an internal control for phagocytosis and oxidative burst. This protocol was described in detail by Hasui et al. (Citation1989) and Quinteiro-Filho et al. (Citation2010).
Statistical analyses
The statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software Inc., San Diego, California, USA). The parametric data were analysed using a two-way analysis of variance (ANOVA) test followed by a Tukey test (stressor and Salmonella factor interaction analyses) and/or a one-way ANOVA followed by a Tukey test. The Mann–Whitney U test was used to compare the non-parametric data from two groups. P < 0.05 was considered to show significance for all of the comparisons. The data are presented as the mean ± standard deviation.
Results
Performance parameters
All of the performance parameters were affected in the chickens subjected to overcrowding from ED1 to ED41 (). The overcrowding stress decreased the body weight gain (F = 6.823; P < 0.05) and feed consumption (g/bird) (F = 5.350; P < 0.01) in the Salmonella-infected (Group POv) and the non-infected (Group Ov) birds. With respect to feed conversion, an interaction between overcrowding and Salmonella infection (F =4.197; P < 0.05) was observed. Although the overcrowding (Group Ov) alone did not affect the feed conversion compared with the control birds (P > 0.05), the birds that were subjected to overcrowding stress and Salmonella Enteritidis infection (Group POv) exhibited an increased feed conversion compared with those of Groups C and PC (F = 4.197; P < 0.05). Mortality was not found in any of the tested groups. There was no difference between groups from day ED1 to ED35.
Table 1. Effects of overcrowding stress (16 birds/m2) on the performance parameters of broiler chickens infected with Salmonella Enteritidis.
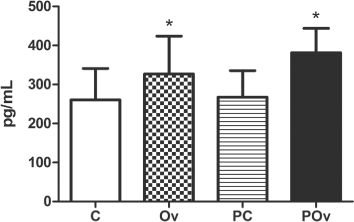
Serum corticosterone determination
As illustrated in , the birds of Groups Ov and POv exhibited high corticosterone serum levels compared with Groups C and PC (Group C: 260.50 ± 80.7, Ov: 326.82 ± 97.14 pg/ml; Group PC: 267.91 ± 67.93, POv: 381.36 ± 62.81 pg/ml; F = 5.28 P < 0.05). No significant differences were observed for this parameter between the birds in Groups Ov and POv.
Lymphoid organ relative weight and IgG, IgM and IgA plasma levels
As shown in , significant differences were not observed in the spleen relative weights. A decreased weight was observed in the bursa of Fabricius from the chickens in Groups Ov and POv compared with Groups C and PC. We observed an increase in the total IgA levels in plasma in Groups Ov, PC and POv compared with Group C (P < 0.05). We also observed a decrease in the total IgG levels and an increase of IgM levels in Group POv compared with Group PC (P < 0.05) ().
Table 2. Effects of overcrowding stress (16 birds/m2) on the relative weight of the lymphoid organs and the plasma immunoglobulin concentration of broiler chickens infected with Salmonella Enteritidis.
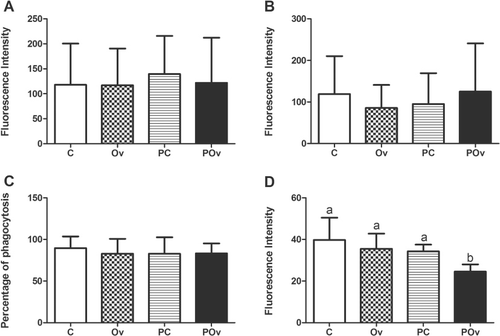
Macrophage activity
Analysis of the macrophage activity showed no alterations in the basal oxidative burst (: Group C, 118.0 ± 82.48; Group Ov, 116.9 ± 73.54; Group PC, 139.5 ± 76.46; Group POv, 121.9 ± 90.24; P > 0.05) or in the S. aureus-induced oxidative burst (: Group C, 119.2 ± 91.10; Group Ov, 85.93 ± 54.86; Group PC, 95.15 ± 74.03; Group POv, 125.0 ± 11.70; P > 0.05) among the groups. The percentage of macrophage phagocytosis (: Group C, 89.55 ± 14.07; Group Ov, 82.81 ± 17.98; Group PC, 82.79 ± 19.81; Group POv, 83.26 ± 11.95; P > 0.05) was similar among the groups. The chickens in Group POv presented a decrease in the intensity of macrophage phagocytosis (Group C, 39.78 ± 10.67; Group Ov, 35.49 ± 7.27; Group PC, 34.29 ± 3.24; Group POv, 24.53 ± 3.47; P < 0.05) when compared with birds in the remaining groups ().
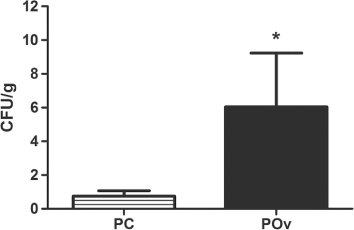
Liver invasion by Salmonella
Salmonella Enteritidis was not observed in the liver and spleen samples from the birds of Group C or Group Ov. Overcrowding stress increased liver Salmonella Enteritidis invasion as detected in birds of Group POv (Group PC, 0.76 ± 0.30; Group POv, 5.58 ± 3.18; P < 0.05) when compared with those of Group PC (). No positive Salmonella Enteritidis counts were observed in the spleens of birds from Group PC or Group POv.
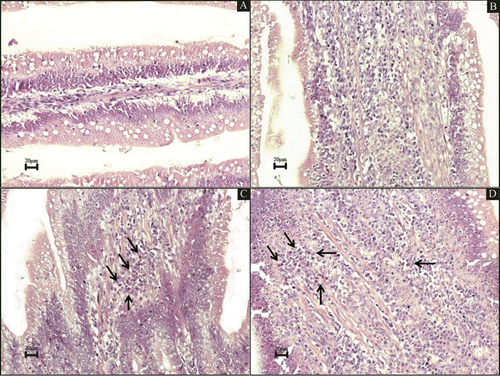
Intestinal histology
Histological intestinal changes were not observed in the chickens in the control group (Group C). No alterations in the morphology (i.e. the crypt depth:villus height ratio, the villus height, the crypt depth and the number of intraepithelial lymphocytes) were found in the duodenum, jejunum or ileum mucosa among the birds of the four groups. A mild increase in the cellularity of the lamina propria was observed in the duodenum of the birds of Groups PC and Ov. This increase in cellularity was characterized by mild acute multifocal lympho-plasmocytic enteritis and by foci of heterophil infiltrates (Groups Ov and PC showed these results in six out of 10 samples; P < 0.05). Histological differences between these groups were not detected. The birds in Group POv exhibited more intense and diffuse multifocal lympho-plasmocytic enteritis with foci of heterophil infiltration in the duodenum portion, which was identified as moderate enteritis (Group POv showed these results in nine out of 10 samples; P < 0.05). illustrates these reported alterations.
Discussion
The results of this study indicate that overcrowding stress decreased performance parameters, induced enteritis in broiler chickens and decreased the bursa:body weight ratio. When the chickens were similarly stressed and infected with Salmonella Enteritidis, an increase of feed conversion, a decrease of IgG plasma levels, an increase of IgA and IgM plasma levels and moderate enteritis throughout the duodenum were observed in the stressed chickens. The overcrowding stress decreased the macrophage phagocytosis intensity and increased Salmonella Enteritidis invasion to the liver of the birds challenged with this pathogenic bacterium. We observed that overcrowding stress increased corticosterone serum levels, demonstrating the role of the HPA axis in the reported data.
The HPA axis is one of the most important systems for the integration of the body and is activated in response to stressful stimuli or homeostatic disturbances (Besedovsky & del Rey, Citation1996; McEwen, Citation2000). One of the most important results of this activation is an increase in plasma corticosterone levels. Different types of stressors, including chemical stressors, prenatal stress, individual housing, inescapable foot shock and others, can activate the HPA axis in rodents (Costa-Pinto & Palermo-Neto, Citation2010). As in heat stress situations (Quinteiro-Filho et al., Citation2010, Citation2012a), the overcrowding stress increased the serum corticosterone levels in broiler chickens. This “stress” hormone can subsequently dysregulate immune responses and may be potentially harmful to the health of the organism.
The literature on high stocking density studies shows no unanimity in relationship to performance parameters in overcrowding-stressed broiler chickens. Our results corroborate with several literature studies that showed an impairment of performance parameters during overcrowding stress (Heckert et al., Citation2002; Dozier III et al., Citation2006; Estevez, Citation2007; Mtileni et al., Citation2007; Tong et al., Citation2012). Our data showed that overcrowding stress decreased weight gain and feed intake in overcrowded chickens. Within this context, we observed that stressed chickens challenged with Salmonella Enteritidis (Group POv) exhibited increased feed conversion compared with birds that were only subjected to overcrowding (Group Ov) or that were only infected with Salmonella (Group PC). These data indicate the existence of a positive interaction between overcrowding stress and Salmonella infection, showing that overcrowding stress-induced disruptions could be more harmful during a pathogenic infection.
The results obtained reinforce the hypothesis that the decreased performance observed in stressed broiler chickens might be related to alterations in the HPA axis function via corticosterone release (Shini & Kaiser, Citation2008; Shini et al., Citation2009; Quinteiro-Filho et al., Citation2010, Citation2012a, Citation2012b). Moreover, the observed intestinal inflammation could lead to a decrease in nutritional absorption because of the presence of intestinal lesions and because of sickness-related behaviour by release of certain interleukins (i.e. IL-1β and IL-6) after stressor application and/or bacterial infection (Mitchell & Carlisle, Citation1992; Burkholder et al., Citation2008; Quinteiro-Filho et al., Citation2010). Indeed, IL-1 may act on the hypothalamic nuclei that regulate food intake and satisfaction, causing a decrease in food consumption and body weight gain (Costa-Pinto et al., Citation2009).
As discussed in our previous work (Quinteiro-Filho et al., Citation2012a), the intestinal mucosa represents the first line of defence against Salmonella spp. (Fagarasan & Honjo, Citation2003; Fagarasan, Citation2006). The primary site of Salmonella spp. colonization is the large blind caeca that branch from the distal ileum immediately before the colon. Following invasion, it is hypothesized that Salmonella spp. are taken up by the macrophages and dendritic cells and subsequently transported via the lymphatic system to the spleen and the liver (Mastroeni & Menager, Citation2003; Chappell et al., Citation2009). We observed that the overcrowding stress decreased the intensity of macrophage phagocytosis in the chickens of Group POv. Other results obtained in our laboratory revealed that different stressors such as footshock, chemical stressors and individual housing decreased the macrophage activity in mice (Fonseca et al., Citation2002; Palermo-Neto et al., Citation2003, Citation2008; Quinteiro-Filho et al., Citation2009; Costa-Pinto & Palermo-Neto, Citation2010). In broiler chickens, we observed that heat stress decreased the macrophage oxidative burst (Quinteiro-Filho et al., Citation2010); this finding was linked to the observed increase of Salmonella migration to the spleen of heat-stressed chickens (Quinteiro-Filho et al., Citation2012a). In our study, overcrowding stress increased the Salmonella Enteritidis invasion to the liver but not to the spleen of the stressed chickens. Indeed, Salmonella migration could happen both to the spleen and liver following invasion since Salmonella is assumed to be taken up by macrophages and transported via the lymphatic system to the spleen and liver (Mastroeni & Menager, Citation2003; Chappell et al., Citation2009). However, there is no firm experimental evidence to define the main target organ of Salmonella invasion (Mastroeni & Menager, Citation2003; Chappell et al., Citation2009). It seems feasible to suggest that bacterial invasion is not controlled by the innate immune system and that Salmonella is able to reach and replicate within several organs (e.g. the liver, as was observed in this model), thereby establishing a systemic infection (Burkholder et al., Citation2008; Chappell et al., Citation2009).
Other important branches of the immune system against bacterial pathogens are humoral immunity and immunoglobulin production. One of the most important lymphoid organs responsible for the adaptative immune function is the bursa of Fabricius. The activity of this organ has been related to signs of immunosuppression in chickens after stressful situations. Heckert et al. (Citation2002) described a progressive decrease in the bursa relative weight in broiler chickens maintained in groups of 10, 15 and 20 birds/m2. Our data strengthen and reinforce those of Heckert et al. (Citation2002) because we observed a decrease in the bursa relative weight in birds exposed to high stocking density, representing one more sign of immunosuppression.
Changes in the integrity and normal function of the bursa of Fabricius could lead to changes in the production of immunoglobulins. We observed a decrease of plasma IgG levels in the birds of Group POv. Caroprese et al. (Citation2009) showed that the concentrations of anti-OVA IgG were primarily influenced by space allowance in dairy ewes, showing higher antibody titres in the low stocking density group than in the high stocking density group. In a heat stress study, Mashaly et al. (Citation2004) reported a decrease in antibody titres to sheep red blood cells in laying hens. These results agree with the findings of Zulkifli et al. (Citation2000), who showed that heat stress caused a reduction in antibody synthesis. Stressors increased serum corticosterone levels (Glaser & Kiecolt-Glaser, Citation2005; Quinteiro-Filho et al., Citation2010, Citation2012a; Shini et al., Citation2010), and this hormone inhibits antibody production by B cells (Gross, Citation1992), which might explain the inverse relationship we found between corticosterone levels and IgG titres.
This decrease in IgG levels might be related to reduced immunological memory, thus leading to an increase in pathogen susceptibility. Overcrowding stress also induced an increase in plasma IgM levels in birds of Group POv. IgM is expressed largely on the surface of B lymphocytes and usually acts as a first antibody response to infection (Ratcliffe, Citation2006). Moreover, overcrowding stress increased plasma IgA levels, suggesting an increase in the levels of this immunoglobulin at the intestinal mucosa. Indeed, IgA is responsible for local protection against bacterial pathogens and parasitic and viral infection (Fagarasan, Citation2006), while protecting the body against non-self peptides (Mestecky et al., Citation1999). We believe that the observed increase in IgM and IgA levels might be a mechanism used by the immune system in an attempt to respond to the Salmonella Enteritidis invasion. Furthermore, stress is known to decrease T-helper 2 cytokines (Wang et al., Citation2001), which are important for antibody production and consequently for pathogen clearance (Lebman & Coffman, Citation1988).
Kiank et al. (Citation2008) showed that commensal bacteria are able to migrate to lymphoid organs and livers of stressed mice. These authors reported that chronically stressed mice spontaneously suffered from increased bacterial load in the liver and lungs, a condition that was sustained for up to 10 days following termination of the stress exposure and which contributed to pneumonia (Kiank et al., Citation2008). These data were related to immune dysfunction in the intestinal barrier. Stressors applied to rats and mice increased their susceptibility to Salmonella (Kuriyama et al., Citation1996) and facilitated bacterial translocation from the mucosal intestinal barrier (Humphrey, Citation2006).
Normal morphology and integrity of the small intestine is important to prevent bacteria translocation from the intestinal tract to the body as well as for the digestion and absorption of nutrients. Numerous authors have reported that environmental stressors affect intestinal barrier integrity, which is composed of enterocytes, tight junctions, secreted mucous and immune cells, such as macrophages (Lambert et al., Citation2002; Prosser et al., Citation2004; Singleton & Wischmeyer, Citation2006; Chappell et al., Citation2009; Lambert, Citation2009).
Loss of intestinal barrier integrity leads to increased intestinal permeability and local intestinal inflammation (Chappell et al., Citation2009). The present data showed mild enteritis in overcrowding-stressed birds (Group Ov). This inflammation was characterized by increased lympho-plasmacytic inflammatory infiltrates in the duodenum. We observed moderate enteritis throughout the small intestine in the birds that were stressed and infected with Salmonella Enteritidis (Group POv). The presence of heterophils in the observed inflammatory infiltrate site reflects bacterial invasion from the epithelium to the lamina propria of the villi. An increased area of inflammation was present in the intestines of the birds that were stressed and infected with Salmonella. In the non-infected and overcrowding-stressed birds (Group Ov) or in the positive control birds (Group PC), only a mild lympho-plasmacytic heterophil infiltration was observed, and large intact areas were observed throughout the intestinal mucosa of birds from these groups.
The observed increase in intestinal inflammatory infiltration (enteritis) may have contributed to the increased production of proinflammatory cytokines (i.e. IL-1). IL-1 was reported to disrupt the tight junctions of the intestinal epithelium and consequently to increase the permeability of the mucosa to the pathogenic bacteria (Al-Sadi & Ma, Citation2007; Al-Sadi et al., Citation2008). Increases in corticosterone release could have decreased the innate immunity of the gut, facilitating Salmonella invasion. High stocking density strongly affected the fingerprint profiles of the bacterial community, changing the commensal microbiota in the caeca of broiler chickens (Guardia et al., Citation2011) and leading to a loss of protection against pathogenic microorganism colonization.
This loss of protection might reflect the competition between the pathogenic and commensal bacteria for the binding of intestinal cell receptors as well as the increased demand for nutrients and the production of bacteriocins (Brisbin et al., Citation2008; Burkholder et al., Citation2008). Microbiota disruption might have consequences on digestive tract physiology, growth performance and decreased intestinal immunity (Brisbin et al., Citation2008; Burkholder et al., Citation2008; Guardia et al., Citation2011).
It is possible that the autonomic nervous system, specifically the sympathetic and parasympathetic arms including the enteric nervous system, may be involved in the relationship between stress and intestinal regulation (Grenham et al., Citation2011). Neuroimmune epithelial interactions play a protective role in the intestines, which ultimately ensure the preservation of the epithelial barrier functions and expel toxic agents from the gut (Bueno, Citation2000; Sharkey & Mawe, Citation2002). It is hypothesized that an increase in noradrenaline is associated with increased bacterial translocation from the gut (Hart & Kamm, Citation2002). Moreover, it seems possible that overcrowding stress might have increased noradrenaline release by the adrenal gland (medullary zone) as reported for corticosterone at the cortical zone.
Overcrowding stress (16 birds/m2) on broiler chickens might have activated the HPA axis in the stressed chickens, increasing the corticosterone serum levels and releasing proinflammatory cytokines. These events might have decreased food intake and body weight gain. Overcrowding stress via the HPA axis and/or the enteric nervous system activities might have influenced the quality of the intestinal immune barrier. This effect allowed pathogenic bacteria to migrate through the intestinal mucosa and resulted in inflammatory infiltration, decreasing the nutrient absorption to an even greater extent. The observed decrease in macrophage activity and total plasma IgG levels may have contributed to the large amount of Salmonella observed in the livers of overcrowding-stressed and infected broiler chickens. The data strengthen the notion that controlling welfare and avoiding overcrowding stress in poultry production are relevant factors for maintaining intestinal integrity, obtaining satisfactory performance indices and decreasing susceptibility to Salmonella infection.
Acknowledgements
This study was financially supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, #09/51886-3 and #09/53532-4) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, #470776/2009-9), to whom the authors wish to express their gratitude.
References
- Al-Sadi, R.M. & Ma, T.Y. (2007). Il-1beta causes an increase in intestinal epithelial tight junction permeability. Journal of Immunology, 178, 4641–4649.
- Al-Sadi, R., Ye, D., Dokladny, K. & Ma, T.Y. (2008). Mechanism of Il-1beta-induced increase in intestinal epithelial tight junction permeability. Journal of Immunology, 180, 5653–5661.
- Barrow, P.A., Jones, M.A., Smith, A.L. & Wigley, P. (2012). The long view: Salmonella - the last forty years. Avian Pathology, 41, 413–420. 10.1080/03079457.2012.718071
- Besedovsky, H.O. & del Rey, A. (1996). Immune-neuro-endocrine interactions: facts and hypotheses. Endocrine Reviews, 17, 64–102.
- Brisbin, J.T., Gong, J. & Sharif, S. (2008). Interactions between commensal bacteria and the gut-associated immune system of the chicken. Animal Health Research Reviews, 9, 101–110. 10.1017/S146625230800145X
- Bueno, L. (2000). Neuroimmune alterations of ENS functioning. Gut, 47, 63–65. 10.1136/gut.47.suppl_4.iv63
- Burkholder, K.M., Thompson, K.L., Einstein, M.E., Applegate, T.J. & Patterson, J.A. (2008). Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella Enteritidis colonization in broilers. Poultry Science, 87, 1734–1741. 10.3382/ps.2008-00107
- Caroprese, M., Annicchiarico, G., Schena, L., Muscio, A., Migliore, R. & Sevi, A. (2009). Influence of space allowance and housing conditions on the welfare, immune response and production performance of dairy ewes. The Journal of Dairy Research, 76, 66–73. 10.1017/S0022029908003683
- Chappell, L., Kaiser, P., Barrow, P., Jones, M.A., Johnston, C. & Wigley, P. (2009). The immunobiology of avian systemic salmonellosis. Veterinary Immunology and Immunopathology, 128, 53–59. 10.1016/j.vetimm.2008.10.295
- Costa-Pinto, F.A., Cohn, D.W., Sa-Rocha, V.M., Sa-Rocha, L.C. & Palermo-Neto, J. (2009). Behavior: a relevant tool for brain-immune system interaction studies. Annals of the New York Academy of Sciences, 1153, 107–119. 10.1111/j.1749-6632.2008.03961.x
- Costa-Pinto, F.A. & Palermo-Neto, J. (2010). Neuroimmune interactions in stress. Neuroimmunomodulation, 17, 196–199. 10.1159/000258722
- Dafwang, I.I., Cook, M.E. & Sunde, M.L. (1987). Interaction of dietary antibiotic supplementation and stocking density on broiler chick performance and immune response. British Poultry Science, 28, 47–55. 10.1080/00071668708416935
- Dawkins, M.S., Donnelly, C.A. & Jones, T.A. (2004). Chicken welfare is influenced more by housing conditions than by stocking density. Nature, 427, 342–344. 10.1038/nature02226
- Dozier III, W.A., Thaxton, J.P., Purswell, J.L., Olanrewaju, H.A., Branton, S.L. & Roush, W.B. (2006). Stocking density effects on male broilers grown to 1.8 kilograms of body weight. Poultry Science, 85, 344–351.
- Dunkley, C.S., McReynolds, J.L., Dunkley, K.D., Njongmeta, L.N., Berghman, L.R., Kubena, L.F., Nisbet, D.J. & Ricke, S.C. (2007). Molting in Salmonella-Enteritidis-challenged laying hens fed alfalfa crumbles. IV. Immune and stress protein response. Poultry Science, 86, 2502–2508. 10.3382/ps.2006-00401
- Ekstrand, C., Carpenter, T.E., Andersson, I. & Algers, B. (1998). Prevalence and control of foot-pad dermatitis in broilers in Sweden. British Poultry Science, 39, 318–324. 10.1080/00071669888845
- Estevez, I. (2007). Density allowances for broilers: where to set the limits? Poultry Science, 86, 1265–1272.
- Fagarasan, S. (2006). Intestinal IgA synthesis: a primitive form of adaptive immunity that regulates microbial communities in the gut. Current Topics in Microbiology and Immunology, 308, 137–153.
- Fagarasan, S. & Honjo, T. (2003). Intestinal IgA synthesis: regulation of front-line body defences. Nature Reviews. Immunology, 3, 63–72. 10.1038/nri982
- Fonseca, E.S., Massoco, C.O. & Palermo-Neto, J. (2002). Effects of prenatal stress on stress-induced changes in behavior and macrophage activity of mice. Physiology & Behavior, 77, 205–215. 10.1016/S0031-9384(02)00812-0
- Gao, J., Zhang, H.J., Yu, S.H., Wu, S.G., Yoon, I., Quigley, J., Gao, Y.P. & Qi, G.H. (2008). Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poultry Science, 87, 1377–1384. 10.3382/ps.2007-00418
- Glaser, R. & Kiecolt-Glaser, J.K. (2005). Stress-induced immune dysfunction: implications for health. Nature Reviews Immunology, 5, 243–251. 10.1038/nri1571
- Grenham, S., Clarke, G., Cryan, J.F. & Dinan, T.G. (2011). Brain-gut-microbe communication in health and disease. Frontiers in Physiology, 2, 94. 10.3389/fphys.2011.00094
- Gross, W.B. (1992). Effect of short-term exposure of chickens to corticosterone on resistance to challenge exposure with Escherichia coli and antibody response to sheep erythrocytes. The American Journal of Veterinary Research, 53, 291–293.
- Guardia, S., Konsak, B., Combes, S., Levenez, F., Cauquil, L., Guillot, J.F., Moreau-Vauzelle, C., Lessire, M., Juin, H. & Gabriel, I. (2011). Effects of stocking density on the growth performance and digestive microbiota of broiler chickens. Poultry Science, 90, 1878–1889. 10.3382/ps.2010-01311
- Hart, A. & Kamm, M.A. (2002). Review article: mechanisms of initiation and perpetuation of gut inflammation by stress. Alimentary Pharmacology & Therapeutics, 16, 2017–2028. 10.1046/j.1365-2036.2002.01359.x
- Hasui, M., Hirabayashi, Y. & Kobayashi, Y. (1989). Simultaneous measurement by flow cytometry of phagocytosis and hydrogen peroxide production of neutrophils in whole blood. Journal of Immunological Methods, 117, 53–58. 10.1016/0022-1759(89)90118-X
- Heckert, R.A., Estevez, I., Russek-Cohen, E. & Pettit-Riley, R. (2002). Effects of density and perch availability on the immune status of broilers. Poultry Science, 81, 451–457.
- Hodges, R.D. (1974). The Histology of the Fowl. London and New York: Academic Press.
- Hoerr, F.J. (2010). Clinical aspects of immunosuppression in poultry. Avian Diseases, 54, 2–15. 10.1637/8909-043009-Review.1
- Houshmand, M., Azhar, K., Zulkifli, I., Bejo, M.H. & Kamyab, A. (2012). Effects of prebiotic, protein level, and stocking density on performance, immunity, and stress indicators of broilers. Poultry Science, 91, 393–401. 10.3382/ps.2010-01050
- Humphrey, T. (2006). Are happy chickens safer chickens? Poultry welfare and disease susceptibility. British Poultry Science, 47, 379–391. 10.1080/00071660600829084
- ISO. (2007). Iso 6579:2002/amd 1:2007 annex d: detection of Salmonella spp. In animal faeces and in environmental samples from the primary production stage. International Organization for Standardization. Geneva, Switzerland.
- Kaiser, P., Wu, Z., Rothwell, L., Fife, M., Gibson, M., Poh, T.Y., Shini, A., Bryden, W. & Shini, S. (2009). Prospects for understanding immune-endocrine interactions in the chicken. General and Comparative Endocrinology, 163, 83–91. 10.1016/j.ygcen.2008.09.013
- Kestin, S.C., Gordon, S., Su, G. & Sorensen, P. (2001). Relationships in broiler chickens between lameness, liveweight, growth rate and age. The Veterinary Record, 148, 195–197. 10.1136/vr.148.7.195
- Kiank, C., Daeschlein, G. & Schuett, C. (2008). Pneumonia as a long-term consequence of chronic psychological stress in BALB/c mice. Brain Behavior and Immunity, 22, 1173–1177. 10.1016/j.bbi.2008.05.003
- Kuriyama, T., Machida, K. & Suzuki, K. (1996). Importance of correlations between phagocytic activity and superoxide production of neutrophils under conditions of voluntary exercise and stress. Journal of Clinical Laboratory Analysis, 10, 458–464. 10.1002/(SICI)1098-2825(1996)10:6%3C458::AID-JCLA25%3E3.0.CO;2-V
- Lambert, G.P. (2009). Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. Journal of Animal Science, 87, 101–108. 10.2527/jas.2008-1339
- Lambert, G.P., Gisolfi, C.V., Berg, D.J., Moseley, P.L., Oberley, L.W. & Kregel, K.C. (2002). Selected contribution: hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. Journal of Applied Physiology, 92, 1750–1761.
- Lay, D.C., Jr., Fulton, R.M., Hester, P.Y., Karcher, D.M., Kjaer, J.B., Mench, J.A., Mullens, B.A., Newberry, R.C., Nicol, C.J., O'Sullivan, N.P. & Porter, R.E. (2011). Hen welfare in different housing systems. Poultry Science, 90, 278–294. 10.3382/ps.2010-00962
- Lebman, D.A. & Coffman, R.L. (1988). Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. The Journal of Experimental Medicine, 168, 853–862. 10.1084/jem.168.3.853
- Martrenchar, A., Boilletot, E., Huonnic, D. & Pol, F. (2002). Risk factors for foot-pad dermatitis in chicken and turkey broilers in France. Preventive Veterinary Medicine, 52, 213–226. 10.1016/S0167-5877(01)00259-8
- Mashaly, M.M., Hendricks, G.L., 3rd, Kalama, M.A., Gehad, A.E., Abbas, A.O. & Patterson, P.H. (2004). Effect of heat stress on production parameters and immune responses of commercial laying hens. Poultry Science, 83, 889–894.
- Mast, J., Goddeeris, B.M., Peeters, K., Vandesande, F. & Berghman, L.R. (1998). Characterisation of chicken monocytes, macrophages and interdigitating cells by the monoclonal antibody KUL01. Veterinary Immunology and Immunopathology, 61, 343–357. 10.1016/S0165-2427(97)00152-9
- Mastroeni, P. & Menager, N. (2003). Development of acquired immunity to Salmonella. Journal of Medical Microbiology, 52, 453–459. 10.1099/jmm.0.05173-0
- McEwen, B.S. (2000). The neurobiology of stress: from serendipity to clinical relevance. Brain Research, 886, 172–189. 10.1016/S0006-8993(00)02950-4
- Mebrhatu, M.T., Cenens, W. & Aertsen, A. (2014). An overview of the domestication and impact of the Salmonella mobilome. Critical Reviews in Microbiology, 40, 63–75.
- Mestecky, J., Russell, M.W., Elson, C.O. (1999). Intestinal IgA: novel views on its function in the defence of the largest mucosal surface. Gut, 44, 2–5. 10.1136/gut.44.1.2
- Mitchell, M.A. & Carlisle, A.J. (1992). The effects of chronic exposure to elevated environmental temperature on intestinal morphology and nutrient absorption in the domestic fowl (Gallus domesticus). Comparative Biochemistry and Physiology. A, Comparative Physiology, 101, 137–142. 10.1016/0300-9629(92)90641-3
- Mtileni, B.J., Nephawe, K.A., Nesamvuni, A.E. & Benyi, K. (2007). The influence of stocking density on body weight, egg weight, and feed intake of adult broiler breeder hens. Poultry Science, 86, 1615–1619.
- Palermo-Neto, J., de Oliveira Massoco, C. & Robespierre de Souza, W. (2003). Effects of physical and psychological stressors on behavior, macrophage activity, and Ehrlich tumor growth. Brain Behavior and Immunity, 17, 43–54. 10.1016/S0889-1591(02)00057-0
- Palermo-Neto, J., Fonseca, E.S., Quinteiro-Filho, W.M., Correia, C.S. & Sakai, M. (2008). Effects of individual housing on behavior and resistance to Ehrlich tumor growth in mice. Physiology & Behavior, 95, 435–440. 10.1016/j.physbeh.2008.07.006
- Prosser, C., Stelwagen, K., Cummins, R., Guerin, P., Gill, N. & Milne, C. (2004). Reduction in heat-induced gastrointestinal hyperpermeability in rats by bovine colostrum and goat milk powders. Journal of Applied Physiology, 96, 650–654. 10.1152/japplphysiol.00295.2003
- Quinteiro-Filho, W.M., Gomes, A.V., Pinheiro, M.L., Ribeiro, A., Ferraz-de-Paula, V., Astolfi-Ferreira, C.S., Ferreira, A.J. & Palermo-Neto, J. (2012a). Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis. Avian Pathology, 41, 421–427. 10.1080/03079457.2012.709315
- Quinteiro-Filho, W.M., Ribeiro, A., Ferraz-de-Paula, V., Pinheiro, M.L., Sakai, M., Sa, L.R., Ferreira, A.J. & Palermo-Neto, J. (2010). Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poultry Science, 89, 1905–1914. 10.3382/ps.2010-00812
- Quinteiro-Filho, W.M., Righi, D.A. & Palermo-Neto, J. (2009). Effect of cyhalothrin on Ehrlich tumor growth and macrophage activity in mice. Brazilian Journal of Medical and Biological Research, 42, 912–917. 10.1590/S0100-879X2009001000006
- Quinteiro-Filho, W.M., Rodrigues, M.V., Ribeiro, A., Ferraz-de-Paula, V., Pinheiro, M.L., Sa, L.R., Ferreira, A.J. & Palermo-Neto, J. (2012b). Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: role of acute HPA axis activation. Journal of Animal Science, 90, 1986–1994. 10.2527/jas.2011-3949
- Qureshi, M.A., Dietert, R.R. & Bacon, L.D. (1986). Genetic variation in the recruitment and activation of chicken peritoneal macrophages. Proceedings of the Society for Experimental Biology and Medicine, 181, 560–568. 10.3181/00379727-181-42293
- Ratcliffe, M.J. (2006). Antibodies, immunoglobulin genes and the bursa of Fabricius in chicken B cell development. Developmental and Comparative Immunology, 30, 101–118. 10.1016/j.dci.2005.06.018
- Riddell, C. (1987). Avian Histopathology. Lawrence, Kansas: American Association of Avian Pathologists. Allen Press Inc.
- Sharkey, K.A. & Mawe, G.M. (2002). Neuroimmune and epithelial interactions in intestinal inflammation. Current Opinion in Pharmacology, 2, 669–677. 10.1016/S1471-4892(02)00215-1
- Shepherd, E.M. & Fairchild, B.D. (2010). Footpad dermatitis in poultry. Poultry Science, 89, 2043–2051. 10.3382/ps.2010-00770
- Shini, S., Huff, G.R., Shini, A. & Kaiser, P. (2010). Understanding stress-induced immunosuppression: exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poultry Science, 89, 841–851. 10.3382/ps.2009-00483
- Shini, S. & Kaiser, P. (2008). Effects of stress, mimicked by administration of corticosterone in drinking water, on the expression of chicken cytokine and chemokine genes in lymphocytes. Stress, 12, 388–399. 10.1080/10253890802526894
- Shini, S., Shini, A. & Huff, G.R. (2009). Effects of chronic and repeated corticosterone administration in rearing chickens on physiology, the onset of lay and egg production of hens. Physiology Behavior, 98, 73–77. 10.1016/j.physbeh.2009.04.012
- Singleton, K.D. & Wischmeyer, P.E. (2006). Oral glutamine enhances heat shock protein expression and improves survival following hyperthermia. Shock, 25, 295–299. 10.1097/01.shk.0000196548.10634.02
- Soleimani, A.F., Zulkifli, I., Hair-Bejo, M., Ebrahimi, M., Jazayeri, S.D., Hashemi, S.R., Meimandipour, A. & Goh, Y.M. (2012). Epigenetic modification: possible approach to reduce Salmonella enterica serovar enteritidis susceptibility under stress conditions. Avian Pathology, 41, 351–354. 10.1080/03079457.2012.691155
- Tachibana, T., Oikawa, D., Takahashi, H., Boswell, T. & Furuse, M. (2007). The anorexic effect of alpha-melanocyte-stimulating hormone is mediated by corticotrophin-releasing factor in chicks. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 147, 173–178. 10.1016/j.cbpa.2006.12.044
- Thaxton, J.P., Olanrewaju, H.A., Branton, S.L. & Roush, W.B. (2005). Stocking density effects on male broilers grown to 1.8 kilograms of body weight. Poultry Science, 708, 344–351.
- Tong, H.B., Lu, J., Zou, J.M., Wang, Q. & Shi, S.R. (2012). Effects of stocking density on growth performance, carcass yield, and immune status of a local chicken breed. Poultry Science, 91, 667–673. 10.3382/ps.2011-01597
- Van Immerseel, F. (2010). Stress-induced survival strategies enable Salmonella Enteritidis to persistently colonize the chicken oviduct tissue and cope with antimicrobial factors in egg white: a hypothesis to explain a pandemic. Gut Pathogens, 2, 23. 10.1186/1757-4749-2-23
- Verbrugghe, E., Boyen, F., Gaastra, W., Bekhuis, L., Leyman, B., Van Parys, A., Haesebrouck, F. & Pasmans, F. (2012). The complex interplay between stress and bacterial infections in animals. Veterinary Microbiology, 155, 115–127. 10.1016/j.vetmic.2011.09.012
- Wang, S., Xu, W. & Cao, Q. (2001). The influence of stress inhibition on the plasma levels of LPS, pro-inflammatory and th1/th2 cytokines in severely scalded rats. Zhonghua Shao Shang Za Zhi, 17, 177–180.
- Weeks, C.A., Danbury, T.D., Davies, H.C., Hunt, P. & Kestin, S.C. (2000). The behaviour of broiler chickens and its modification by lameness. Applied Animal Behaviour Science, 67, 111–125. 10.1016/S0168-1591(99)00102-1
- Zulkifli, I., Abdulllah, N., Azrin, N.M. & Ho, Y.W. (2000). Growth performance and immune response of two commercial broiler strains fed diets containing lactobacillus cultures and oxytetracycline under heat stress conditions. British Poultry Science, 41, 593–597. 10.1080/713654979